Corneal Penetration of Low-Dose Atropine Eye Drops
Abstract
1. Introduction
2. Materials and Methods
2.1. Liquid Chromatography
2.2. Atropine Preparations
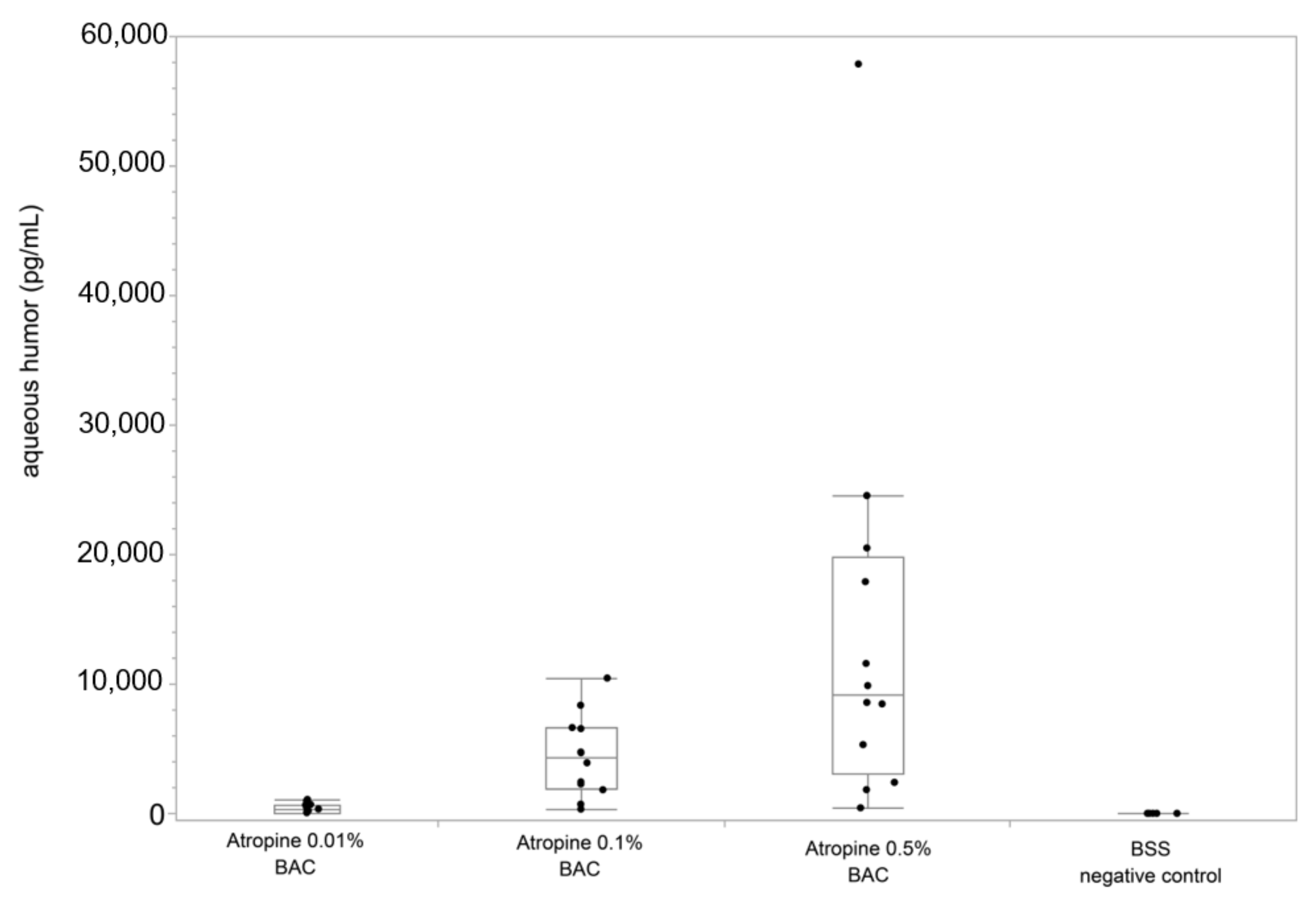
3. Results
4. Discussion
4.1. Local Compatibility and Safety
4.2. Mechanisms of Myopia Inhibition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pineles, S.L.; Kraker, R.T.; VanderVeen, D.K.; Hutchinson, A.K.; Galvin, J.A.; Wilson, L.B.; Lambert, S.R. Atropine for the prevention of myopia progression in children: A report by the American Academy of Ophthalmology. Ophthalmology 2017, 124, 1857–1866. [Google Scholar] [CrossRef]
- Gong, Q.; Janowski, M.; Luo, M.; Wei, H.; Chen, B.; Yang, G.; Liu, L. Efficacy and adverse effects of atropine in childhood myopia: A meta-analysis. JAMA Ophthalmol. 2017, 135, 624–630. [Google Scholar] [CrossRef]
- Prousali, E.; Haidich, A.-B.; Fontalis, A.; Ziakas, N.; Brazitikos, P.; Mataftsi, A. Efficacy and safety of interventions to control myopia progression in children: An overview of systematic reviews and meta-analyses. BMC Ophthalmol. 2019, 19, 106. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; Liu, R.-B.; Pan, J.-H.; Zhang, L.-L.; Xu, Z.-P.; Lu, X.-J. Atropine 0.01% eye drops slow myopia progression: A systematic review and meta-analysis. Int. J. Ophthalmol. 2019, 12, 1337–1343. [Google Scholar] [CrossRef]
- Walline, J.J.; Lindsley, K.; Vedula, S.S.; Cotter, S.A.; Mutti, D.O.; Twelker, J.D. Interventions to slow progression of myopia in children. Cochrane Database Syst. Rev. 2011, 12, CD004916. [Google Scholar] [CrossRef] [PubMed]
- Derby, H. On the atropine treatment of acquired and progressive myopia. Trans. Am. Ophthalmol. Soc. 1874, 2, 139–154. [Google Scholar] [PubMed]
- Sun, H.-Y.; Lu, W.-Y.; You, J.-Y.; Kuo, H.-Y. Peripheral refraction in myopic children with and without atropine usage. J. Ophthalmol. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mathis, U.; Feldkaemper, M.; Wang, M.; Schaeffel, F. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Beuerman, R.W. Biological mechanisms of atropine control of myopia. Eye Contact Lens 2020, 46, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bian, J.; Lu, D.; Wang, Q.; Gong, B.; Li, K.-K.; Yu, F.; Cheung, J.K.-W.; Ji, X.; Zhang, H.; et al. Combined retinal proteome datasets in response to atropine treatment using iTRAQ and SWATH-MS based proteomics approaches in guinea pig myopia model. Data Brief. 2020, 33, 106526. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Mihara, K.; Ramachandran, R.; Saifeddine, M.; Nathanson, N.M.; Stell, W.K.; Hollenberg, M.D. Myopia-inhibiting concentrations of muscarinic receptor antagonists block ac-tivation of Alpha2A-adrenoceptors in vitro. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2778–2791. [Google Scholar] [CrossRef]
- Luft, W.A.; Ming, Y.; Stell, W.K. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Investig. Opthalmology Vis. Sci. 2003, 44, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Phillips, J.R. Which low-dose atropine for myopia control? Clin. Exp. Optom. 2020, 103, 230–232. [Google Scholar] [CrossRef]
