Trends and Clinical Impact of Gastrointestinal Endoscopic Procedures on Acute Heart Failure in Spain (2002–2017)
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting and Participants
2.2. Study Variables
2.3. PSM Method
2.4. Statistical Methods
2.5. Sensitivity Analysis
2.6. Ethical Aspects
3. Results
3.1. Heart Failure Hospitalizations
3.2. Distribution of Study Covariates among Patients Hospitalized with Heart Failure with or without a Gastroscopy
3.3. Distribution of Study Covariates among Patients Hospitalized with Heart Failure That Did or Did Not Undergo Colonoscopy
3.4. Multivariable Logistic Regression Analysis of the Factors Associated with IHM in Patients Hospitalized with Heart Failure Who Underwent Gastroscopy or Colonoscopy
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, M.C.; Winters, M.E. Congestive Heart Failure. Emerg. Med. Clin. N. Am. 2015, 33, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; McAlister, F.A. Armstrong PW-Anemia is common in heart failure and is associated with poor outcomes: Insights from a cohort of 12,065 patients with new-onset heart failure. Circulation 2003, 107, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, H.F.; Januzzi, J.L.; Damman, K.; van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J.; van der Meer, D. Anemia and Mortality in Heart Failure Patients- A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2008, 52, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, O.; Floria, M.; Dascalita, P.; Stoica, A.; Adascalitei, P.; Sorodoc, V.; Sorodoc, L. Anemia in heart failure-from guidelines to controversies and challenges. Anatol. J. Cardiol. 2018, 20, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.J.; Yeo, P.S.; Wong, R.C.-C.; Ong, H.Y.; Leong, K.T.; Jaufeerally, F.; Sim, D.; Santhanakrishnan, R.; Lim, S.L.; MY Chan, M.; et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: Prevalence, clinical correlates, functional significance and prognosis. Eur. J. Heart Fail. 2014, 16, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2017, 73, 1–9. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J 2013, 165, 575–582.e3. [Google Scholar] [CrossRef]
- Zain, E.A.S.; Mohammad, A.G.; Lobna, A.W.; Elham, A.H.; Khaled, M.A. Upper Gastrointestinal Mucosal Changes in Patients with Congestive Heart Failure. Med. J. Cairo Univ. 2013, 81, 1009–1014. [Google Scholar]
- Romeiro, F.G.; Okoshi, K.; Zornoff, L.A.; Okoshi, M.P. Gastrointestinal changes associated to heart failure. Arq. Bras. Cardiol. 2012, 98, 273–277. [Google Scholar] [PubMed]
