Long-Term Technical Performance of the Osypka QT-5® Ventricular Pacemaker Lead
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Device Follow-Up Examinations
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Patient Characteristics
3.2. Perioperative Complications
3.3. Long-Term Outcomes
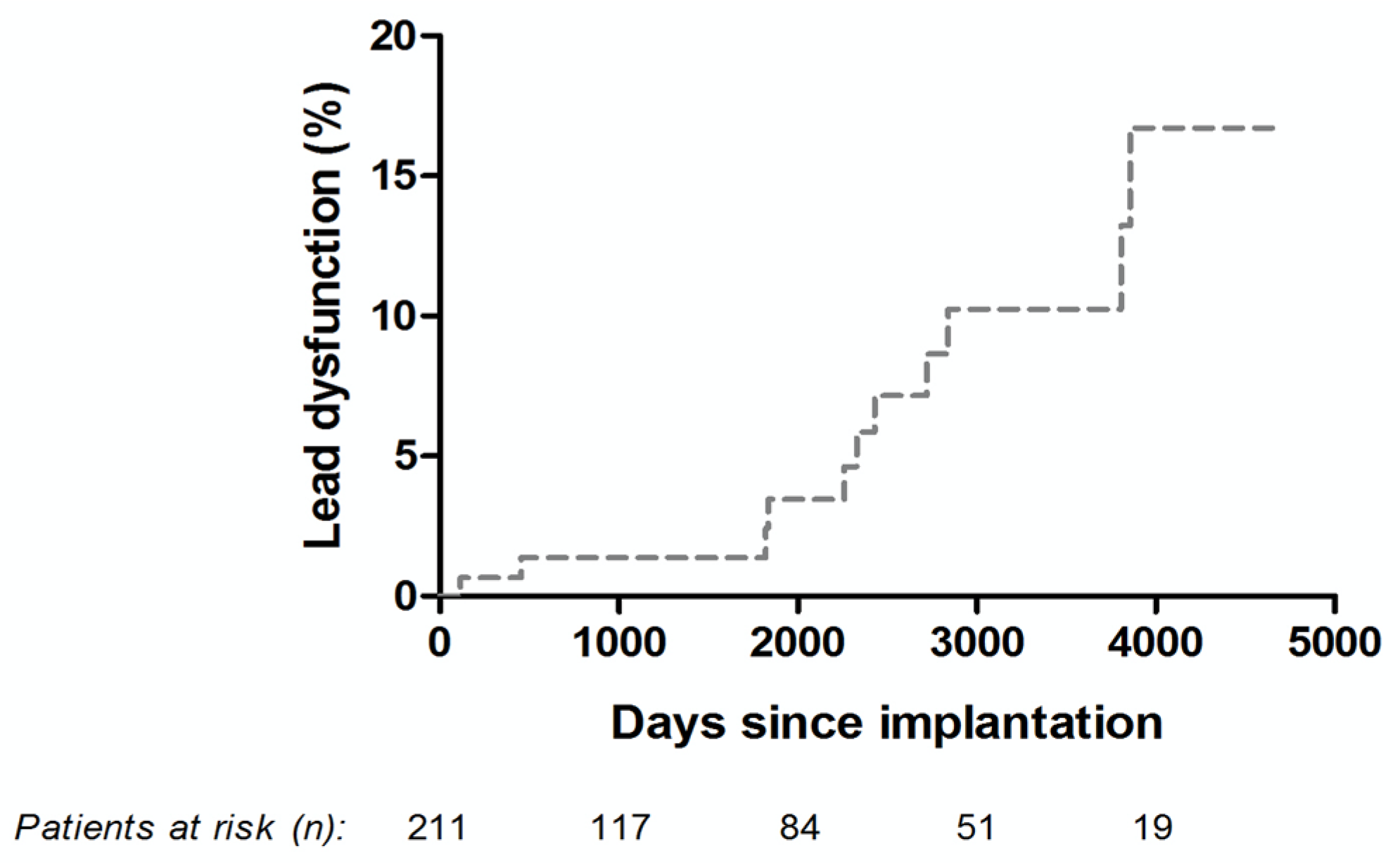
3.4. Long-Term Technical Performance of the Osypka QT-5® Ventricular Lead
3.5. Long-Term Pacemaker Reoperation and Osypka QT-5® Ventricular Lead Explantation Rates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidhu, S.; Marine, J.E. Evaluating and managing bradycardia. Trends Cardiovasc. Med. 2019, 30, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Langenfeld, H.; Grimm, W.; Maisch, B.; Koghsiek, K. Course of Symptoms and Spontaneous ECG in Pacemaker Patients: A 5-Year Follow-up Study. Pacing Clin. Electrophysiol. 1988, 11, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Alboni, P.; Menozzi, C.; Brignole, M.; Paparella, N.; Gaggioli, G.; Lolli, G.; Cappato, R. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome the THEOPACE study: A randomized controlled trial. Circulation 1997, 96, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Edhag, O.; Swahn, A. Prognosis of patients with complete heart block or arrhythmic syncope who were not treated with artificial pacemakers. A long-term follow-up study of 101 patients. Acta Medica Scand. 1976, 200, 457–463. [Google Scholar] [CrossRef]
- Udo, E.O.; Zuithoff, N.P.; Van Hemel, N.M.; De Cock, C.C.; Hendriks, T.; Doevendans, P.A.; Moons, K.G. Incidence and predictors of short- and long-term complications in pacemaker therapy: The Followpace study. Hearth Rhythm. 2012, 9, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Moller, M.; Arnsbo, P.; Nielsen, J.C. Risk factors for lead complications in cardiac pacing: A population-based cohort study of 28,860 Danish patients. Hearth Rhythm. 2011, 8, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A Leadless Intracardiac Transcatheter Pacing System. N. Engl. J. Med. 2016, 374, 533–541. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Prat, X.V.; Jacobsenet, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018, 15, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.S.; Blais, P.; Hinberg, I.; Johnson, D. Degradation of Explanted Polyurethane Cardiac Pacing Leads and of Polvurethane. Biomater. Artif. Cells Artif. Organs 1988, 16, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, M.J.; Wilkoff, B.; Anderson, J.M.; Hiltner, A. Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J. Biomed. Mater. Res. 2001, 58, 302–307. [Google Scholar] [CrossRef]