- Fang, Y.-T.; Chou, Y.-J.; Pu, C.; Lin, P.-J.; Liu, T.-L.; Huang, N.; Chou, P. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: A nationwide study. Eye 2013, 27, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Polling, J.R.; Tan, E.; Driessen, S.; Loudon, S.E.; Wong, H.-L.; van der Schans, A.; Tideman, J.W.L.; Klaver, C.C.W. A 3-year follow-up study of atropine treatment for progressive myopia in Europeans. Eye 2020, 34, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Chia, A.; Chua, W.-H.; Cheung, Y.-B.; Wong, W.-L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef]
- Chua, W.-H.; Balakrishnan, V.; Chan, Y.-H.; Tong, L.; Ling, Y.; Quah, B.-L.; Tan, D. Atropine for the treatment of childhood myopia. Ophthalmology 2006, 113, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Brennan, N.A.; Toubouti, Y.M.; Cheng, X.; Bullimore, M.A. Efficacy in myopia control. Prog. Retin. Eye Res. 2020, 2020, 100923. [Google Scholar] [CrossRef] [PubMed]
- Yam, J.C.; Li, F.F.; Zhang, X.; Tang, S.M.; Yip, B.H.K.; Kam, K.W.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: Phase 2 report. Ophthalmology 2020, 127, 910–919. [Google Scholar] [CrossRef]
- Li, F.F.; Kam, K.W.; Zhang, Y.; Tang, S.M.; Young, A.L.; Chen, L.J.; Tham, C.C.; Pang, C.P.; Yam, J.C. Differential effects on ocular biometrics by 0.05%, 0.025%, and 0.01% atropine: Low-concentration atropine for myopia progression study. Ophthalmology 2020, 127, 1603–1611. [Google Scholar] [CrossRef]
- Lyu, Y.; Ji, N.; Fu, A.-C.; Wang, W.-Q.; Wei, L.; Qin, J.; Zhao, B.-X. Comparison of administration of 0.02% atropine and orthokeratology for myopia control. Eye Contact Lens 2020, 47, 81–85. [Google Scholar] [CrossRef]
- Larkin, G.L.; Tahir, A.; Epley, K.D.; Beauchamp, C.L.; Tong, J.T.; Clark, R.A. Atropine 0.01% eye drops for myopia control in american children: A multiethnic sample across three US sites. Ophthalmol. Ther. 2019, 8, 589–598. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Parra, M.M.; Merayo-Lloves, J.; Larrea, J.; Rodriguez, C.J.; Camacho, P.A. Topical atropine in the control of myopia. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 78–88. [Google Scholar] [PubMed]
- Azuara-Blanco, A.; Logan, N.S.; Strang, N.; Saunders, K.; Allen, P.M.; Weir, R.; Doherty, P.; Adams, C.; Gardner, E.; Hogg, R.E.; et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: A multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)—Study protocol. Br. J. Ophthalmol. 2019, 104, 950–955. [Google Scholar] [CrossRef]
- Sacchi, M.; Serafino, M.; Villani, E.; Tagliabue, E.; Luccarelli, S.; Bonsignore, F.; Nucci, P. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 2019, 97, e1136–e1140. [Google Scholar] [CrossRef] [PubMed]
- Kedvessy, G.; De Grosz, S.; Szepesy, A. Preparation of ophthalmic solutions: Modern concepts: I. Atropine sulphate. Br. J. Ophthalmol. 1950, 34, 228–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latreille, P.-L.; Banquy, X. A simple method for the subnanomolar quantitation of seven ophthalmic drugs in the rabbit eye. Anal. Bioanal. Chem. 2015, 407, 3567–3578. [Google Scholar] [CrossRef] [PubMed]