- Ministry of Health. Spanish National Hospital Discharge Database (Conjunto Minimo Basico de Datos). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 15 April 2020).
- Bonow, R.O.; Bennett, S.; Casey, D.E., Jr.; Ganiats, T.G.; Hlatky, M.A.; Konstam, M.A.; Lambrew, C.T.; Normand, S.-L.T.; Pina, I.L.; Radford, M.J.; et al. ACC/AHA clinical performance measures for adults with chronic heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): Endorsed by the Heart Failure Society of America. Circulation 2005, 112, 1853–1887. [Google Scholar] [PubMed]
- Mehta, A.B.; Syeda, S.N.; Wiener, R.S.; Walkey, A.J. Epidemiological trends in invasive mechanical ventilation in the United States: A population-based study. J. Crit. Care 2015, 30, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Austin, P.C. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat. Med. 2011, 30, 1292–1301. [Google Scholar] [CrossRef]
- Cosma, A.; Bănescub, C.; Mocan, S.; Balla, B.; Negovan, A. Congestive Heart Failure and Upper Digestive Endoscopic Lesions. Acta Med. Marisiensis 2019, 65, 19–24. [Google Scholar] [CrossRef]
- Valkhoff, V.E.; Sturkenboom, M.C.; Kuipers, E.J. Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pr. Res. Clin. Gastroenterol. 2012, 26, 125–140. [Google Scholar] [CrossRef]
- García-Rayado, G.; Sostres, C.; Lanas, A. Aspirin and Omeprazole for Secondary Prevention of Cardiovascular Disease in Patients at Risk for Aspirin-associated Gastric Ulcers. Expert. Rev. Clin. Pharmacol. 2017, 10, 875–888. [Google Scholar] [CrossRef]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients with heart failure have an increased risk of incident cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yates, J.M.; Logan, E.C.; Stewart, R.M. Iron deficiency anaemia in general practice: Clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad. Med. J. 2004, 80, 405–410. [Google Scholar] [CrossRef]
- Lucas, C.A.; Logan, E.C.; Logan, R.F. Audit of the investigation and outcome of iron-deficiency anaemia in one health district. J. R Coll. Physicians Lond. 1996, 30, 33–36. [Google Scholar]
- Martens, P.; Minten, L.; Dupont, M.; Mullens, W. Prevalence of underlying gastrointestinal malignancies in iron-deficient heart failure. ESC Heart Fail. 2019, 6, 37–44. [Google Scholar] [CrossRef]
- Moratti, R.; Tramarin, R.; Tavazzi, L. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur. Heart J. 2005, 26, 2232–2237. [Google Scholar]
