Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
3. Results
3.1. Patient Characteristics
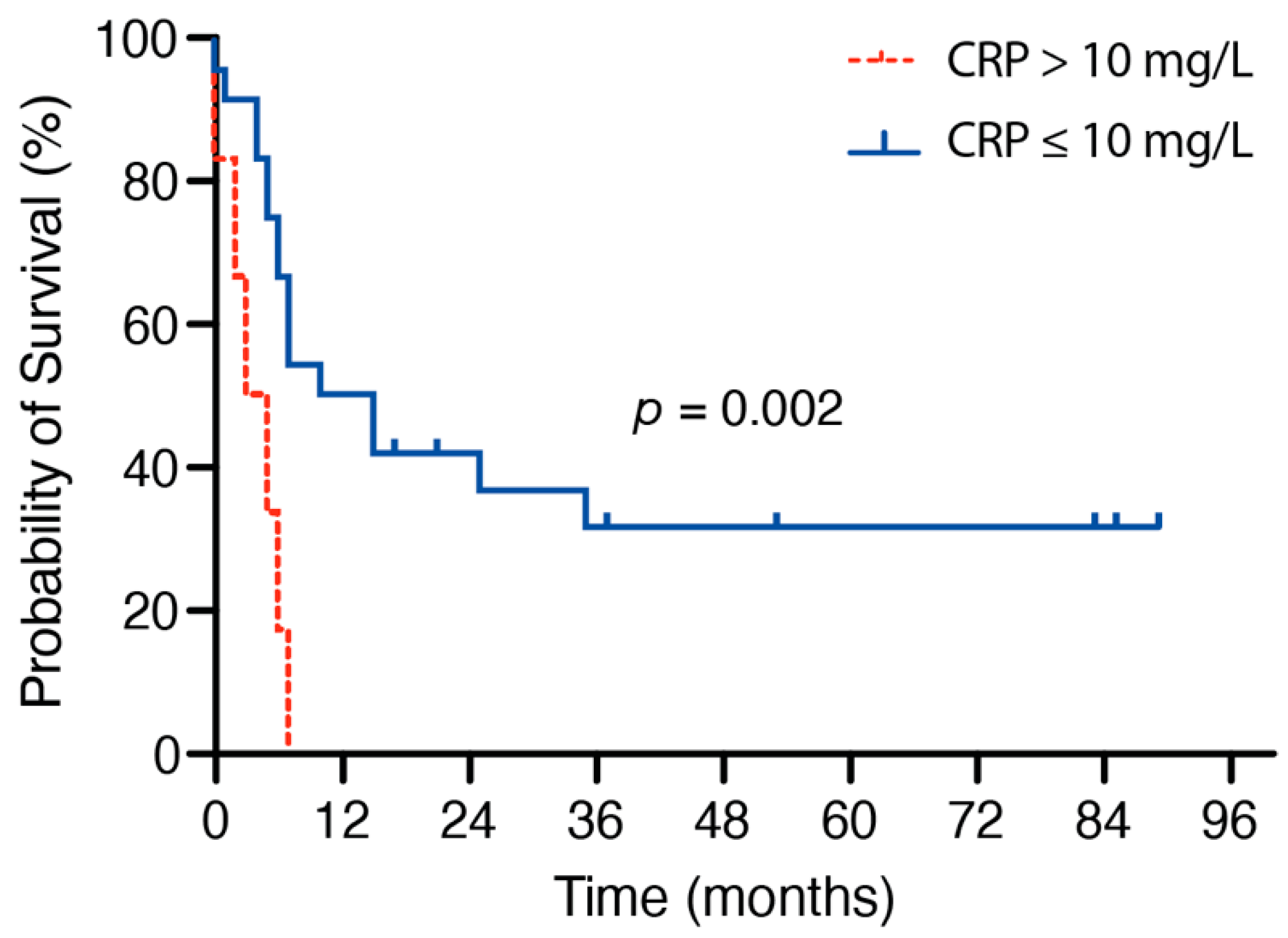
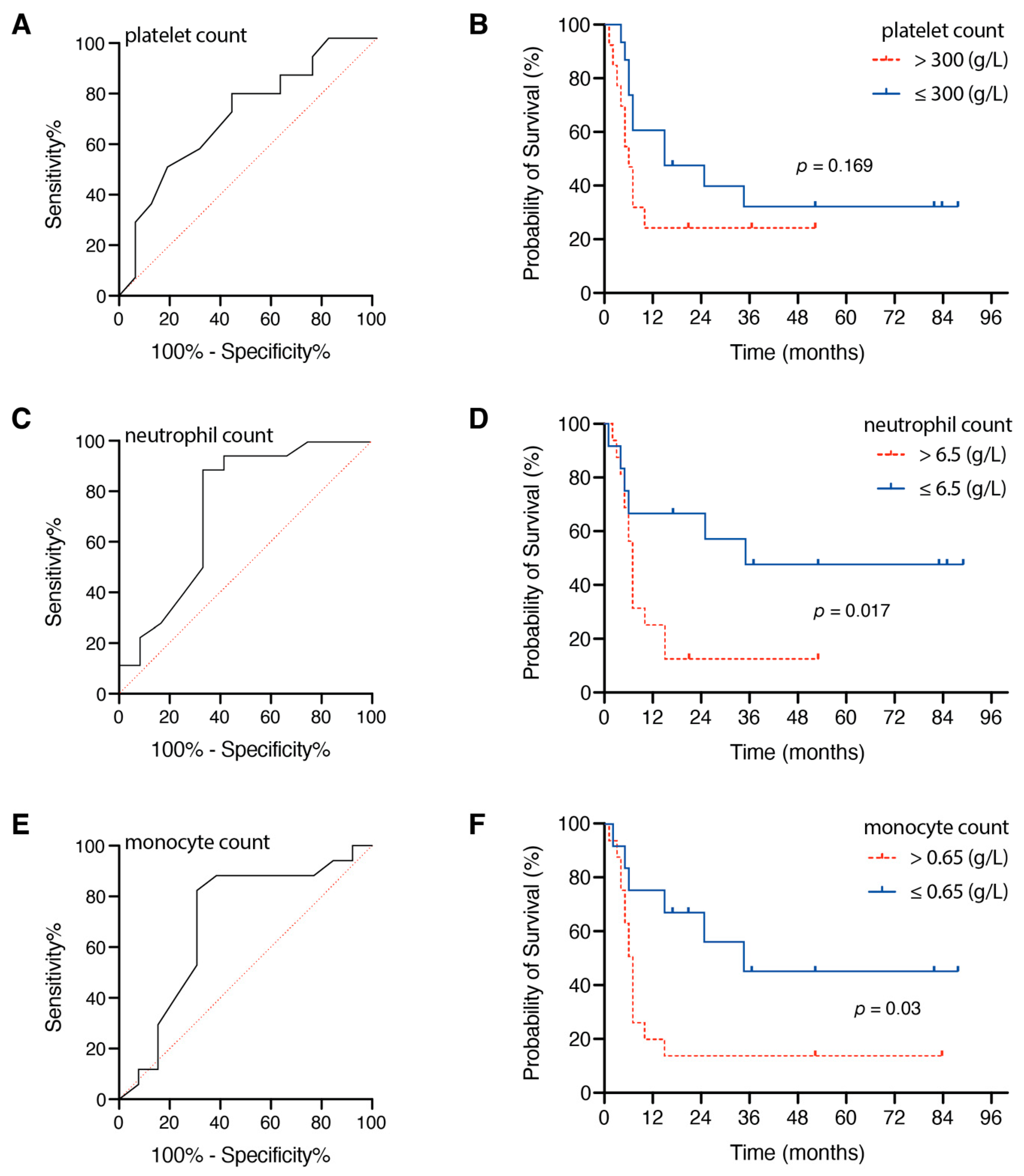
3.2. Influence of Preoperative Inflammatory Markers
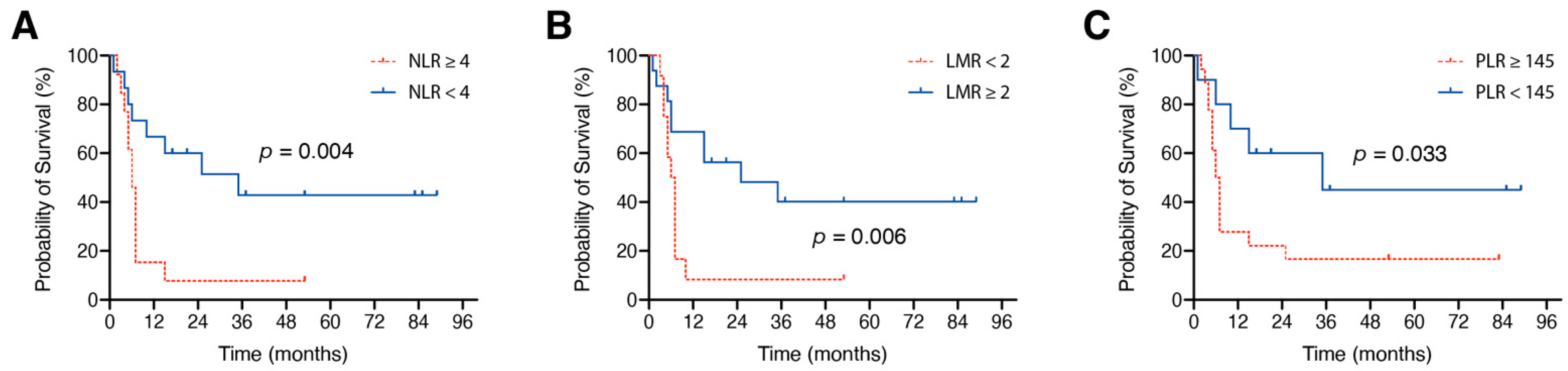
3.3. Influence of Preoperative Inflammatory Marker Ratios
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Nieblas-Bedolla, E.; Nayyar, N.; Singh, M.; Sullivan, R.J.; Brastianos, P.K. Emerging Immunotherapies in the Treatment of Brain Metastases. Oncologist 2020. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Pierscianek, D.; Ahmadipour, Y.; Michel, A.; Chihi, M.; Oppong, M.D.; Kebir, S.; Glas, M.; Stuschke, M.; Sure, U.; Jabbarli, R. Preoperative Survival Prediction in Patients With Glioblastoma by Routine Inflammatory Laboratory Parameters. Anticancer Res. 2020, 40, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, M.; Switchenko, J.M.; Press, R.H.; Jhaveri, J.; Buchwald, Z.S.; Blumenfeld, P.A.; Marwaha, G.; Diaz, A.; Wang, D.; Abrams, R.A.; et al. Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery. J. Neurooncol. 2018, 139, 689–697. [Google Scholar] [CrossRef]
- Park, B.K.; Park, J.W.; Han, E.C.; Ryoo, S.B.; Han, S.W.; Kim, T.Y.; Chie, E.K.; Jeong, S.Y.; Park, K.J. Systemic inflammatory markers as prognostic factors in stage IIA colorectal cancer. J. Surg. Oncol. 2016, 114, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, K.; Matsumoto, S.; Migita, K.; Kunishige, T.; Nakade, H.; Miyao, S.; Sho, M. Prognostic value of the fibrinogen-to-platelet ratio as an inflammatory and coagulative index in patients with gastric cancer. Surg. Today 2019, 49, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Xu, W.; Wang, H.; Huang, Y.; Che, G. Prognostic value of a novel scoring system using inflammatory response biomarkers in non-small cell lung cancer: A retrospective study. Thorac. Cancer 2019, 10, 1402–1411. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Fu, X.; Wang, A.; Yang, Y.; Shang, Y.; Gao, Q. Platelet-to-lymphocyte ratio in peripheral blood: A novel independent prognostic factor in patients with melanoma. Int. Immunopharmacol. 2018, 56, 143–147. [Google Scholar] [CrossRef]