- Cottriall, C.; McBrien, N.; Annies, R.; Leech, E. Prevention of form-deprivation myopia with pirenzepine: A study of drug delivery and distribution. Ophthalmic Physiol. Opt. 1999, 19, 327–335. [Google Scholar] [CrossRef]
- Barathi, V.A.; Weon, S.R.; Beuerman, R.W. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol. Vis. 2009, 15, 1277–1293. [Google Scholar] [PubMed]
- Hsiao, Y.-T.; Chang, W.-A.; Kuo, M.-T.; Lo, J.; Lin, H.-C.; Yen, M.-C.; Jian, S.-F.; Chen, Y.-J.; Kuo, P.-L. Systematic analysis of transcriptomic profile of the effects of low dose atropine treatment on scleral fibroblasts using next-generation sequencing and bioinformatics. Int. J. Med. Sci. 2019, 16, 1652–1667. [Google Scholar] [CrossRef]
- Chang, W.-A.; Hsiao, Y.-T.; Lin, H.-C.; Jian, S.-F.; Chen, Y.-J.; Kuo, P.-L. Deduction of novel genes potentially involved in the effects of very low dose atropine (0.003%) treatment on corneal epithelial cells using next-generation sequencing and bioinformatics approaches. Medicina 2019, 55, 589. [Google Scholar] [CrossRef]
- Salazar, M.; Patil, P.N. An explanation for the long duration of mydriatic effect of atropine in eye. Investig. Ophthalmol. 1976, 15, 671–673. [Google Scholar]
- Rimpelä, A.-K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Van Der Bijl, P.; Engelbrecht, A.H.; Van Eyk, A.D.; Meyer, D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J. Ocul. Pharmacol. Ther. 2002, 18, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Hurst, J.; De Vries, J.W.; Ullah, S.; Gruszka, A.; Kwak, M.; Löscher, M.; Dammeier, S.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; et al. Self-assembled DNA nanoparticles loaded with travoprost for glaucoma-treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102260. [Google Scholar] [CrossRef]
- Willem de Vries, J.; Schnichels, S.; Hurst, J.; Strudel, L.; Gruszka, A.; Kwak, M.; Batz-Schmidt, K.-U.; Spitzer, M.A.; Herrmann, A. DNA nanoparticles for ophthalmic drug delivery. Biomaterials 2018, 157, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Johansen, R.; Schafer, N.C.; Brown, P.I. Effect of extreme temperatures on drugs for prehospital ACLS. Am. J. Emerg. Med. 1993, 11, 450–452. [Google Scholar] [CrossRef]
- Kinoshita, N.; Konno, Y.; Hamada, N.; Kanda, Y.; Shimmura-Tomita, M.; Kaburaki, T.; Kakehashi, A. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: A 2-year randomised trial. Sci. Rep. 2020, 10, 12750. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Imaizumi, H.; Yamatani, A. Physical, chemical, and microbiological stability study of diluted atropine eye drops. J. Pharm. Health Care Sci. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Berton, B.; Chennell, P.; Yessaad, M.; Bouattour, Y.; Jouannet, M.; Wasiak, M.; Sautou, V. Stability of ophthalmic atropine solutions for child myopia control. Pharmaceutics 2020, 12, 781. [Google Scholar] [CrossRef]
- Maurice, D.M.; Mishima, S. Ocular pharmacokinetics. Botulinum Toxin Ther. 1984, 1984, 19–116. [Google Scholar] [CrossRef]
- Q1A(R2) Stability Testing of New Drug Substances and Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1ar2-stability-testing-new-drug-substances-and-products (accessed on 28 December 2020).
