The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline †
Abstract
1. Introduction
2. Methods
3. Cancer Risks in Peutz–Jeghers Syndrome
4. Recommendations and Statements
4.1. Clinical Genetic Management
| If the clinical diagnostic criteria for PJS are met, genetic germline screening of the STK11 gene is warranted regardless of age. A patient meeting the clinical criteria should be regarded as having PJS, even if an underlying causative germline variant is not identified. Level of evidence: moderate Strength of recommendation: strong |
| The detection rate of pathogenic STK11 variants in patients with a clinical diagnosis of PJS is high (up to 100%), using techniques that detect single nucleotide changes as well as larger deletions and duplications in the STK11 gene. Currently, there is no evidence for genetic heterogeneity in patients fulfilling the diagnostic clinical criteria for PJS without a germline STK11 PV. Thus pathogenic variants that cannot be identified by up-to-date methods in routine diagnostics should be considered in these cases. Level of evidence: moderate Strength of recommendation: strong |
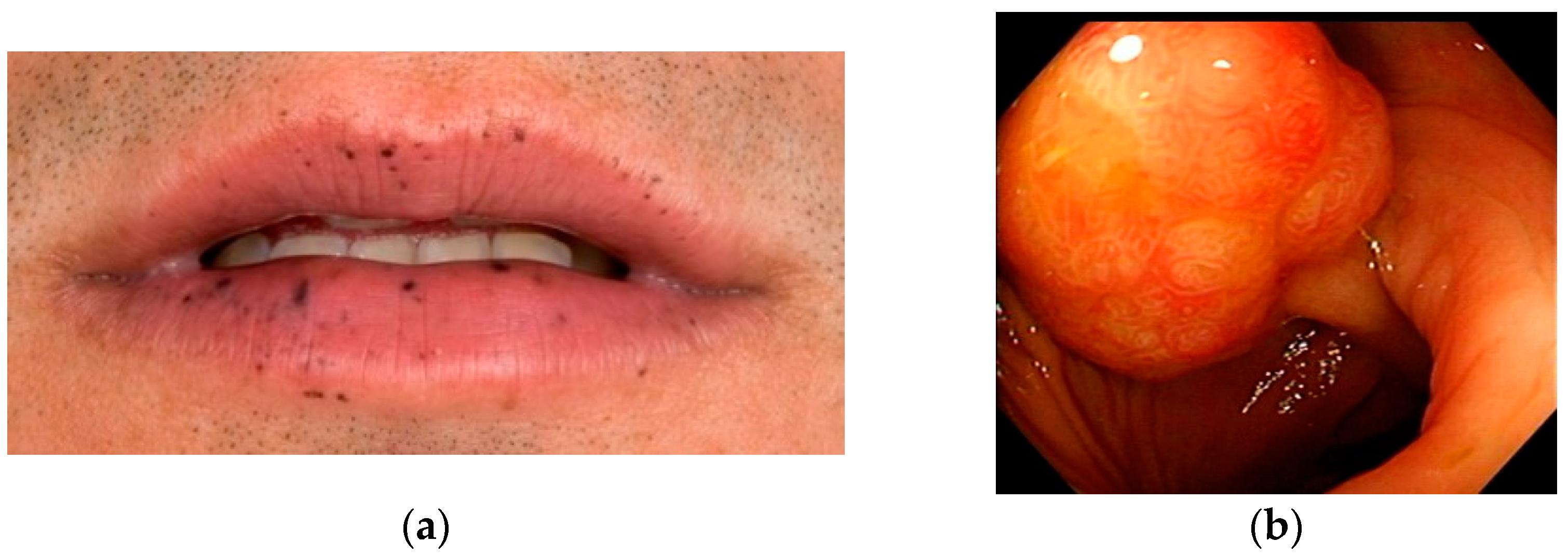
| Based on recent recommendations of the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPHGAN), genetic germline screening of the STK11 gene is warranted in children and adolescents with typical perioral pigmentation. Genetic screening may be considered in adults with isolated typical perioral pigmentation but is less likely to yield a pathogenic variant with increasing age. Level of evidence: low Strength of recommendation: moderate |
| Genetic germline screening of the STK11 gene is warranted in children and adolescents with one PJ polyp. Genetic screening may be considered in adults with a confident diagnosis of solitary polyp but is less likely to yield a pathogenic variant with increasing age. Level of evidence: low Strength of recommendation: moderate |
| If no pathogenic variant in STK11 can be identified in a patient not fulfilling the clinical diagnostic criteria for PJS, the patient should not be considered as having PJS. Level of evidence: low Strength of recommendation: moderate |
4.2. Gastrointestinal Management
| Based on recent recommendations of the European Society of Gastrointestinal Endoscopy (ESGE), a baseline oesophagogastroduodenoscopy and colonoscopy is recommended at the age of 8 years in asymptomatic individuals with PJS. If polyps are detected at the baseline endoscopy, a 1–3 yearly interval based on phenotype for oesophagogastroduodenoscopy and/or colonoscopy is recommended. Routine oesophagogastroduodenoscopy and colonoscopy surveillance is recommended at the age of 18 if the baseline endoscopy is negative. Level of evidence: low Strength of recommendation: strong |
| Based on recent recommendations of the European Society of Gastrointestinal Endoscopy (ESGE), small bowel surveillance is recommended from the age of 8 years in asymptomatic individuals with PJS. A 1–3 yearly interval is recommended based on phenotype for small-bowel surveillance. Either MRI studies or video capsule enteroscopy is recommended for small-bowel surveillance. Level of evidence: moderate Strength of recommendation: strong |
