The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking
Abstract
1. Introduction
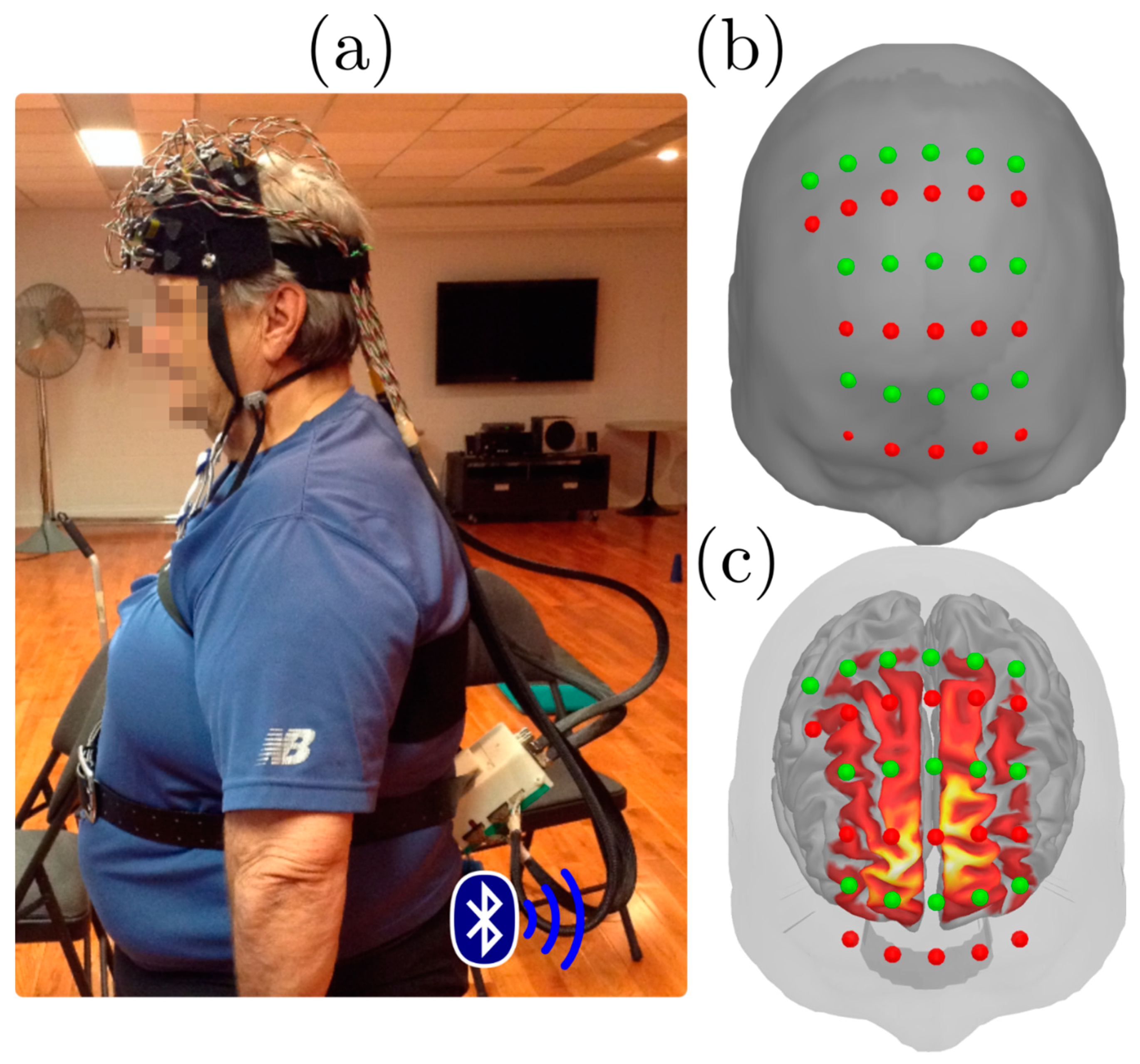
2. Experimental Section
3. Results
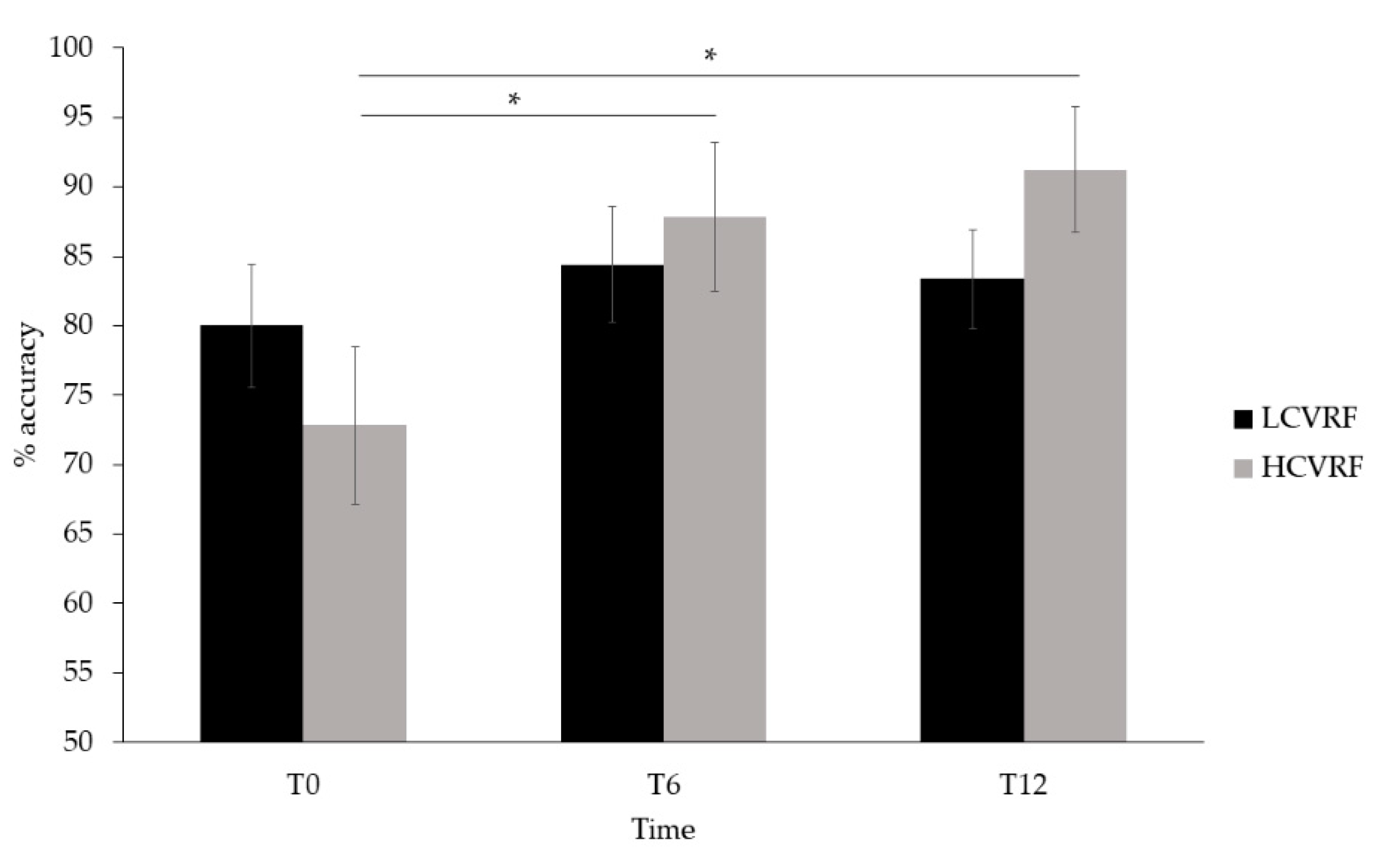
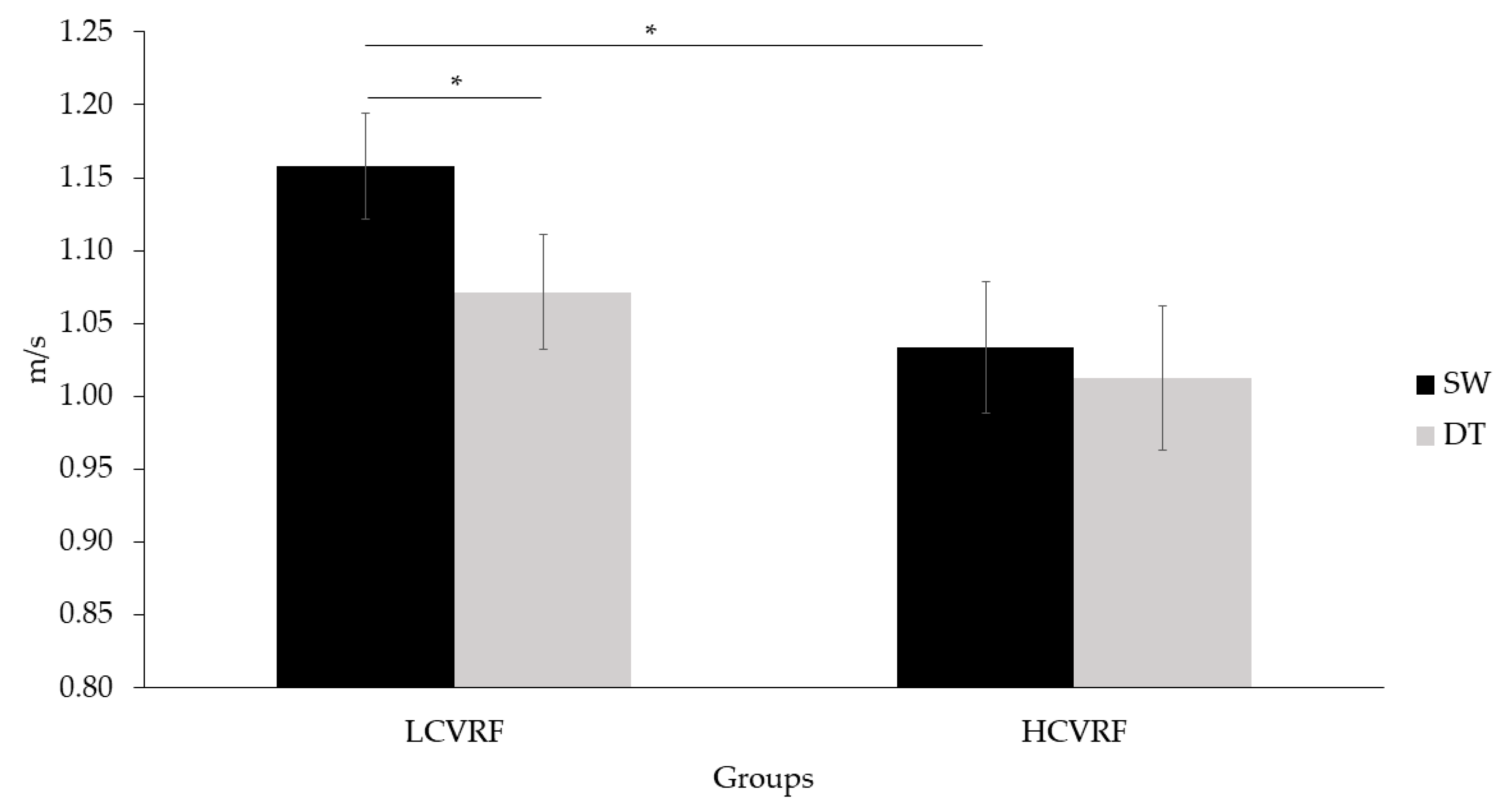
3.1. Behavioral Results
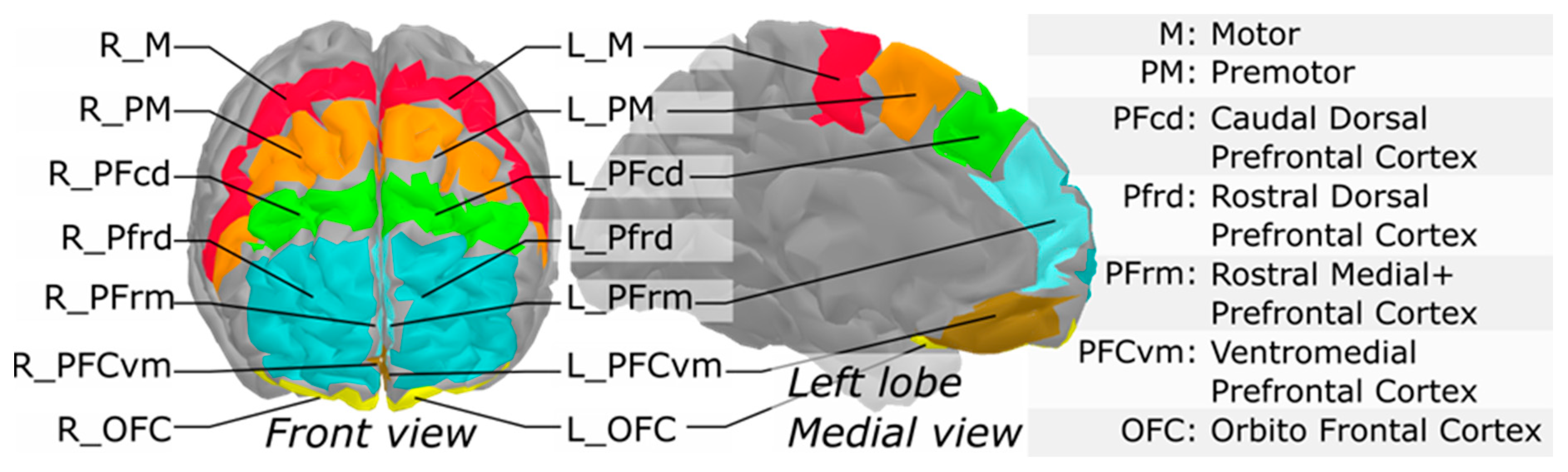
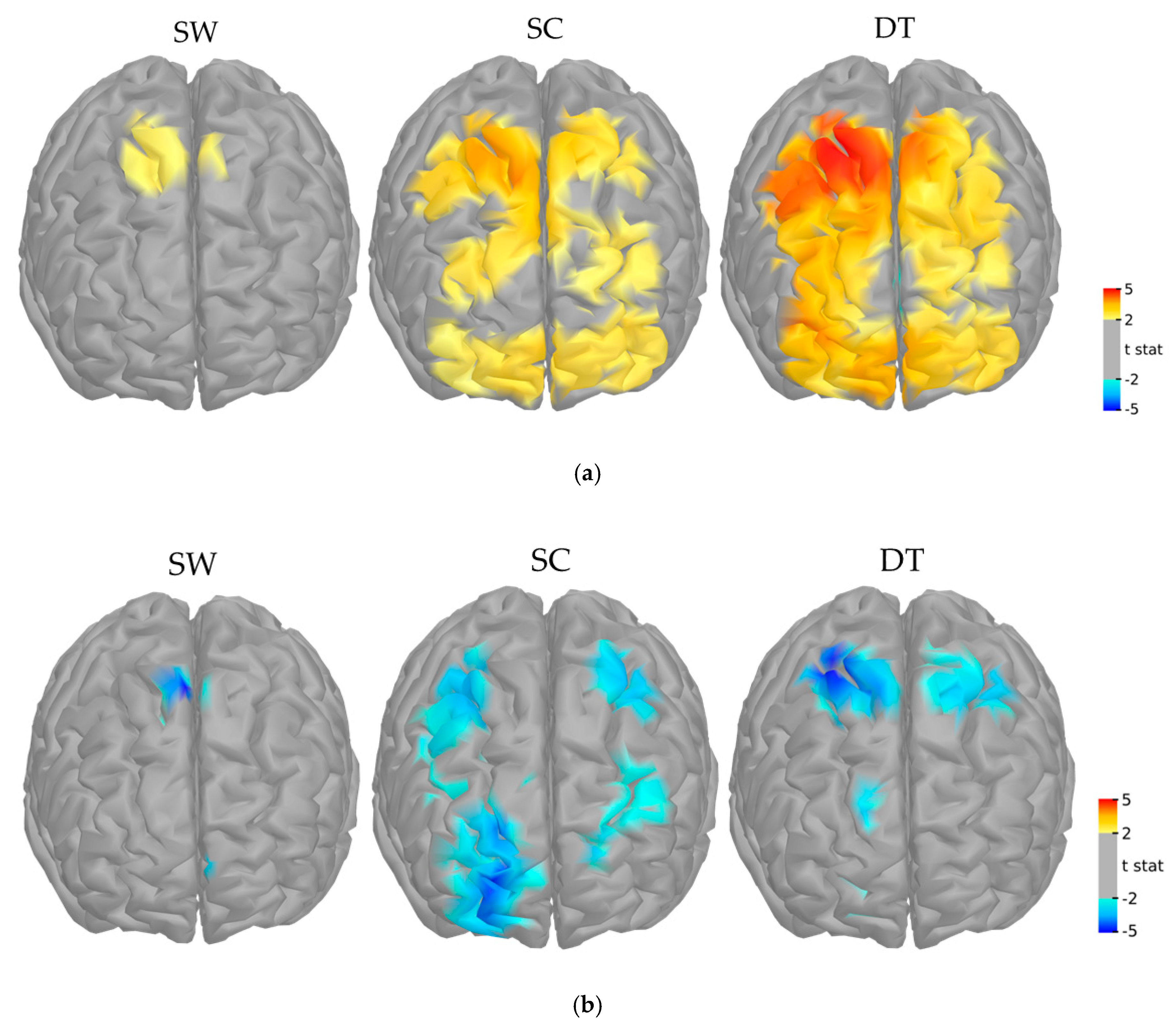
3.2. fNIRS Main Effect and Functional Mask
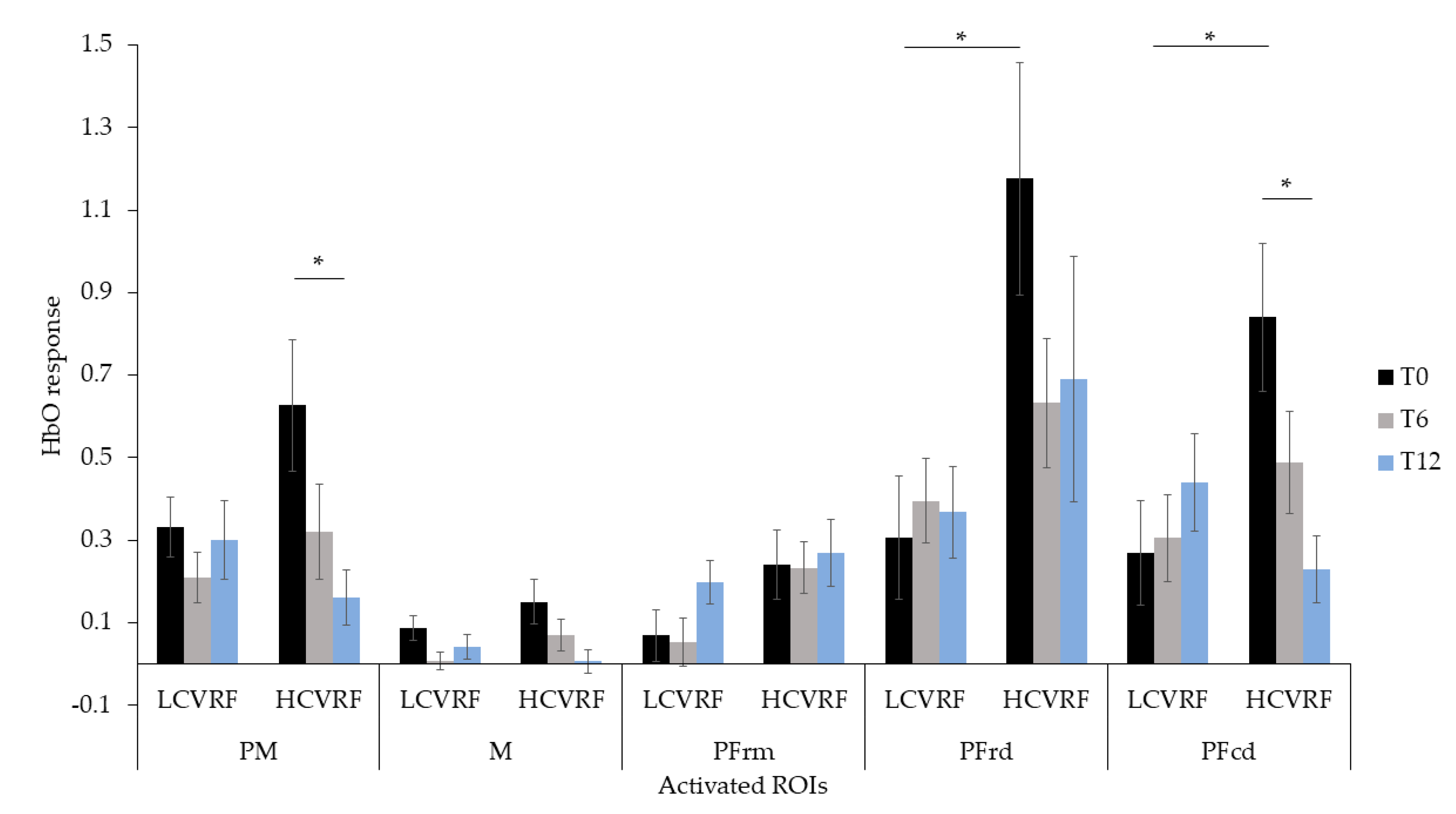
3.3. Interaction between fNIRS, Clinical Group and Time
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; Mcintosh, A.R. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage 2002, 17, 1394–1402. [Google Scholar] [CrossRef]
- Hayes, S.M.; Alosco, M.L.; Forman, D.E. The effects of aerobic exercise on cognitive and neural decline in aging and cardiovascular disease. Curr. Geriatr. Rep. 2014, 3, 282–290. [Google Scholar] [CrossRef]
- Yaffe, K.; Vittinghoff, E.; Pletcher, M.J.; Hoang, T.D.; Launer, L.J.; Whitmer, R.A.; Coker, L.H.; Sidney, S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014, 129, 1560–1567. [Google Scholar] [CrossRef]
