Laboratory and Instrumental Risk Factors Associated with a Sudden Cardiac Death Prone ECG Pattern in the General Population: Data from the Brisighella Heart Study
Abstract
1. Introduction
2. Patients and Methods
2.1. The Brisighella Heart Study
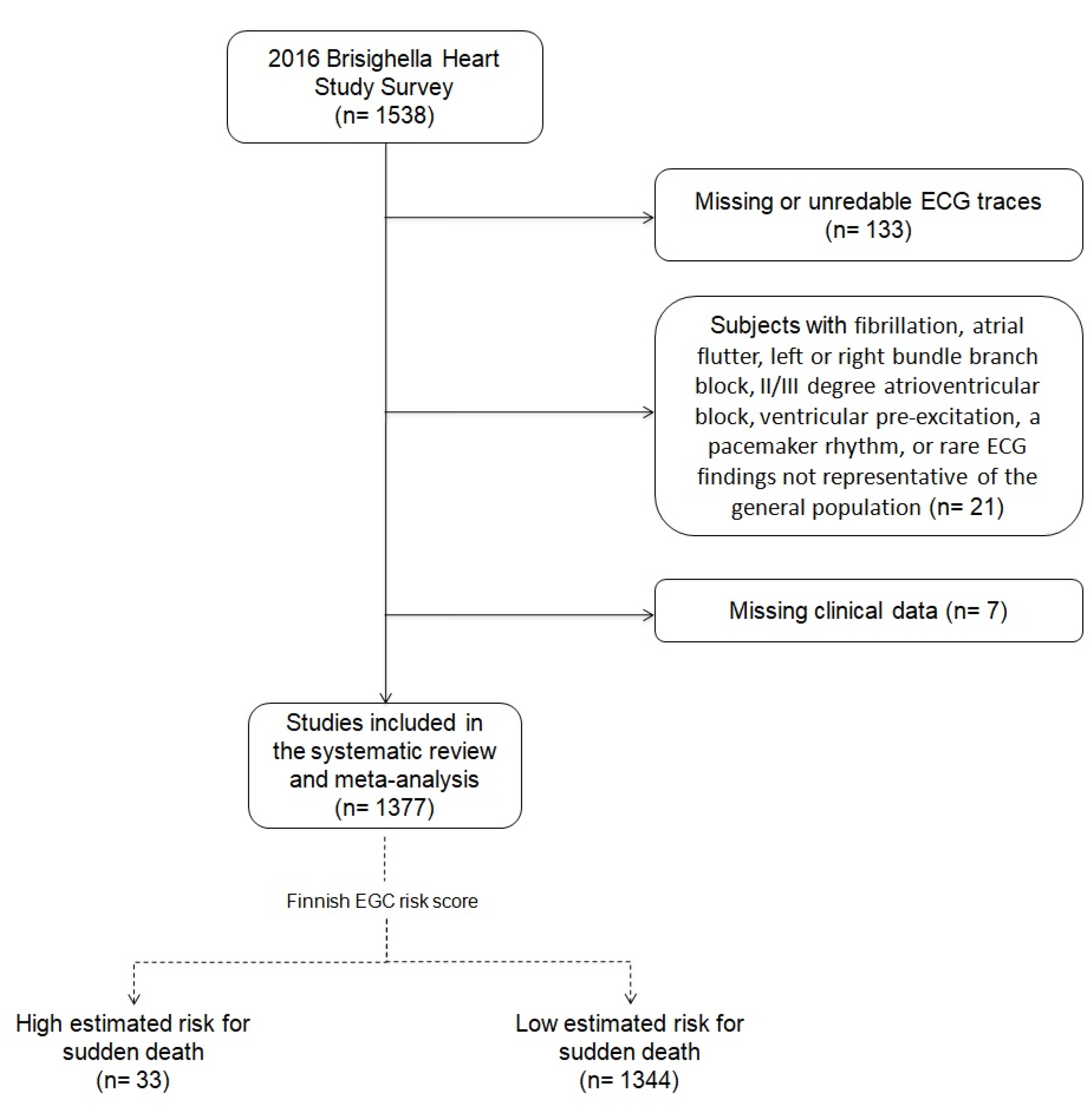
2.2. The 2016 Brisighella Heart Study Survey
2.3. ECG Risk Score
2.4. Statistical Analysis
3. Results
3.1. High-Risk Subjects Identification
3.2. High-Risk Subjects’ History
3.3. High-Risk Subjects’ Anthropometric Data
3.4. High-Risk Subjects’ Hemodynamic Parameters
3.5. High-Risk Subjects’ Laboratory Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priori, S.G.; Aliot, E.; Blomstrom-Lundqvist, C.; Bossaert, L.; Breithardt, G.; Brugada, P.; Camm, A.J.; Cappato, R.; Cobbe, S.M.; Di Mario, C.; et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur. Heart J. 2001, 22, 1374–1450. [Google Scholar] [CrossRef]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of Sudden Cardiac Death: Clinical and Research Implications. Prog. Cardiovasc. Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Holkeri, A.; Eranti, A.; Haukilahti, M.A.E.; Kerola, T.; Kenttä, T.V.; Tikkanen, J.T.; Anttonen, O.; Noponen, K.; Seppänen, T.; Rissanen, H.; et al. Predicting sudden cardiac death in a general population using an electrocardiographic risk score. Heart 2020, 106, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; D’Addato, S.; Santi, F.; Ferroni, A.; Borghi, C.; Brisighella Heart Study. Leisure-time physical activity and cardiovascular disease mortality: The Brisighella Heart Study. J. Cardiovasc. Med. 2012, 13, 559–564. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; D’Addato, S.; Borghi, C.; for the Brisighella Heart Study group. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension. J. Hypertens. 2019, 37, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Rosticci, M.; Fogacci, F.; Grandi, E.; D’Addato, S.; Borghi, C. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur. J. Intern. Med. 2017, 37, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hickson, S.; Butlin, M.; Broad, J.; Avolio, A.P.; Wilkinson, I.B.; McEniery, C.M. Validity and repeatability of the Vicorder apparatus: A comparison with the SphygmoCor device. Hypertens. Res. 2009, 32, 1079–1085. [Google Scholar] [CrossRef]
- Parsons, T.J.; Sartini, C.; Ellins, E.A.; Halcox, J.P.J.; Smith, K.E.; Ash, S.; Lennon, L.T.; Wannamethee, S.G.; Lee, I.M.; Whincup, P.H.; et al. Objectively measured physical activity, sedentary time and subclinical vascular disease: Cross-sectional study in older British men. Prev. Med. 2016, 89, 194–199. [Google Scholar] [CrossRef]