- Van der Meer, P.; Voors, A.A.; Lipsic, E.; Smilde, T.D.; van Gilst, W.H.; van Veldhuisen, D.J. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 44, 63–67. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; van Veldhuisen, D.J.; van der Meer, P. Anemia in Heart Failure: Still Relevant? JACC Heart Fail. 2018, 6, 201–208. [Google Scholar] [CrossRef]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- Abu Ghanimeh, M.; Albadarinb, S.; Kaddourah, O.; Alturkmani, H.; Abughanimeh, O.; Tahboub, M.; Derbas, L.; Clarkston, W. Safety of Gastrointestinal Endoscopic Procedures in Patients with Severe Heart Failure. Am. J. Gastroenterol. 2017, 11, S265–S266. [Google Scholar] [CrossRef]
- Sivananthan, A.; Glover, B.; Ayaru, L.; Patel, K.; Darzi, A.; Patel, N. The evolution of lower gastrointestinal endoscopy: Where are we now? Ther. Adv. Gastrointest Endosc. 2020, 13, 1–16. [Google Scholar]
- Graham, D.G.; Banks, M.R. Advances in upper gastrointestinal endoscopy. F1000Research 2015, 4. [Google Scholar] [CrossRef]
- ASGE Technology Committee. High-definition and high-magnification endoscopes. Gastrointest. Endosc. 2014, 80, 919–927. [Google Scholar] [CrossRef]
- Patel, N.; Seneci, C.; Yang, G.-Z.; Darzi, A.; Teare, J. Flexible platforms for natural orifice transluminal and endoluminal surgery. Endosc. Int. Open 2014, 2, E117–E123. [Google Scholar] [CrossRef]
- Spurr, C., Jr. History of the instruments and techniques of gastrointestinal endoscopy. In Diagnostic and Therapeutic Procedures in Gastroenterology: An Illustrated Guide, 2nd ed.; Sridhar, S., Wu, G., Eds.; Humana Press: Totowa, NJ, USA, 2018; pp. 3–13. [Google Scholar]
- Manzano, L.; González-Franco, Á.; Cerqueiro, J.M.; Montero Pérez-Barquero, M. Heart Failure Programs/Units. A Multidisciplinary Approach. Rev. Esp. Cardiol. 2017, 70, 410. [Google Scholar] [CrossRef]
- Montminy, E.M.; Jang, A.; Conner, M.; Karlitz, J.J. Screening for Colorectal Cancer. Med. Clin. N. Am. 2020, 104, 1023–1036. [Google Scholar] [CrossRef]
| Variables | 2002–2003 | 2004–2005 | 2006–2007 | 2008–2009 | 2010–2011 | 2012–2013 | 2014–2015 | 2016–2017 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number of hospital admissions | 322,404 | 374,205 | 419,752 | 472,706 | 516,195 | 559,658 | 608,784 | 617,914 | 3891,618 |
| Incidence per 100,000 population * | 468.96 | 524.85 | 568.31 | 621.88 | 673.07 | 730.20 | 799.05 | 809.85 | 653.24 |
| Female sex, n (%) * | 171,366 (53.15) | 197,542 (52.79) | 221,102 (52.67) | 249,179 (52.71) | 270,879 (52.48) | 292,693 (52.3) | 316,917 (52.06) | 322,561 (52.2) | 2042,239 (52.48) |
| Age, years, mean (SD) * | 76.49 (10.9) | 76.92 (10.86) | 77.46 (10.9) | 78.01 (10.84) | 78.61 (10.77) | 79.09 (10.75) | 79.54 (10.77) | 80.11 (10.77) | 78.53 (10.87) |