- Wang, Y.; LaPointe, G. Arabinogalactan Utilization by Bifidobacterium longum subsp. longum NCC 2705 and Bacteroides caccae ATCC 43185 in Monoculture and Coculture. Microorganisms 2020, 8, 1703. [Google Scholar] [CrossRef] [PubMed]
- Schuss, P.; Güresir, Á.; Schneider, M.; Velten, M.; Vatter, H.; Güresir, E. Factors influencing early postoperative complications following surgery for symptomatic spinal metastasis: A single-center series and multivariate analysis. Neurosurg. Rev. 2020, 43, 211–216. [Google Scholar] [CrossRef]
- Schneider, M.; Heimann, M.; Schaub, C.; Eichhorn, L.; Potthoff, A.L.; Giordano, F.A.; Güresir, E.; Ko, Y.D.; Landsberg, J.; Lehmann, F.; et al. Comorbidity Burden and Presence of Multiple Intracranial Lesions Are Associated with Adverse Events after Surgical Treatment of Patients with Brain Metastases. Cancers 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Lewis, C.; Collins, H.H.; Shabto, J.M.; Akce, M.; Kissick, H.T.; Carthon, B.C.; Shaib, W.L.; et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 2019, 125, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Papale, M.; Buccarelli, M.; Mollinari, C.; Russo, M.A.; Pallini, R.; Ricci-Vitiani, L.; Tafani, M. Hypoxia, Inflammation and Necrosis as Determinants of Glioblastoma Cancer Stem Cells Progression. Int. J. Mol. Sci. 2020, 21, 2660. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Ravishankaran, P.; Karunanithi, R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J. Surg. Oncol. 2011, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Nordestgaard, B.G.; Flyger, H.; Bojesen, S.E. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: A cohort study. Breast Cancer Res. BCR 2011, 13, R55. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Walsh, D.; Bennani-Baiti, N.; Thomas, S.; Lorton, C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS ONE 2015, 10, e0143080. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, J.A.; Scrimini, S.; Sauleda, J.; de Borja García-Cosío, F.; Noguera, A.; Iglesias, A.; Ríos, A.; Cόrdova, R.; Agustí, A. Role of C reactive protein in non small cell lung cancer staging. Eur. Respir. J. 2011, 38 (Suppl. 55), 2806. [Google Scholar]
- Schuss, P.; Hadjiathanasiou, A.; Brandecker, S.; Güresir, Á.; Vatter, H.; Güresir, E. Elevated C-reactive protein and white blood cell count at admission predict functional outcome after non-aneurysmal subarachnoid hemorrhage. J. Neurol. 2018, 265, 2944–2948. [Google Scholar] [CrossRef]
- Emerging Risk Factors, C.; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Cho, U.; Park, H.S.; Im, S.Y.; Yoo, C.Y.; Jung, J.H.; Suh, Y.J.; Choi, H.J. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE 2018, 13, e0200936. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Yang, Y.; Zhang, T.; Ma, X. Prognostic Value of Peripheral Inflammatory Markers in Preoperative Mucosal Melanoma: A Multicenter Retrospective Study. Front. Oncol. 2019, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Hamashima, T.; Yamamoto, S.; Sasahara, M. Pathogenetic significance and possibility as a therapeutic target of platelet derived growth factor. Pathol. Int. 2017, 67, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. European Organization for R, Treatment of Cancer Soft T, Bone Sarcoma G Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lino-Silva, L.S.; Salcedo-Hernandez, R.A.; Garcia-Perez, L.; Meneses-Garcia, A.; Zepeda-Najar, C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res. 2017, 27, 140–144. [Google Scholar] [CrossRef]
- Wade, R.G.; Robinson, A.V.; Lo, M.C.I.; Keeble, C.; Marples, M.; Dewar, D.J.; Moncrieff, M.D.S.; Peach, H. Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios as Biomarkers of Survival in Cutaneous Melanoma: A Multicenter Cohort Study. Ann. Surg. Oncol. 2018, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