- Müller, J.; Ewert, P.; Hager, A. Increased aortic blood pressure augmentation in patients with congenital heart defects e a crosssectional study in 1125 patients and 322 controls. Int. J. Cardiol. 2015, 184, 225–229. [Google Scholar] [CrossRef]
- Kong, M.H.; Fonarow, G.C.; Peterson, E.D.; Curtis, A.B.; Hernandez, A.F.; Sanders, G.D.; Thomas, K.L.; Hayes, D.L.; Al-Khatib, S.M. Systematic review of the incidence of sudden cardiac death in the United States. J. Am. Coll. Cardiol. 2011, 57, 794–801. [Google Scholar] [CrossRef]
- Shimizu, M.; Koizumi, J.; Miyamoto, S.; Origasa, H.; Mabuchi, H.; HOLICOS-PAT Study Group. Electrocardiographic events and cholesterol reduction with pravastatin in patients with hypercholesterolemia: The Hokuriku Lipid Coronary Heart Disease Study-Pravastatin Atherosclerosis Trial. Int. J. Cardiol. 2005, 99, 395–401. [Google Scholar] [CrossRef]
- Cicero, A.F.; Rosticci, M.; Tocci, G.; Bacchelli, S.; Urso, R.; D’Addato, S.; Borghi, C. Serum uric acid and other short-term predictors of electrocardiographic alterations in the Brisighella Heart Study cohort. Eur. J. Intern. Med. 2015, 26, 255–258. [Google Scholar] [CrossRef]
- İlgenli, T.F.; Tokatlı, A.; Akpınar, O.; Kılıçaslan, F. The Effects of Cigarette Smoking on the Tp-e Interval, Tp-e/QT Ratio and Tp-e/QTc Ratio. Adv. Clin. Exp. Med. 2015, 24, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.B.; Arab, C.; DeFilippis, A.P.; Lorkiewicz, P.; Keith, R.J.; Xie, Z.; Bhatnagar, A.; Carll, A.P. Smoking Accelerates Atrioventricular Conduction in Humans Concordant with Increased Dopamine Release. Cardiovasc. Toxicol. 2021, 21, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Z.; Liu, Y.; Yu, S.; Yang, H.; Zheng, L.; Zhang, Y.; Sun, Y. Sex-specific association between serum uric acid and prolonged corrected QT interval: Result from a general rural Chinese population. Medicine 2016, 95, e5568. [Google Scholar] [CrossRef]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Mozos, I. The link between ventricular repolarization variables and arterial function. J. Electrocard. 2015, 48, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.L.; Palmisano, J.N.; Benjamin, E.J. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events: The Framingham Heart Study. Circ. Cardiovasc. Imaging 2016, 9, e004979. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Rosticci, M.; Reggi, A.; Derosa, G.; Parini, A.; Grandi, E.; D’Addato, S.; Borghi, C. Relationship Between Serum Uric Acid and Electrocardiographic Alterations in a Large Sample of General Population: Data from the Brisighella Heart Study. High Blood. Press. Cardiovasc. Prev. 2015, 22, 129–134. [Google Scholar] [CrossRef]