| < 60 years, n (%) * | 23,924 (7.42) | 26,848 (7.17) | 29,295 (6.98) | 30,665 (6.49) | 31,264 (6.06) | 32,826 (5.87) | 34,348 (5.64) | 32,953 (5.33) | 242,123 (6.22) |
| 60–75 years, n (%) * | 105,639 (32.77) | 115,387 (30.84) | 119,520 (28.47) | 124,686 (26.38) | 124,547 (24.13) | 125,540 (22.43) | 133,222 (21.88) | 130,904 (21.18) | 979,445 (25.17) |
| 76–85 years, n (%) * | 129,197 (40.07) | 155,867 (41.65) | 178,213 (42.46) | 202,388 (42.81) | 221,751 (42.96) | 238,998 (42.7) | 249,420 (40.97) | 241,085 (39.02) | 1616,919 (41.55) |
| > 85 years, n (%) * | 63,644 (19.74) | 76,103 (20.34) | 92,724 (22.09) | 114,967 (24.32) | 138,633 (26.86) | 162,294 (29) | 191,794 (31.5) | 212,972 (34.47) | 1053,131 (27.06) |
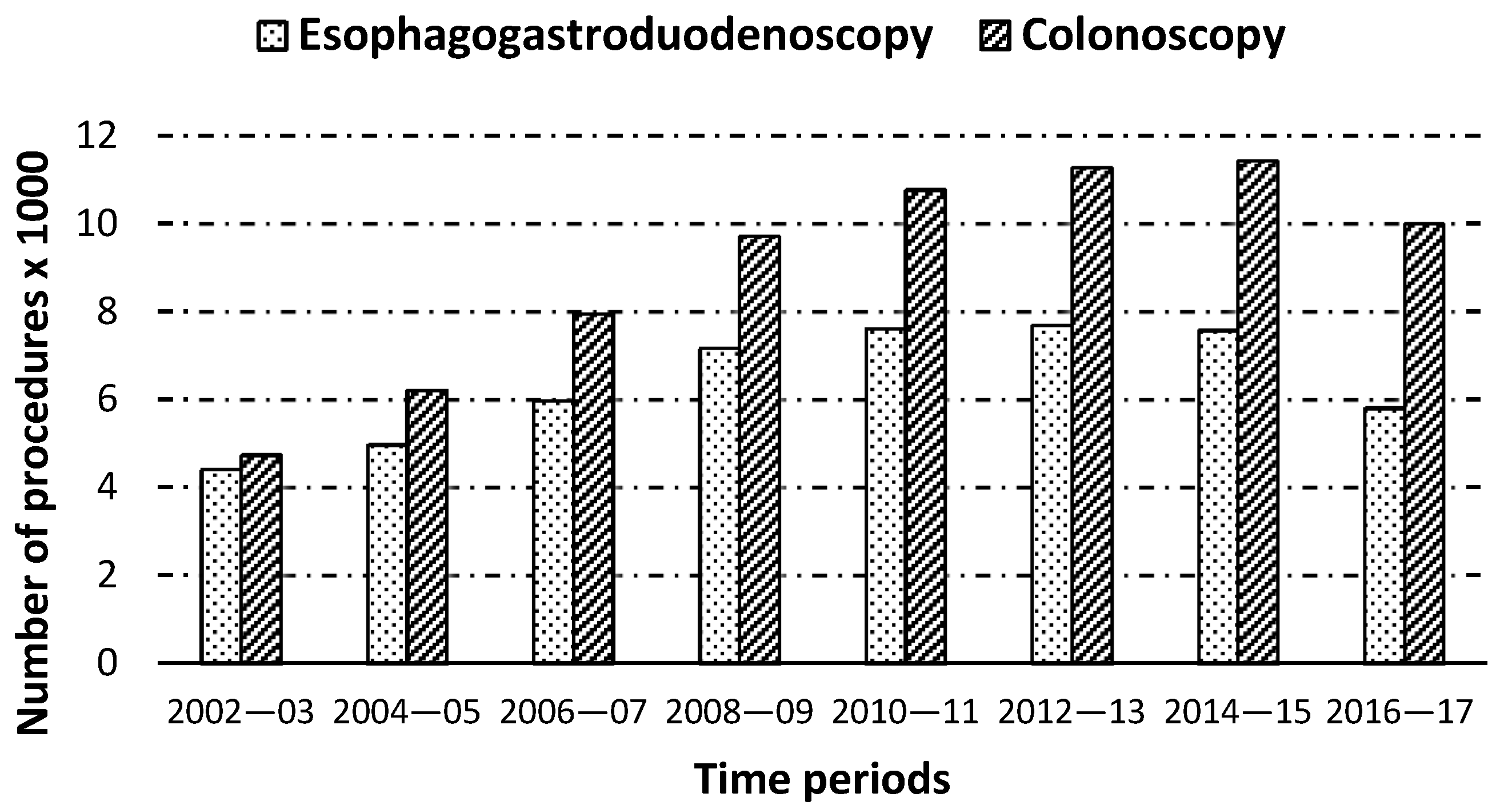
| EGD, n (%) * | 4409 (1.37) | 4972 (1.33) | 5969 (1.42) | 7166 (1.52) | 7612 (1.47) | 7687 (1.37) | 7568 (1.24) | 5804 (0.94) | 51,187 (1.32) |
| Colonoscopy, n (%) * | 4739 (1.47) | 6213 (1.66) | 7953 (1.89) | 9712 (2.05) | 10,768 (2.09) | 11,267 (2.01) | 11,428 (1.88) | 9996 (1.62) | 72,076 (1.85) |
| CCI, mean (SD) * | 2.19 (0.98) | 2.28 (1.01) | 2.3 (1.01) | 2.35 (1.02) | 2.39 (1.02) | 2.44 (1.04) | 2.46 (1.04) | 2.49 (1.11) | 2.38 (1.04) |
| Ischemic coronary disease, n (%) * | 97,548 (30.26) | 114,861 (30.69) | 124,593 (29.68) | 136,082 (28.79) | 141,906 (27.49) | 152,626 (27.27) | 159,558 (26.21) | 153,259 (24.8) | 1080,433 (27.76) |
| Atrial fibrillation, n (%) * | 119,835 (37.17) | 147,922 (39.53) | 172,769 (41.16) | 200,508 (42.42) | 224,191 (43.43) | 250,202 (44.71) | 277,822 (45.64) | 295,617 (47.84) | 1688,866 (43.4) |
| Anemia, n (%) * | 719 (0.22) | 871 (0.23) | 1068 (0.25) | 1316 (0.28) | 1497 (0.29) | 1741 (0.31) | 1828 (0.30) | 1789 (0.29) | 10,829 (0.28) |
| Chronic liver disease, n (%) * | 8949 (2.78) | 11,538 (3.08) | 13,449 (3.2) | 15,531 (3.29) | 17,488 (3.39) | 20,229 (3.61) | 22,984 (3.78) | 12,201 (1.97) | 122,369 (3.14) |
| Angiodysplasia, n (%) | 965 (0.3) | 1478 (0.39) | 1894 (0.45) | 2402 (0.51) | 3054 (0.59) | 3618 (0.65) | 4128 (0.68) | 4445 (0.72) | 21,984 (0.56) |
| Acute renal failure, n (%) * | 49,726 (15.42) | 66,197 (17.69) | 80,701 (19.23) | 104,535 (22.11) | 128,249 (24.85) | 156,387 (27.94) | 184,424 (30.29) | 205,242 (33.22) | 975,461 (25.07) |
| T2DM, n (%) * | 95,182 (29.52) | 122,448 (32.72) | 142,169 (33.87) | 166,936 (35.31) | 185,036 (35.85) | 202,878 (36.25) | 220,319 (36.19) | 231,517 (37.47) | 1366,485 (35.11) |
| COPD, n (%) * | 71,136 (22.06) | 81,937 (21.9) | 82,076 (19.55) | 89,205 (18.87) | 97,518 (18.89) | 104,762 (18.72) | 111,752 (18.36) | 171,698 (27.79) | 810,084 (20.82) |