- Tohfafarosh, M.; Sevit, A.; Patel, J.; Kiel, J.W.; Greenspon, A.; Prutkin, J.M.; Kurtz, S.M. Characterization of Outer Insulation in Long-Term-Implanted Leads. J. Autom. Inf. Sci. 2016, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Haeberlin, A.; Anwander, M.-T.; Kueffer, T.; Tholl, M.; Baldinger, S.; Servatius, H.; Lam, A.; Franzeck, F.; Asatryan, B.; Zurbuchen, A.; et al. Unexpected high failure rate of a specific MicroPort/LivaNova/Sorin pacing lead. Hearth Rhythm 2021, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | |
|---|---|
| Age, years (IQR) | 78.2 (72.0–83.5) |
| Male, n/% | 109/51.7% |
| Height, cm (IQR) | 168 (160–175) |
| Weight, kg (IQR) | 76.0 (66.0–86.0) |
| Body surface area, cm2 | 1.9 (1.7–2.0) |
| Body mass index | 27.0 (24.1–29.4) |
| Hypertension, n/% | 162/76.8% |
| Diabetes, n/% | 53/25.1% |
| Chronic kidney disease, n/% | 42/19.9% |
| Coronary artery disease, n/% | 83/39.3% |
| Previous stroke, n/% | 19/9.0% |
| Left bundle branch block, n/% | 16/7.6% |
| Right bundle branch block, n/% | 38/18.0% |
| First pacemaker implantation, n/% | 195/92.4% |
| Primary indication | |
| Higher degree atrioventricular block, n/% | 79/37.4% |
| Sick sinus syndrome, n/% | 47/22.2% |
| Higher degree atrioventricular block + sick sinus syndrome, n/% | 7/3.3% |
| AF with bradycardia, n/% | 55/26.1% |
| Bradycardia-tachycardia syndrome, n/% | 23/10.9% |
| Characteristics | |
|---|---|
| DDD-R n/% | 139/65.9% |
| VVI-R n/% | 72/34.1% |
| Pacemaker implanted 1 | |
| Sensia®, n/% | 43/20.4% |
| Adapta®, n/% | 42/19.9% |
| Nexus®, n/% | 37/17.5% |
| Zephyr®, n/% | 17/8.1% |
| Altrua®, n/% | 16/7.6% |
| Reply®, n/% | 15/7.1% |
| Cylos®, n/% | 10/4.7% |
| Other, n/% | 31/14.7% |
| Right atrial lead 2 | |
| 4592 CapSure SP Novus®, n/% | 123/88.5% |
| 1944 IsoFlex®, n/% | 6/4.3% |
| Other, n/% | 10/7.2% |
| Primary pacing mode programmed | |
| DDD, n/% | 138/65.4% |
| VVI, n/% | 73/34.6% |
| Perioperative complications | |
| Dislocation, n/% | 4/1.9% |
| Hematoma, n/% | 9/4.3% |
| Pneumothorax, n/% | 7/3.3% |
| Infection, n/% | 2/0.9% |
| Loose set screw, n/% | 1/0.5% |
| Any reoperation, n/% | 78/37.0% |
| Any reoperation without revision of the ventricular lead, n/% | 42/19.9% |
| Battery replacement, n/% | 36/17.1% |
| Upgrade to cardiac resynchronization therapy with pacemaker, n/% | 4/1.9% |
| Dislocation of atrial lead, n/% | 1/0.5% |
| Chronic pocket pain, n/% | 1/0.5% |
| Any reoperation with revision of the ventricular lead, n/% | 36/17.1% |
| Battery replacement with precautionary revising of the ventricular lead due to low impedance, n/% | 5/2.4% |
| Upgrade to cardiac resynchronization therapy with defibrillator and necessity to implant an ICD lead, n/% | 6/2.8% |
| Dislocation of ventricular lead, n/% | 4/1.9% |
| Infection of pacemaker system, n/% | 2/0.9% |
| Atrial lead dysfunction with revision of ventricular lead, n/% | 2/0.9% |