- Mantovani, A.; Rigolon, R.; Pichiri, I.; Morani, G.; Bonapace, S.; Dugo, C.; Zoppini, G.; Bonora, E.; Targher, G. Relation of elevated serum uric acid levels to first-degree heart block and other cardiac conduction defects in hospitalized patients with type 2 diabetes. J. Diabetes Complicat. 2017, 12, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.F.; Grupper, A.; Chernichovski, T.; Grupper, A.; Hillel, O.; Engel, A.; Schwartz, D. Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J. Vasc. Res. 2011, 48, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Suzuki, H.; Kamioka, M.; Kamiyama, Y.; Saitoh, S.; Takeishi, Y. Uric acid increases the incidence of ventricular arrhythmia in patients with left ventricular hypertrophy. Fukushima J. Med. Sci. 2012, 58, 101–106. [Google Scholar] [CrossRef][Green Version]
- Silbernagel, G.; Hoffmann, M.M.; Grammer, T.B.; Boehm, B.O.; März, W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, Y.; Horio, T.; Kamide, K.; Rakugi, H.; Ogihara, T.; Kawano, Y. Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension 2006, 47, 195–202. [Google Scholar] [CrossRef]
- Cicero, A.; Fogacci, F.; Grandi, E.; Rizzoli, E.; Bove, M.; D’Addato, S.; Borghi, C. Prevalent Seasoning and Cooking Fats, Arterial Stiffness and Blood Lipid Pattern in a Rural Population Sample: Data from the Brisighella Heart Study. Nutrients 2020, 12, 3063. [Google Scholar] [CrossRef]
- Cicero, A.; Fogacci, F.; Desideri, G.; Grandi, E.; Rizzoli, E.; D’Addato, S.; Borghi, C. Arterial Stiffness, Sugar-Sweetened Beverages and Fruits Intake in a Rural Population Sample: Data from the Brisighella Heart Study. Nutrients 2019, 11, 2674. [Google Scholar] [CrossRef]
| Long-Term NSAIDs Use | ||||
|---|---|---|---|---|
| No | Yes | |||
| Estimated risk of sudden death | Low | Number of subjects | 1189 | 95 |
| % within risk of sudden death | 92.6% | 7.4% | ||
| % in long-term NSAIDs use | 98.2% | 91.3% | ||
| % of the total | 90.4% | 7.2% | ||
| High | Number of subjects | 22 | 9 | |
| % within risk of sudden death | 71% | 29.0% | ||
| % in Long-term NSAIDs use | 1.8% | 8.7% | ||
| % of the total | 1.7% | 0.7% | ||
| Whole sample | Number of subjects | 1211 | 104 | |