- Lund, W.; Waaler, T.; Meisalo, V.; Kelly, P. The kinetics of atropine and apoatropine in aqueous solutions. Acta Chem. Scand. 1968, 22, 3085–3097. [Google Scholar] [CrossRef] [PubMed]
- Schier, J.G.; Ravikumar, P.R.; Nelson, L.S.; Heller, M.B.; Howland, M.A.; Hoffman, R.S. Preparing for chemical terrorism: Stability of injectable atropine sulfate. Acad. Emerg. Med. 2004, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Dimmel, A.; Jüttemeyer, S.; Springer, D.; Loch, M. Rapid resolution liquid chromatography for monitoring the quality of stockpiled atropine preparations for injection. Drug Test. Anal. 2012, 4, 222–228. [Google Scholar] [CrossRef]
- Keller, N.; Moore, D.; Carper, D.; Longwell, A. Increased corneal permeability induced by the dual effects of transient tear film acidification and exposure to benzalkonium chloride. Exp. Eye Res. 1980, 30, 203–210. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, J.-X. Will the long-term use of atropine eye drops in children increase the risk of dry eye? Med. Hypotheses 2019, 132, 109331. [Google Scholar] [CrossRef]
- Tan, D.; Tay, S.A.; Loh, K.-L.; Chia, A. Topical atropine in the control of myopia. Asia-Pac. J. Ophthalmol. 2016, 5, 424–428. [Google Scholar] [CrossRef]
- Kothari, M.; Jain, R.; Khadse, N.; Rathod, V.; Mutha, S. Allergic reactions to atropine eye drops for retardation of progressive myopia in children. Indian J. Ophthalmol. 2017, 66, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.R.; Shan, X.F.; Cai, Z.G.; Zhang, X.; Yu, G.Y. A new treatment for epiphora secondary to submandibular gland transplantation: Transcutaneous atropine gel. Ocul. Surf. 2014, 12, 221–226. [Google Scholar] [CrossRef]
- Ding, C.; Cong, X.; Zhang, Y.; Yang, N.; Li, S.; Wu, L.; Yu, G. Hypersensitive mAChRs are involved in the epiphora of transplanted glands. J. Dent. Res. 2014, 93, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Boudet, J.; Qing, W.; Boyer-Chammard, A.; Del Franco, G.; Bergougnan, J.L.; Rosen, P.; Meyer, P. Dose-response effects of atropine in human volunteers. Fundam. Clin. Pharmacol. 1991, 5, 635–640. [Google Scholar] [CrossRef]
- Lönnerholm, G.; Widerlöv, E. Effect of intravenous atropine and methylatropine on heart rate and secretion of saliva in man. Eur. J. Clin. Pharmacol. 1975, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Pfister, R.R.; Burstein, N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: A scanning electron microscope study. Investig. Ophthalmol. 1976, 15, 246–259. [Google Scholar]
- Yu, T.C.; Wu, T.E.; Wang, Y.S.; Cheng, S.F.; Liou, S.W. A STROBE-compliant case-control study: Effects of cumulative doses of topical atropine on intraocular pressure and myopia progression. Medicine 2020, 99, e22745. [Google Scholar] [CrossRef] [PubMed]
- Burstein, N.L. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Investig. Ophthalmol. Vis. Sci. 1980, 19, 308–313. [Google Scholar]
- Wilson, W.S.; Duncan, A.J.; Jay, J.L. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br. J. Ophthalmol. 1975, 59, 667–669. [Google Scholar] [CrossRef]
- Leopold, I.H. Local toxic effect of detergents on ocular structures. Arch. Ophthalmol. 1945, 34, 99–102. [Google Scholar] [CrossRef]
- De Saint Jean, M.; Brignole, F.; Bringuier, A.F.; Bauchet, A.; Feldmann, G.; Baudouin, C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 619–630. [Google Scholar]