| Colon cancer, n (%) * | 1413 (0.44) | 1960 (0.52) | 2328 (0.55) | 2817 (0.6) | 3187 (0.62) | 3674 (0.66) | 3999 (0.66) | 4152 (0.67) | 23,530 (0.6) |
| Stomach cancer, n (%) * | 702 (0.22) | 789 (0.21) | 905 (0.22) | 1067 (0.23) | 1164 (0.23) | 1191 (0.21) | 1237 (0.2) | 1235 (0.2) | 8290 (0.21) |
| Gastrointestinal bleeding, n (%) * | 6074 (1.88) | 7167 (1.92) | 7963 (1.9) | 8888 (1.88) | 9713 (1.88) | 10,333 (1.85) | 11,134 (1.83) | 13,201 (2.14) | 74,473 (1.91) |
| Inflammatory bowel disease, n (%) * | 556 (0.17) | 708 (0.19) | 861 (0.21) | 1100 (0.23) | 1212 (0.23) | 1430 (0.26) | 1828 (0.3) | 1958 (0.32) | 9653 (0.25) |
| Red cell transfusion, n (%) * | 19,991 (6.2) | 24,620 (6.58) | 30,054 (7.16) | 36,601 (7.74) | 42,832 (8.3) | 47,414 (8.47) | 50,074 (8.23) | 33,505 (5.42) | 285,091 (7.33) |
| IHM, n (%) * | 44,461 (13.79) | 49,725 (13.29) | 54,075 (12.88) | 60,551 (12.81) | 65,041 (12.6) | 69,594 (12.44) | 75,129 (12.34) | 76,670 (12.41) | 495.246 (12.73) |
| LOHS, mean (SD) | 11.29 (10.94) | 11.12 (10.85) | 10.89 (10.47) | 10.74 (10.57) | 10.32 (10.24) | 9.84 (9.29) | 9.74 (9.25) | 9.78 (9.38) | 10.34 (10.35) |
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| EGD | No EGD | p-Value | EGD | No EGD | p-Value | |
| 2002–2003, n (%) | 4409 (8.61) | 317,995 (8.28) | <0.001 | 4409 (8.61) | 4632 (9.05) | 0.083 |
| 2004–2005, n (%) | 4972 (9.71) | 369,233 (9.61) | 4972 (9.71) | 5156 (10.07) | ||
| 2006–2007, n (%) | 5969 (11.66) | 413,783 (10.77) | 5969 (11.66) | 6024 (11.77) | ||
| 2008–2009, n (%) | 7166 (14) | 465,540 (12.12) | 7166 (14) | 7010 (13.69) | ||
| 2010–2011, n (%) | 7612 (14.87) | 508,583 (13.24) | 7612 (14.87) | 7532 (14.71) | ||
| 2012–2013, n (%) | 7687 (15.02) | 551,971 (14.37) | 7687 (15.02) | 7572 (14.79) | ||
| 2014–2015, n (%) | 7568 (14.79) | 601,216 (15.65) | 7568 (14.79) | 7507 (14.67) | ||
| 2016–2017, n (%) | 5804 (11.34) | 612,110 (15.94) | 5804 (11.34) | 5754 (11.24) | ||
| Female sex, n (%) | 25715 (50.24) | 2016,524 (52.51) | <0.001 | 25,715 (50.24) | 25,949 (50.69) | 0.144 |
| Age, years, and mean (SD) | 77.51 (9.68) | 78.54 (10.88) | <0.001 | 77.51 (9.68) | 77.71 (9.94) | <0.001 |
| < 60 years, n (%) | 2753 (5.38) | 239,370 (6.23) | <0.001 | 2753 (5.38) | 2782 (5.43) | <0.001 |
| 60–75 years, n (%) | 14,473 (28.27) | 964,972 (25.13) | 14,473 (28.27) | 14,060 (27.47) | ||
| 76–85 years, n (%) | 24,642 (48.14) | 1592,277 (41.46) | 24,642 (48.14) | 24,344 (47.56) | ||
| 85 years, n (%) | 9319 (18.21) | 1043,812 (27.18) | 9319 (18.21) | 10,001 (19.54) | ||