| Patients with Melanoma and BM (n = 30) | |
|---|---|
| Median age at surgery (years) | 62 (43–84 1) |
| Female sex | 11 (37%) |
| Median GPA index | 2.5 (0.5–4.0) |
| Median preoperative KPS | 80 (50–100) |
| Previous oncological therapy 2 | 12 (40%) |
| Preoperative corticosteroid intake | 13 (43%) |
| Multiple BM | 4 (13%) |
| Admission median CRP (mg/L) | 12.3 (0.2–102.8) |
| Admission median WBC (g/L) | 12.9 (4.3–32.2) |
| Admission median neutrophils (g/L) | 8.4 (2.04–30.02) |
| Admission median lymphocytes (g/L) | 1.7 (0.25–3.26) |
| Admission median monocytes (g/L) | 0.79 (0.2–1.83) |
| Admission median platelets (g/L) | 306.8 (179–603) |
| Median OS (months) | 7 (95% CI 5.7–8.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; Heimann, M.; Ko, Y.-D.; Lehmann, F.; et al. Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases. J. Clin. Med. 2021, 10, 634. https://doi.org/10.3390/jcm10040634
Schneider M, Schäfer N, Bode C, Borger V, Eichhorn L, Giordano FA, Güresir E, Heimann M, Ko Y-D, Lehmann F, et al. Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases. Journal of Clinical Medicine. 2021; 10(4):634. https://doi.org/10.3390/jcm10040634
Chicago/Turabian StyleSchneider, Matthias, Niklas Schäfer, Christian Bode, Valeri Borger, Lars Eichhorn, Frank A. Giordano, Erdem Güresir, Muriel Heimann, Yon-Dschun Ko, Felix Lehmann, and et al. 2021. "Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases" Journal of Clinical Medicine 10, no. 4: 634. https://doi.org/10.3390/jcm10040634
APA StyleSchneider, M., Schäfer, N., Bode, C., Borger, V., Eichhorn, L., Giordano, F. A., Güresir, E., Heimann, M., Ko, Y.-D., Lehmann, F., Potthoff, A.-L., Radbruch, A., Schaub, C., Schwab, K. S., Weller, J., Vatter, H., Herrlinger, U., Landsberg, J., & Schuss, P. (2021). Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases. Journal of Clinical Medicine, 10(4), 634. https://doi.org/10.3390/jcm10040634