| % within risk of sudden death | 92.1% | 7.9% | ||
| % in long-term NSAIDs use | 100% | 100% | ||
| % of the total | 92.1% | 7.9% | ||
| Estimated Risk of Sudden Death | ||||
|---|---|---|---|---|
| Low | High | |||
| Antihypertensive drugs use | No | Number of subjects | 909 | 12 |
| % within antihypertensive drugs use | 98.7% | 1.3% | ||
| % within risk of sudden death | 67.7% | 36.4% | ||
| % of the total | 66.1% | 0.9% | ||
| Yes | Number of subjects | 434 | 21 | |
| % within antihypertensive drugs use | 95.4% | 4.6% | ||
| % within risk of sudden death | 32.3% | 63.6% | ||
| % of the total | 31.5% | 1.5% | ||
| Whole sample | Number of subjects | 1343 | 33 | |
| % within antihypertensive drugs use | 97.6% | 2.4% | ||
| % within risk of sudden death | 100% | 100% | ||
| % of the total | 97.6% | 2.4% | ||
| Low Estimated Risk for Sudden Death (Mean ± Standard Deviation) | High Estimated Risk for Sudden Death (Mean ± Standard Deviation) | Mean Differences | 95% Confidence Interval of the Differences | Sig. (2-Tailed) | ||
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Waist circumference (cm) | 92.03 ± 13.22 | 97.27 ± 8.98 | −5.23 | −9.85 | −0.62 | 0.026 |
| Hip circumference (cm) | 100.88 ± 11.17 | 103.71 ± 6.92 | −2.83 | −6.66 | 1.01 | 0.148 |
| Body Mass Index (kg/m2) | 26.68 ± 4.64 | 27.63 ± 2.84 | −0.95 | −2.55 | 0.64 | 0.241 |
| Waist/Hip ratio | 0.91 ± 0.09 | 0.93 ± 0.08 | −0.03 | −0.06 | 0.01 | 0.109 |
| Index of Central Obesity | 0.56 ± 0.08 | 0.60 ± 0.06 | −0.04 | −0.07 | −0.01 | 0.005 |
| Wrist circumference (cm) | 17.16 ± 1.79 | 17.38 ± 1.26 | −0.22 | −0.84 | 0.39 | 0.479 |
| Arm circumference (cm) | 28.46 ± 3.39 | 27.91 ± 2.65 | 0.55 | −0.62 | 1.72 | 0.356 |
| Neck circumference (cm) | 36.91 ± 4.15 | 38.47 ± 3.43 | −1.56 | −2.99 | −0.13 | 0.032 |
| Low Estimated Risk for Sudden Death (Mean ± Standard Deviation) | High Estimated Risk for Sudden Death (Mean ± Standard Deviation) | Mean Differences | 95% Confidence Interval of the Differences | Sig. (2-Tailed) | ||
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Systolic blood pressure (mmHg) | 140.23 ± 20.69 | 151.10 ± 28.2 | −10.87 | −18.44 | −3.3 | 0.005 |
| Diastolic blood pressure (mmHg) | 73.22 ± 9.53 | 78.77 ± 15.66 | −5.55 | −9.07 | −2.03 | 0.002 |
| Pulse pressure (mmHg) | 67.01 ± 16.83 | 72.33 ± 20.73 | −5.32 | −11.45 | 0.82 | 0.089 |
| Aortic blood pressure (mmHg) | 137.27 ± 20.82 | 147.53 ± 27.88 | −10.26 | −17.88 | −2.65 | 0.008 |
| Aortic pulse pressure (mmHg) | 64.06 ± 16.82 | 68.77 ± 20.76 | −4.71 | −10.84 | 1.43 | 0.133 |