- Cheng, J.; Yang, Y.; Kong, X.; Zeng, L.; Chen, Z.; Xu, J.; Chaoran, Z. The effect of 0.01% atropine eye drops on the ocular surface in children for the control of myopia—The primary results from a six-month prospective study. Ther. Clin. Risk Manag. 2020, 16, 735–740. [Google Scholar] [CrossRef]
- Cho, W.-H.; Fang, P.-C.; Yu, H.-J.; Lin, P.-W.; Huang, H.-M.; Kuo, M.-T. Analysis of tear film spatial instability for pediatric myopia under treatment. Sci. Rep. 2020, 10, 14789. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, A.; Ponto, K.A.; Hoehn, R.; Zwiener, I.; Zeller, T.; Lackner, K.; Beutel, M.E.; Pfeiffer, N. Myopia and level of education results from the Gutenberg health study. Ophthalmology 2014, 121, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Krause, L.; Kuchenbäcker, C.; Prütz, F.; Elflein, H.M.; Pfeiffer, N.; Urschitz, M.S. Prävalenz von Kurzsichtigkeit und deren Veränderung bei Kindern und Ju-gendlichen. Dtsch Arztebl Int. 2020, 117, 855–860. [Google Scholar] [CrossRef]
- Ziemssen, F.; Lagreze, W.; Voykov, B. Secondary diseases in high myopia. Ophthalmologe 2017, 114, 30–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tideman, J.W.L.; Polling, J.R.; Vingerling, J.R.; Jaddoe, V.W.V.; Williams, C.; Guggenheim, J.A.; Klaver, C.C.W. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018, 96, 301–309. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhou, X.; Qu, X. Analysis of factors that may affect the effect of atropine 0.01% on myopia control. Front. Pharmacol. 2020, 11, 01081. [Google Scholar] [CrossRef]
- Mathis, U.; Ziemssen, F.; Schaeffel, F. Effects of a human VEGF antibody (Bevacizumab) on deprivation myopia and choroidal thickness in the chicken. Exp. Eye Res. 2014, 127, 161–169. [Google Scholar] [CrossRef]
- McBrien, N.A.; Moghaddam, H.O.; Reeder, A.P. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Investig. Ophthalmol. Vis. Sci. 1993, 34, 205–215. [Google Scholar]
- Diether, S.; Schaeffel, F. Long-term changes in retinal contrast sensitivity in chicks from frosted occluders and drugs: Relations to myopia? Vis. Res. 1999, 39, 2499–2510. [Google Scholar] [CrossRef][Green Version]
- Carr, B.J.; Stell, W.K. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.-H.; Turnbull, P.R.K.; Phillips, J.R. Additive effect of atropine eye drops and short-term retinal defocus on choroidal thickness in children with myopia. Sci. Rep. 2020, 10, 18130. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Zhu, X.; Wallman, J. Effects of muscarinic agents on chick choroids in intact eyes and eyecups: Evidence for a muscarinic mechanism in choroidal thinning. Ophthalmic Physiol. Opt. 2013, 33, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wallman, J.; Wildsoet, C.; Xu, A.; Gottlieb, M.D.; Nickla, D.L.; Marran, L.; Krebs, W.; Christensen, A.M. Moving the retina: Choroidal modulation of refractive state. Vis. Res. 1995, 35, 37–50. [Google Scholar] [CrossRef]
- Read, S.A.; Fuss, J.A.; Vincent, S.J.; Collins, M.J.; Alonso-Caneiro, D. Choroidal changes in human myopia: Insights from optical coherence tomography imaging. Clin. Exp. Optom. 2019, 102, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Chrai, S.S.; Makoid, M.C.; Eriksen, S.P.; Robinson, J.R. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J. Pharm. Sci. 1974, 63, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Pellinen, P.; Lokkila, J. Corneal penetration into rabbit aqueous humor is comparable between preserved and preservative-free tafluprost. Ophthalmic Res. 2009, 41, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Hippalgaonkar, K.; Repka, M.A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 2008, 348, 175–178. [Google Scholar] [CrossRef]