| Colonoscopy, n (%) | 16,521 (32.28) | 55,555 (1.45) | <0.001 | 16,521 (32.28) | 16,230 (31.71) | 0.051 |
| CCI, mean (SD) | 2.5 (1.06) | 2.38 (1.04) | <0.001 | 2.5 (1.06) | 2.41 (1.03) | <0.001 |
| Ischemic coronary disease, n (%) | 11,595 (22.65) | 1068,838 (27.83) | <0.001 | 11,595 (22.65) | 10,746 (20.99) | <0.001 |
| Atrial fibrillation, n (%) | 21,575 (42.15) | 1667,291 (43.41) | <0.001 | 21,575 (42.15) | 21,691 (42.38) | 0.463 |
| Anemia, n (%) | 312 (0.61) | 10,517 (0.27) | 0.001 | 310 (0.61) | 331 (0.66) | 0.405 |
| Chronic liver disease, n (%) | 4551 (8.89) | 117,818 (3.07) | <0.001 | 4551 (8.89) | 4786 (9.35) | <0.001 |
| Angiodysplasia, n (%) | 4023 (7.86) | 17,961 (0.47) | <0.001 | 4023 (7.86) | 3236 (6.32) | 0.355 |
| Acute renal failure, n (%) | 13,096 (25.58) | 962,365 (25.06) | 0.006 | 13,096 (25.58) | 12,967 (25.33) | 0.002 |
| Type 2 diabetes, n (%) | 17,023 (33.26) | 1349,462 (35.14) | <0.001 | 17,023 (33.26) | 16,564 (32.36) | <0.001 |
| COPD, n (%) | 8990 (17.56) | 801,094 (20.86) | <0.001 | 8990 (17.56) | 8399 (16.41) | 0.051 |
| Colon cancer, n (%) | 817 (1.6) | 22,713 (0.59) | <0.001 | 817 (1.6) | 897 (1.75) | 0.743 |
| Stomach cancer, n (%) | 1385 (2.71) | 6905 (0.18) | <0.001 | 1385 (2.71) | 1368 (2.67) | 0.038 |
| Gastrointestinal bleeding, n (%) | 11,554 (22.57) | 62,919 (1.64) | <0.001 | 11,554 (22.57) | 11,832 (23.12) | 0.517 |
| Inflammatory bowel disease, n (%) | 166 (0.32) | 9487 (0.25) | <0.001 | 166 (0.32) | 178 (0.35) | <0.001 |
| Red cell transfusion, n (%) | 21,229 (41.47) | 263,862 (6.87) | <0.001 | 21,229 (41.47) | 22,110 (43.19) | <0.001 |
| LOHS, n (%) | 17.51 (16.08) | 10.25 (10.24) | <0.001 | 17.51 (16.08) | 14.87 (14.36) | <0.001 |
| IHM, n (%) | 4956 (9.68) | 490,290 (12.77) | <0.001 | 4956 (9.68) | 7662 (14.97) | <0.001 |
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Colonoscopy | No Colonoscopy | p-Value | Colonoscopy | No Colonoscopy | p-Value | |
| 2002–2003, n (%) | 4739 (6.58) | 317,665 (8.32) | <0.001 | 4739 (6.58) | 5126 (7.11) | <0.001 |
| 2004–2005, n (%) | 6213 (8.62) | 367,992 (9.63) | 6213 (8.62) | 6482 (8.99) | ||
| 2006–2007, n (%) | 7953 (11.03) | 411,799 (10.78) | 7953 (11.03) | 7996 (11.09) | ||
| 2008–2009, n (%) | 9712 (13.47) | 462,994 (12.12) | 9712 (13.47) | 9482 (13.16) | ||
| 2010–2011, n (%) | 10,768 (14.94) | 505,427 (13.23) | 10,768 (14.94) | 10,395 (14.42) | ||
| 2012–2013, n (%) | 11,267 (15.63) | 548,391 (14.36) | 11,267 (15.63) | 10,942 (15.18) | ||
| 2014–2015, n (%) | 11,428 (15.86) | 597,356 (15.64) | 11,428 (15.86) | 11,205 (15.55) | ||
| 2016–2017, n (%) | 9996 (13.87) | 607,918 (15.92) | 9996 (13.87) | 10,448 (14.5) | ||