- Dregan, A.; Stewart, R.; Gulliford, M.C. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: A population-based cohort study. Age Ageing 2013, 42, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Kåreholt, I.; Ngandu, T.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Serum cholesterol changes after midlife and late-life cognition. Neurology 2007, 68, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Xue, Q.L.; Masaki, K.; Petrovitch, H.; Ross, G.W.; White, L.R.; Launer, L.J. Change in blood pressure and incident dementia: A 32-year prospective study. Hypertension 2009, 54, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. Br. Med. J. 2001, 322, 1447–1451. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Johnston, S.C.; Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef]
- Vuorinen, M.; Solomon, A.; Rovio, S.; Nieminen, L.; Kåreholt, I.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Changes in vascular risk factors from midlife to late life and white matter lesions: A 20-year follow-up study. Dement. Geriatr. Cogn. Disord. 2011, 31, 119–125. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Eldreth, D.; Erickson, K.I.; Varma, V.; Harris, G.; Fried, L.P.; Rebok, G.W.; Tanner, E.K.; Carlson, M.C. Cardiovascular risks and brain function: A functional magnetic resonance imaging study of executive function in older adults. Neurobiol. Aging 2014, 35, 1396–1403. [Google Scholar] [CrossRef][Green Version]
- Knopman, D.S.; Penman, A.D.; Catellier, D.J.; Coker, L.H.; Shibata, D.K.; Sharrett, A.R.; Mosley, T.H. Vascular risk factors and longitudinal changes on brain MRI: The ARIC study. Neurology 2011, 76, 1879–1885. [Google Scholar] [CrossRef]
- Friedman, J.I.; Tang, C.Y.; de Haas, H.J.; Changchien, L.; Goliasch, G.; Dabas, P.; Wang, V.; Fayad, Z.A.; Fuster, V.; Narula, J. Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC Cardiovasc. Imaging 2014, 7, 1039–1053. [Google Scholar] [CrossRef]
- Beason-Held, L.L.; Thambisetty, M.; Deib, G.; Sojkova, J.; Landman, B.A.; Zonderman, A.B.; Ferrucci, L.; Kraut, M.A.; Resnick, S.M. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke 2012, 43, 1542–1547. [Google Scholar] [CrossRef]