- Tan, Q.; Ng, A.L.; Choy, B.N.; Cheng, G.P.; Woo, V.C.; Cho, P. One-year results of 0.01% atropine with orthokeratology (AOK) study: A randomised clinical trial. Ophthalmic Physiol. Opt. 2020, 40, 557–566. [Google Scholar] [CrossRef]
- Sánchez-González, J.-M.; De-Hita-Cantalejo, C.; Baustita-Llamas, M.-J.; Sánchez-González, M.C.; Capote-Puente, R. The combined effect of low-dose atropine with orthokeratology in pediatric myopia control: Review of the current treatment status for myopia. J. Clin. Med. 2020, 9, 2371. [Google Scholar] [CrossRef]

| Formulation | Ingredients | Volume | Instructions for Use (Technical Information) | Shelf Life | Pharmacy |
|---|---|---|---|---|---|
| 0.01% F1 | Atropine sulphate 0.1 g/g, benzalkonium chloride 0.005%, sodium chloride, water | 10 mL | Storage at 15–25 °C Can be used for 4 weeks after opening. | 12 months | Bergapotheke |
| 0.01% F2 | Atropine sulphate 0.01%, benzalkonium chloride 0.005%, sodium chloride 0.9%, water for injection | 10 mL | Keep protected from children Can be used for 4 weeks after opening. | 8 months | Alte Apotheke |
| 0.01% F3 | Atropine sulphate 0.1 mg, sodium chloride, sodium edetate, preserved with 0.005% benzalkonium chloride | 10 mL | Store below 25 °C Can be used for 4 weeks after opening. | 8 months | University Pharmacy |
| 0.01% PF1 | Atropine sulphate 0.1 mg, sodium chloride, hydrochlorid acid, water for injection | 0.5 mL | For single use. Discard the rest! Store at 15–25 °C. | 12 months | Bergapotheke |
| 0.01% PF2 | Atropine sulphate 0.1 mg, sodium chloride, hydrochlorid acid, water for injection | 10 mL | Store below 25 °C. Use 24 h after opening. | 1 month | University Pharmacy |
| 0.01% PF3 | Atropine sulphate 0.1 mg n.d. | 0.25 mL | Store in refrigerator 2–8 °C. After opening the sachet, the contents of the intact single-dose containers can be used for 1 month. | 3 months | Study Medication |
| 0.1% | Atropine sulphate 1 mg, sodium chloride, sodium edetate, preserved with 0.005% benzalkonium chloride | 10 mL | Store below 25 °C Can be used for 4 weeks after opening. | 8 months | University Pharmacy |
| 0.5% | Atropine sulphate 5 mg/mL, benzalkonium chloride 0.05 mg/mL, sodium chloride, water | 10 mL | Do not store above 25 °C. Use 4 weeks after opening. | 18 months | Ursapharm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austermann, H.; Schaeffel, F.; Mathis, U.; Hund, V.; Mußhoff, F.; Ziemssen, F.; Schnichels, S. Corneal Penetration of Low-Dose Atropine Eye Drops. J. Clin. Med. 2021, 10, 588. https://doi.org/10.3390/jcm10040588
Austermann H, Schaeffel F, Mathis U, Hund V, Mußhoff F, Ziemssen F, Schnichels S. Corneal Penetration of Low-Dose Atropine Eye Drops. Journal of Clinical Medicine. 2021; 10(4):588. https://doi.org/10.3390/jcm10040588
Chicago/Turabian StyleAustermann, Henning, Frank Schaeffel, Ute Mathis, Verena Hund, Frank Mußhoff, Focke Ziemssen, and Sven Schnichels. 2021. "Corneal Penetration of Low-Dose Atropine Eye Drops" Journal of Clinical Medicine 10, no. 4: 588. https://doi.org/10.3390/jcm10040588
APA StyleAustermann, H., Schaeffel, F., Mathis, U., Hund, V., Mußhoff, F., Ziemssen, F., & Schnichels, S. (2021). Corneal Penetration of Low-Dose Atropine Eye Drops. Journal of Clinical Medicine, 10(4), 588. https://doi.org/10.3390/jcm10040588