| Augmentation Index (%) | 25.62 ± 9.28 | 24.63 ± 11.27 | 0.98 | −2.40 | 4.37 | 0.569 |
| Cardiac Output (L/min) | 7.05 ± 2.28 | 8.09 ± 2.8 | −1.04 | −1.87 | −0.21 | 0.015 |
| Total Peripheral Resistance (PRU) | 0.93 ± 0.29 | 0.88 ± 0.28 | 0.05 | −0.05 | 0.16 | 0.314 |
| Stroke Volume (mL) | 112.56 ± 34.88 | 114.53 ± 36.84 | −1.97 | −14.64 | 10.7 | 0.760 |
| Mean Arterial Pressure (mmHg) | 95.56 ± 11.84 | 102.87 ± 18.25 | −7.32 | −11.67 | −2.96 | 0.001 |
| Carotid-femoral Pulse Wave Velocity (m/s) | 9 ± 2.30 | 10.23 ± 3.34 | −1.3 | −2.14 | −0.45 | 0.003 |
| Pulse Pressure Index | 0.47 ± 0.06 | 0.47 ± 0.08 | −0.001 | −0.024 | 0.023 | 0.955 |
| Right Ankle-Brachial Index | 1.13 ± 0.16 | 1.16 ± 0.15 | −0.03 | −0.1 | 0.03 | 0.281 |
| Left Ankle-Brachial Index | 1.13 ± 0.16 | 1.15 ± 0.13 | −0.03 | −0.09 | 0.04 | 0.432 |
| Cardiac Index | 4.01 ± 1.46 | 4.53 ± 2.08 | −0.53 | −1.64 | 0.58 | 0.351 |
| Low Estimated Risk for Sudden Death (Mean ± Standard Deviation) | High Estimated Risk for Sudden Death (Mean ± Standard Deviation) | Mean Differences | 95% Confidence Interval of the Differences | Sig. (2-Tailed) | ||
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Total Cholesterol (mg/dL) | 217.27 ± 40.63 | 217.09 ± 39.78 | 0.18 | −13.86 | 14.22 | 0.980 |
| Triglycerides (mg/dL) | 118.57 ± 68.09 | 161.03 ± 144.63 | −42.46 | −66.95 | −17.98 | 0.001 |
| High-Density Lipoprotein Cholesterol (mg/dL) | 51.99 ± 15.01 | 53.21 ± 19.82 | −1.22 | −6.46 | 4.01 | 0.647 |
| Low-Density Lipoprotein Cholesterol (mg/dL) | 141.77 ± 36.79 | 135.92 ± 38.85 | 5.85 | −7.28 | 18.98 | 0.382 |
| Serum uric acid (mg/dL) | 5.27 ± 1.33 | 5.86 ± 1.35 | −0.59 | −1.05 | −0.13 | 0.013 |
| Fasting plasma glucose (mg/dL) | 95.18 ± 20.32 | 98.48 ± 35.19 | −3.3 | −10.49 | 3.89 | 0.368 |
| Apolipoprotein A1 (mg/dL) | 154.75 ± 28.51 | 150.06 ± 28.47 | 4.69 | −5.16 | 14.55 | 0.350 |
| Apolipoprotein B-100 (mg/dL) | 91.96 ± 20.58 | 89.3 ± 21.24 | 2.65 | −4.47 | 9.77 | 0.465 |
| Creatinine (mg/dL) | 1.03 ± 0.19 | 1.15 ± 0.38 | −0.12 | −0.19 | −0.05 | 0.001 |
| Aspartate aminotransferase (U/L) | 23.48 ± 17.91 | 24.27 ± 8.11 | −0.8 | −6.93 | 5.34 | 0.799 |
| Alanine aminotransferase (U/L) | 24.55 ± 20.2 | 22.06 ± 7.72 | 2.49 | −4.42 | 9.4 | 0.480 |
| Creatinine Phosphokinase (U/L) | 130.02 ± 146.85 | 119.82 ± 63.42 | 10.2 | −40.08 | 60.48 | 0.691 |
| Gamma-Glutamyl-Transferase (U/L) | 26.8 ± 26.34 | 32.03 ± 40.76 | −5.23 | −14.48 | 4.03 | 0.268 |
| Low-Density Lipoprotein Cholesterol/Apolipoprotein B-100 | 1.56 ± 0.32 | 1.53 ± 0.26 | 0.03 | −0.08 | 0.15 | 0.576 |