| Female sex, n (%) | 36,626 (50.82) | 2005,613 (52.51) | <0.001 | 36,626 (50.82) | 36,564 (50.73) | 0.744 |
| Age, years, mean (SD) | 77.65 (9.14) | 78.54 (10.9) | <0.001 | 77.65 (9.14) | 77.72 (9.79) | 0.818 |
| < 60 years, n (%) | 3254 (4.51) | 238,869 (6.25) | <0.001 | 3254 (4.51) | 3686 (5.11) | <0.001 |
| 60–75 years, n (%) | 20,558 (28.52) | 958,887 (25.1) | 20,558 (28.52) | 19,904 (27.62) | ||
| 76–85 years, n (%) | 35,765 (49.62) | 1581,154 (41.4) | 35,765 (49.62) | 34,658 (48.09) | ||
| > 85 years, n (%) | 12,499 (17.34) | 1040,632 (27.24) | 12,499 (17.34) | 13,828 (19.19) | ||
| EGD, n (%) | 16,521 (22.92) | 34,666 (0.91) | <0.001 | 16,521 (22.92) | 14,996 (20.81) | <0.001 |
| CCI, mean (SD) | 2.30 (1.03) | 2.38 (1.04) | <0.001 | 2.30 (1.03) | 2.41 (1.05) | <0.001 |
| Ischemic coronary disease, n (%) | 16,587 (23.01) | 1063,846 (27.85) | <0.001 | 16,587 (23.01) | 15,500 (21.51) | <0.001 |
| Atrial fibrillation, n (%) | 30,979 (42.98) | 1657,887 (43.41) | 0.023 | 30,979 (42.98) | 30,602 (42.46) | 0.045 |
| Anemia, n (%) | 480 (0.71) | 10,317 (0.27) | <0.001 | 509 (0.71) | 525 (0.73) | <0.662 |
| Chronic liver disease, n (%) | 4349 (6.03) | 118,020 (3.09) | <0.001 | 4349 (6.03) | 4690 (6.51) | <0.001 |
| Angiodysplasia, n (%) | 4300 (5.97) | 17,684 (0.46) | <0.001 | 4300 (5.97) | 3856 (5.35) | <0.001 |
| Acute renal failure, n (%) | 19,022 (26.39) | 956,439 (25.04) | <0.001 | 19,022 (26.39) | 18,764 (26.03) | 0.122 |
| Type 2 diabetes, n (%) | 25,478 (35.35) | 1341,007 (35.11) | 0.182 | 25,478 (35.35) | 24,592 (34.12) | 0.182 |
| COPD, n (%) | 13,383 (18.57) | 796,701 (20.86) | <0.001 | 13,383 (18.57) | 12,750 (17.69) | <0.001 |
| Colon cancer, n (%) | 4711 (6.54) | 18,819 (0.49) | <0.001 | 4711 (6.54) | 5099 (7.07) | <0.001 |
| Stomach cancer, n (%) | 390 (0.54) | 7900 (0.21) | <0.001 | 390 (0.54) | 513 (0.71) | <0.001 |
| Gastrointestinal bleeding, n (%) | 14,997 (20.81) | 59,476 (1.56) | <0.001 | 14,997 (20.81) | 15,495 (21.5) | <0.001 |
| Inflammatory bowel disease, n (%) | 996 (1.38) | 8657 (0.23) | <0.001 | 996 (1.38) | 1179 (1.64) | <0.001 |
| Red cell transfusion, n (%) | 26,369 (36.58) | 258,722 (6.77) | <0.001 | 26,369 (36.58) | 27,837 (38.62) | <0.001 |
| LOHS, n (%) | 18.56 (16.43) | 10.19 (10.18) | <0.001 | 18.56 (16.43) | 13.68 (12.1) | <0.001 |
| IHM, n (%) | 5672 (7.87) | 48,9574 (12.82) | <0.001 | 5672 (7.87) | 11,688 (16.22) | <0.001 |
| Variables | EGD OR (95%CI) | Colonoscopy OR (95%CI) |
|---|---|---|
| Year | 0.97 (0.96–0.99) | 0.93 (0.92–0.94) |
| Female sex | 0.83 (0.77–0.88) | 0.82 (0.77–0.87) |