- Holtzer, R.; George, C.J.; Izzetoglu, M.; Wang, C. The effect of diabetes on prefrontal cortex activation patterns during active walking in older adults. Brain Cogn. 2018, 125, 14–22. [Google Scholar] [CrossRef]
- Osofundiya, O.; Benden, M.E.; Dowdy, D.; Mehta, R.K. Obesity-specific neural cost of maintaining gait performance under complex conditions in community-dwelling older adults. Clin. Biomech. 2016, 35, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Niermeyer, M.A. Cognitive and gait decrements among non-demented older adults with Type 2 diabetes or hypertension: A systematic review. Clin. Neuropsychol. 2018, 32, 1256–1281. [Google Scholar] [CrossRef]
- Tanne, D.; Freimark, D.; Poreh, A.; Merzeliak, O.; Bruck, B.; Schwammenthal, Y.; Schwammenthal, E.; Motro, M.; Adler, Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int. J. Cardiol. 2005, 103, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Pühse, U.; Looser, V.N.; Kamijo, K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat. Hum. Behav. 2020, 4, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013. [Google Scholar] [CrossRef]
- Swardfager, W.; Herrmann, N.; Marzolini, S.; Saleem, M.; Kiss, A.; Shammi, P.; Oh, P.I.; Lanctot, K.L. Cardiopulmonary fitness is associated with cognitive performance in patients with coronary artery disease. J. Am. Geriatr. Soc. 2010, 58, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Jousilahti, P.; Barengo, N.C.; Qiao, Q.; Lakka, T.A.; Tuomilehto, J. Physical activity, cardiovascular risk factors, and mortality among finnish adults with diabetes. Diabetes Care 2005, 28, 799–805. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; John, P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 2013, 347, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, J.; Mccoy, T.P.; Letvak, S.; Ivanov, L. Associations of lifestyle intervention effect with blood pressure and physical activity among community-dwelling older Americans with hypertension in Southern California. Int. J. Environ. Res. Public Health 2020, 17, 5673. [Google Scholar] [CrossRef]
- Rêgo, M.L.M.; Cabral, D.A.R.; Costa, E.C.; Fontes, E.B. Physical Exercise for Individuals with hypertension: It is time to emphasize its benefits on the brain and cognition. Clin. Med. Insights Cardiol. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Gordon, T. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease; US Department of Health, Education, and Welfare, National Institutes of Health: Bethesda, MD, USA, 1970.
- Li, K.Z.H.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive involvement in balance, gait and dual-tasking in aging: A focused review from a neuroscience of aging perspective. Front Neurol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- American College of Sport Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Young, J.; Angevaren, M.; Rusted, J.; Tabet, N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment (Review). Cochrane Database Syst. Rev. 2015, 4. [Google Scholar] [CrossRef]
- Lareau, E.; Lesage, F.; Pouliot, P.; Nguyen, D.; Le Lan, J.; Sawan, M. Multichannel wearable system dedicated for simultaneous electroencephalography/near-infrared spectroscopy real-time data acquisitions. J. Biomed. Opt. 2011, 16, 096014. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011. [Google Scholar] [CrossRef] [PubMed]
- GitHub. Available online: Github.com/Nirstorm/nirstorm#nirstorm (accessed on 22 December 2020).