| Low impedance of ventricular lead, n/% | 2/0.9% |
| Dislocation of atrial and ventricular lead, n/% | 2/0.9% |
| Cut of ventricular lead during tricuspid valve repair, n/% | 1/0.5% |
| Loose set screw, n/% | 1/0.5% |
| Ventricular lead dysfunction, n/% | 11/5.2% |
| Days after Implant | R-Wave Sensing (mV) | Lead Impedance (Ω) | Pacing Threshold (V/ms) | Malfunction and Consequence |
|---|---|---|---|---|
| 3858 | Not measurable | >3000 | Not measurable | Lead fracture/lead replacement |
| 2429 | 10.0 | 730 | 2.10 at 0.4 ms | Increasing pacing threshold/replaced as part of battery exchange |
| 3807 | Not measurable | 200 | 0.75 at 0.4 ms | Impedance drop/replaced as part of battery exchange |
| 1834 | 5.9 | 280 | Not measurable | Exit block/lead replacement |
| 2259 | 7.2 | 240 | 1.50 at 0.4 ms | Artefact oversensing and impedance drop/lead replacement |
| 2840 | 1.5 | 200 | Not measurable | Impedance drop and exit block/lead replacement |
| 455 | 12.0 | 200 | 0.75 at 0.4 ms | Impedance drop/lead replacement |
| 2720 | 5.4 | 350 | Not measurable | Artefact oversensing and exit block/lead replacement |
| 116 | Not measurable | >3000 | Not measurable | Lead fracture/lead replacement |
| 2329 | 6.3 | 200 | Missing value | Impedance drop/replaced as part of battery exchange |
| 1821 | 3.7 | 259 | 2.10 at 0.4 ms | Impedance drop and increasing pacing threshold/replaced as part of battery exchange |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semmler, G.; Barbieri, F.; Thudt, K.; Vock, P.; Mörtl, D.; Mayr, H.; Wollmann, C.G.; Adukauskaite, A.; Pfeifer, B.; Senoner, T.; et al. Long-Term Technical Performance of the Osypka QT-5® Ventricular Pacemaker Lead. J. Clin. Med. 2021, 10, 639. https://doi.org/10.3390/jcm10040639
Semmler G, Barbieri F, Thudt K, Vock P, Mörtl D, Mayr H, Wollmann CG, Adukauskaite A, Pfeifer B, Senoner T, et al. Long-Term Technical Performance of the Osypka QT-5® Ventricular Pacemaker Lead. Journal of Clinical Medicine. 2021; 10(4):639. https://doi.org/10.3390/jcm10040639
Chicago/Turabian StyleSemmler, Georg, Fabian Barbieri, Karin Thudt, Paul Vock, Deddo Mörtl, Harald Mayr, Christian Georg Wollmann, Agne Adukauskaite, Bernhard Pfeifer, Thomas Senoner, and et al. 2021. "Long-Term Technical Performance of the Osypka QT-5® Ventricular Pacemaker Lead" Journal of Clinical Medicine 10, no. 4: 639. https://doi.org/10.3390/jcm10040639
APA StyleSemmler, G., Barbieri, F., Thudt, K., Vock, P., Mörtl, D., Mayr, H., Wollmann, C. G., Adukauskaite, A., Pfeifer, B., Senoner, T., & Dichtl, W. (2021). Long-Term Technical Performance of the Osypka QT-5® Ventricular Pacemaker Lead. Journal of Clinical Medicine, 10(4), 639. https://doi.org/10.3390/jcm10040639