| High-Density Lipoprotein Cholesterol/Apolipoprotein A1 | 0.33 ± 0.07 | 0.35 ± 0.08 | −0.01 | −0.04 | 0.01 | 0.241 |
| Lipoprotein(a) (mg/dL) | 22.72 ± 30.06 | 20.84 ± 29.22 | 1.88 | −8.5 | 12.27 | 0.722 |
| Visceral Adiposity Index | 3.27 ± 1.67 | 3.8 ± 1.33 | −0.55 | −1.5 | 0.41 | 0.259 |
| eGFR-CKD Epi (mL/min) | 71.32 ± 15.52 | 62.65 ± 17.56 | 8.68 | 3.3 | 14.06 | 0.002 |
| eGFR-MDRD (mL/min) | 70.20 ± 13.81 | 65.12 ± 16.79 | 5.08 | 0.28 | 9.89 | 0.038 |
| eGFR-Cockcroft Gault (mL/min) | 75.24 ± 24.1 | 62.58 ± 25.75 | 12.66 | 4.31 | 21 | 0.003 |
| eGFR-Mayo Quadratic Formula (mL/min) | 89.92 ± 17.3 | 79.76 ± 22.37 | 10.16 | 4.13 | 16.19 | 0.001 |
| Lipid Accumulation Product | 44.4 ± 36.49 | 65.23 ± 58.6 | −20.82 | −33.86 | −7.79 | 0.002 |
| Hepatic Steatosis Index | 37.8 ± 5.83 | 37.48 ± 3.84 | 0.32 | −1.69 | 2.32 | 0.754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, P.; Cicero, A.F.G.; Fogacci, F.; D’Addato, S.; Bacchelli, S.; Borghi, C.; on behalf of the Brisighella Heart Study Group. Laboratory and Instrumental Risk Factors Associated with a Sudden Cardiac Death Prone ECG Pattern in the General Population: Data from the Brisighella Heart Study. J. Clin. Med. 2021, 10, 640. https://doi.org/10.3390/jcm10040640
Coppola P, Cicero AFG, Fogacci F, D’Addato S, Bacchelli S, Borghi C, on behalf of the Brisighella Heart Study Group. Laboratory and Instrumental Risk Factors Associated with a Sudden Cardiac Death Prone ECG Pattern in the General Population: Data from the Brisighella Heart Study. Journal of Clinical Medicine. 2021; 10(4):640. https://doi.org/10.3390/jcm10040640
Chicago/Turabian StyleCoppola, Pierangelo, Arrigo Francesco Giusepp Cicero, Federica Fogacci, Sergio D’Addato, Stefano Bacchelli, Claudio Borghi, and on behalf of the Brisighella Heart Study Group. 2021. "Laboratory and Instrumental Risk Factors Associated with a Sudden Cardiac Death Prone ECG Pattern in the General Population: Data from the Brisighella Heart Study" Journal of Clinical Medicine 10, no. 4: 640. https://doi.org/10.3390/jcm10040640
APA StyleCoppola, P., Cicero, A. F. G., Fogacci, F., D’Addato, S., Bacchelli, S., Borghi, C., & on behalf of the Brisighella Heart Study Group. (2021). Laboratory and Instrumental Risk Factors Associated with a Sudden Cardiac Death Prone ECG Pattern in the General Population: Data from the Brisighella Heart Study. Journal of Clinical Medicine, 10(4), 640. https://doi.org/10.3390/jcm10040640