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649–662. [Google Scholar] [CrossRef]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W.; Evans, A.C. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. Neuroimage 2009, 44, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Auzias, G.; Coulon, O.; Brovelli, A. MarsAtlas: A cortical parcellation atlas for functional mapping. Hum. Brain Mapp. 2016, 37, 1573–1592. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behavior; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitiv. Lancet 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Espeland, M.A.; Lipska, K.; Miller, M.E.; Rushing, J.; Cohen, R.A.; Verghese, J.; McDermott, M.M.; King, A.C.; Strotmeyer, E.S.; Blair, S.N.; et al. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 861–866. [Google Scholar] [CrossRef]
- Plummer, P.; Apple, S.; Dowd, C.; Keith, E. Texting and walking: Effect of environmental setting and task prioritization on dual-task interference in healthy young adults. Gait Posture 2015, 41, 46–51. [Google Scholar] [CrossRef]
- Smith, E.; Cusack, T.; Blake, C. The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait Posture 2016, 44, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Nieuwhof, F.; Reelick, M.; Giladi, N.; Hausdorff, J.M. Increased frontal brain activation during walking while dual tasking: An fNIRS study in healthy young adults. J. Neuroeng. Rehabil. 2014, 11, 1–7. [Google Scholar] [CrossRef]
- Dumontheil, I.; Gilbert, S.J.; Frith, C.D.; Burgess, P.W. Recruitment of lateral rostral prefrontal cortex in spontaneous and task-related thoughts. Q. J. Exp. Psychol. 2010, 63, 1740–1756. [Google Scholar] [CrossRef] [PubMed]
- Luria, A.R. The frontal lobes and the regulation of behavior. In Psychophysiology of the Frontal Lobes; Pribram, K.H., Luria, A., Eds.; Academic Press: Cambridge, MA, USA, 1973; pp. 3–26. [Google Scholar] [CrossRef]
- Szameitat, A.J.; Schubert, T.; Müller, K.; Von Yves Cramon, D. Localization of executive functions in dual-task performance with fMRI. J. Cogn. Neurosci. 2002, 14, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, G.A. Human Memory, 2nd ed.; Routledge: New York, NY, USA, 2016. [Google Scholar]
- Clark, D.J. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Coetsee, C.; Terblanche, E. Cerebral oxygenation during cortical activation: The differential influence of three exercise training modalities. A randomized controlled trial. Eur. J. Appl. Physiol. 2017, 117, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, U.C.; Shoemaker, J.K.; Suskin, N.; St Lawrence, K.S. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. NeuroImage Clin. 2013, 3, 388–395. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62. [Google Scholar] [CrossRef]
- Ruffieux, J.; Keller, M.; Lauber, B.; Taube, W. Changes in standing and walking performance under dual-task conditions across the lifespan. Sport Med. 2015, 45, 1739–1758. [Google Scholar] [CrossRef] [PubMed]
- Marusic, U.; Taube, W.; Morrison, S.A.; Biasutti, L.; Grassi, B.; De Pauw, K.; Meeusen, R.; Pisot, R.; Ruffieux, J. Aging effects on prefrontal cortex oxygenation in a posture-cognition dual-task: An fNIRS pilot study. Eur. Rev. Aging Phys. Act. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2018, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, A.; Van Beek, A.H.E.A.; Rikkert, M.G.M.O.; Claassen, J.A.H.R.; Kessels, R.P.C. Effects of Aging on Cerebral Oxygenation during Working-Memory Performance: A Functional Near- Infrared Spectroscopy Study. PLoS ONE 2012, 7, e0046210. [Google Scholar] [CrossRef]