| 60–75 years, | 0.77 (0.67–0.87) | 1.03 (0.89–1.19) |
| 76–85 years | 0.86 (0.76–0.98) | 1.12 (0.97–1.28) |
| >85 years | 1.03 (0.9–1.18) | 1.34 (1.15–1.55) |
| Colonoscopy | 0.45 (0.41–0.49) | NA |
| EGD | NA | 0.6 (0.55–0.64) |
| CCI | 1.52 (1.46–1.58) | 1.44 (1.39–1.5) |
| Ischemic coronary disease | 0.75 (0.7–0.82) | 0.73 (0.68–0.78) |
| Atrial fibrillation | 0.78 (0.73–0.83) | 0.85 (0.8–0.9) |
| Chronic liver disease | 1.07 (0.97–1.18) | 0.84 (0.75–0.95) |
| Angiodysplasia, n (%) | 0.67 (0.58–0.77) | 0.63 (0.54–0.73) |
| Acute renal failure | 0.71 (0.65–0.76) | 0.75 (0.69–0.8) |
| Type 2 diabetes | 0.44 (0.41–0.48) | 0.46 (0.43–0.5) |
| COPD | 0.61 (0.55–0.66) | 0.65 (0.6–0.71) |
| Colon cancer | 1.57 (1.26–1.96) | 1.67 (1.53–1.83) |
| Stomach cancer | 1.91 (1.67–2.18) | 1.78 (1.33–2.38) |
| Gastrointestinal bleeding | 1.86 (1.74–1.99) | 1.39 (1.31–1.49) |
| Inflammatory bowel disease | 1.41 (0.86–2.32) | 1.78 (1.47–2.16) |
| Red blood cell transfusion | 1.1 (1.04–1.17) | 1.04 (0.98–1.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Bailón, M.; Jiménez-García, R.; Muñoz-Rivas, N.; Hernández-Barrera, V.; de Miguel-Yanes, J.M.; de Miguel-Díez, J.; Andrès, E.; Lorenzo-Villalba, N.; López-de-Andrés, A. Trends and Clinical Impact of Gastrointestinal Endoscopic Procedures on Acute Heart Failure in Spain (2002–2017). J. Clin. Med. 2021, 10, 546. https://doi.org/10.3390/jcm10030546
Méndez-Bailón M, Jiménez-García R, Muñoz-Rivas N, Hernández-Barrera V, de Miguel-Yanes JM, de Miguel-Díez J, Andrès E, Lorenzo-Villalba N, López-de-Andrés A. Trends and Clinical Impact of Gastrointestinal Endoscopic Procedures on Acute Heart Failure in Spain (2002–2017). Journal of Clinical Medicine. 2021; 10(3):546. https://doi.org/10.3390/jcm10030546
Chicago/Turabian StyleMéndez-Bailón, Manuel, Rodrigo Jiménez-García, Nuria Muñoz-Rivas, Valentín Hernández-Barrera, José Maria de Miguel-Yanes, Javier de Miguel-Díez, Emmanuel Andrès, Noel Lorenzo-Villalba, and Ana López-de-Andrés. 2021. "Trends and Clinical Impact of Gastrointestinal Endoscopic Procedures on Acute Heart Failure in Spain (2002–2017)" Journal of Clinical Medicine 10, no. 3: 546. https://doi.org/10.3390/jcm10030546
APA StyleMéndez-Bailón, M., Jiménez-García, R., Muñoz-Rivas, N., Hernández-Barrera, V., de Miguel-Yanes, J. M., de Miguel-Díez, J., Andrès, E., Lorenzo-Villalba, N., & López-de-Andrés, A. (2021). Trends and Clinical Impact of Gastrointestinal Endoscopic Procedures on Acute Heart Failure in Spain (2002–2017). Journal of Clinical Medicine, 10(3), 546. https://doi.org/10.3390/jcm10030546