- Holtzer, R.; Izzetoglu, M.; Chen, M.; Wang, C. Distinct fNIRS-derived HbO2 trajectories during the course and over repeated walking trials under single-and dual-task conditions: Implications for within session learning and prefrontal cortex efficiency in older adults. J. Gerontol. Ser. A 2018, 74, 1076–1083. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
| Variables | LCVRF (14) | HCVRF (9) |
|---|---|---|
| Female n (%) | 10 (71.4%) | 5 (55.6%) |
| Age (years) | 66.86 ± 5.63 | 69.78 ± 5.89 |
| Education | 16.64 ± 4.03 | 13.78 ± 4.18 |
| MMSE | 28.57 ± 1.16 | 28.11 ± 0.93 |
| GDS | 3.71 ± 3.15 | 6.00 ± 4.24 |
| PASE | 127.43 ± 63.90 | 105.64 ± 57.59 |
| Smoking n (%) | 1 (7.10%) | 2 (22.20%) |
| Blood Parameters: | ||
| Resting SBP (mmHg) | 127.54 ± 13.87 | 135.11 ± 15.14 |
| Resting DBP (mmHg) | 74.92 ± 6.61 | 79.89 ± 9.09 |
| Total cholesterol (mmol/L) | 4.33 ± 1.25 | 4.04 ± 0.95 |
| LDL-cholesterol (mmol/L) | 2.30 ± 1.03 | 2.23 ± 0.66 |
| HDL-cholesterol (mmol/L) | 1.47 ± 0.47 | 1.27 ± 0.31 |
| Medications | 0.43 ± 0.94 | 2.67 ± 1.32 |
| Characteristics of the 1-year physical activity: | ||
| Frequency (n° visits/week) | 1.82 ± 0.84 | 1.73 ± 0.92 |
| Duration (min/week) | 221.38 ± 138.18 | 161.33 ± 82.53 |
| Intensity (Borg’s scale) | 4.47 ± 2.21 | 4.00 ± 1.68 |
| Experimental Condition | T0 | T6 | T12 | |||
|---|---|---|---|---|---|---|
| LCVRF | HCVRF | LCVRF | HCVRF | LCVRF | HCVRF | |
| Single Cognitive: | ||||||
| PM | 0.332 ± 0.271 | 0.627 ± 0.478 | 0.210 ± 0.228 | 0.321 ± 0.347 | 0.300 ± 0.356 | 0.162 ± 0.202 |
| M | 0.087 ± 0.115 | 0.151 ± 0.167 | 0.008 ± 0.082 | 0.070 ± 0.112 | 0.041 ± 0.109 | 0.006 ± 0.083 |
| PFrm | 0.069 ± 0.235 | 0.241 ± 0.248 | 0.052 ± 0.218 | 0.233 ± 0.186 | 0.198 ± 0.201 | 0.269 ± 0.241 |
| PFrd | 0.306 ± 0.557 | 1.176 ± 0.844 | 0.395 ± 0.382 | 0.632 ± 0.469 | 0.368 ± 0.415 | 0.691 ± 0.892 |
| PFcd | 0.270 ± 0.474 | 0.840 ± 0.541 | 0.305 ± 0.396 | 0.488 ± 0.374 | 0.440 ± 0.443 | 0.229 ± 0.241 |
| Single Walking: | ||||||
| PM | 0.116 ± 0.492 | 0.331 ± 0.553 | −0.021 ± 0.311 | 0.117 ± 0.360 | 0.158 ± 0.415 | 0.065 ± 0.235 |
| Right M | 0.054 ± 0.179 | 0.052 ± 0.198 | 0.001 ± 0.069 | 0.022 ± 0.146 | 0.0133 ± 0.121 | 0.055 ± 0.102 |
| Right PFcd | 0.092 ± 0.712 | 0.465 ± 0.771 | −0.330 ± 0.697 | 0.055 ± 0.759 | -0.024 ± 0.628 | 0.052 ± 0.264 |
| Dual Task: | ||||||
| PM | 0.703 ± 0.684 | 1.193 ± 0.821 | 0.225 ± 0.413 | 0.439 ± 0.493 | 0.555 ± 0.621 | 0.477 ± 0.557 |
| M | 0.126 ± 0.173 | 0.253 ± 0.262 | 0.021 ± 0.107 | 0.078 ± 0.148 | 0.097 ± 0.201 | 0.110 ± 0.228 |
| PFrm | 0.154 ± 0.488 | 0.481 ± 0.472 | −0.082 ± 0.468 | 0.321 ± 0.622 | −0.054 ± 0.398 | 0.131 ± 0.276 |
| PFrd | 0.864 ± 0.962 | 1.899 ± 1.410 | 0.342 ± 0.562 | 0.760 ± 0.897 | 0.555 ± 0.760 | 1.092 ± 0.943 |
| PFcd | 0.754 ± 0.970 | 1.341 ± 1.036 | 0.355 ± 0.732 | 0.396 ± 0.796 | 0.671 ± 0.862 | 0.438 ± 0.823 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talamonti, D.; Vincent, T.; Fraser, S.; Nigam, A.; Lesage, F.; Bherer, L. The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking. J. Clin. Med. 2021, 10, 579. https://doi.org/10.3390/jcm10040579
Talamonti D, Vincent T, Fraser S, Nigam A, Lesage F, Bherer L. The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking. Journal of Clinical Medicine. 2021; 10(4):579. https://doi.org/10.3390/jcm10040579
Chicago/Turabian StyleTalamonti, Deborah, Thomas Vincent, Sarah Fraser, Anil Nigam, Frédéric Lesage, and Louis Bherer. 2021. "The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking" Journal of Clinical Medicine 10, no. 4: 579. https://doi.org/10.3390/jcm10040579
APA StyleTalamonti, D., Vincent, T., Fraser, S., Nigam, A., Lesage, F., & Bherer, L. (2021). The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking. Journal of Clinical Medicine, 10(4), 579. https://doi.org/10.3390/jcm10040579