| Based on recent recommendations of the European Society of Gastrointestinal Endoscopy (ESGE) elective polypectomy should be performed for small-bowel polyps > 15–20 mm to prevent intussusception. In a symptomatic patient, smaller polyps causing obstructive symptoms should be removed. Level of evidence: low Strength of recommendation: strong |
| Based on recent recommendations of the European Society of Gastrointestinal Endoscopy (ESGE), device-assisted enteroscopy for the removal of polyps is recommended. Based on phenotype, intraoperative enteroscopy could be considered. Level of evidence: moderate Strength of recommendation: strong |
| In case of symptoms, an oesophagogastroduodenoscopy, small bowel investigation, or colonoscopy should be performed earlier rather than waiting for routine surveillance. Level of evidence: low Strength of recommendation: strong |
| For patients with a confident diagnosis of a solitary PJ polyp, routine endoscopic surveillance is not recommended. Level of evidence: low Strength of recommendation: strong |
| Routine haemoglobin testing in children with PJS is not recommended, as there are no data reporting on its utility and outcome. Haemoglobin testing may be useful in the symptomatic setting. Level of evidence: low Strength of recommendation: weak |
4.3. Surgical Management
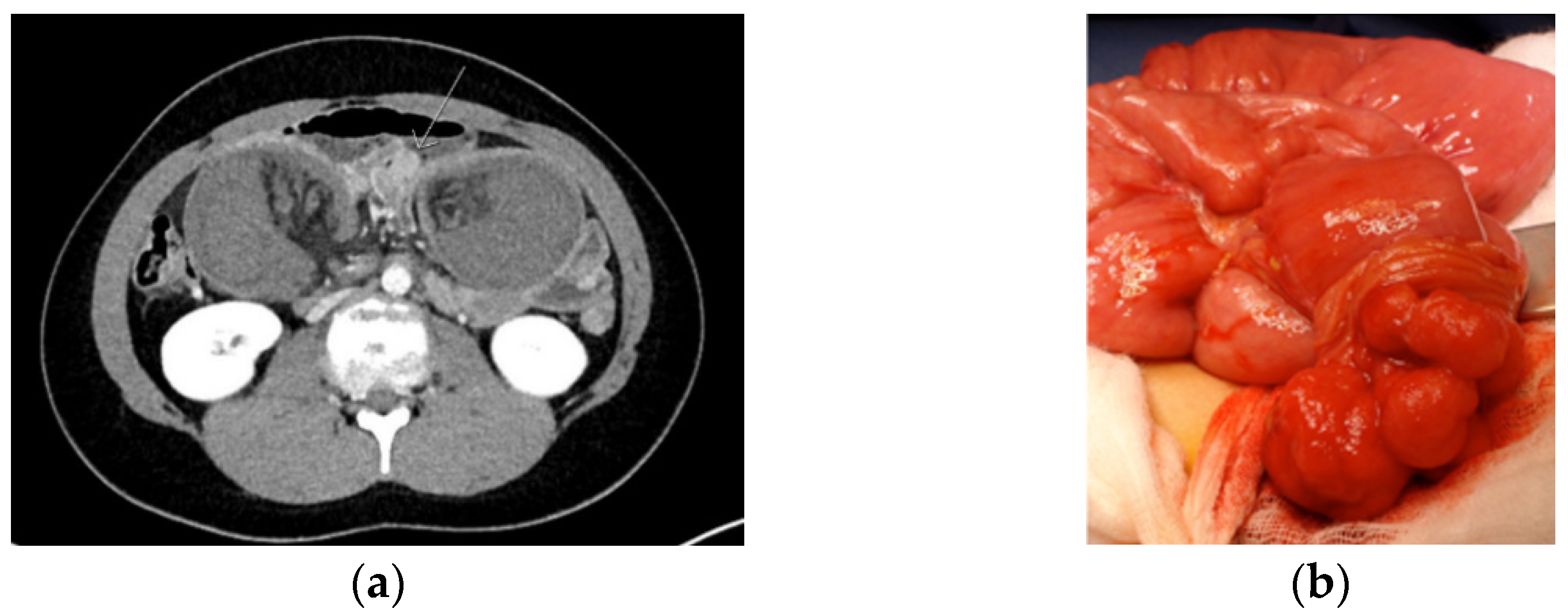
| PJS patients with an episode of acute severe abdominal pain and/or suspicion of intussusception should urgently be referred to a surgical unit, preferably a dedicated center. If, after clinical and diagnostic evaluation the event of small bowel intussusception is not ruled out, emergency surgery (even in diagnostic intent) is recommended. Level of evidence: moderate/low Strength of recommendation: strong |
| At surgery, the preferred strategy of treating an intussusception is to dessuscept, if safe to do so. If successful, the polyp that acts as a hypomochlion should be removed by enterotomy with resection of the (pedunculated) polyp at the base. In addition, the entire small bowel should be critically inspected for further relevant polyps, and all polyps > 15 mm should be removed by enterotomy or by intraoperative enteroscopy. Depending on the distance between the polyps, an enterotomy in between polyps allowing for removal of multiple polyps via one enterotomy is preferred. Level of evidence: moderate/low Strength of recommendation: strong |
4.4. Pancreatic Management
| Although PJS is considered a hereditary condition that carries some of the highest lifetime risks for developing pancreatic cancer, it should be discussed with patients that the benefits and harms of pancreatic cancer surveillance are not well established yet and under investigation. Therefore, it is recommended that surveillance is conducted at centers of expertise in the framework of a study or registry. Level of evidence: moderate/low Strength of recommendation: strong |
| Based on recent recommendations of the International Cancer of the Pancreas (CAPS) Consortium, patients with PJS are eligible for pancreatic surveillance in the framework of a study or registry, irrespective of patients’ family history of pancreatic cancer (PDAC), because of an estimated lifetime risk to develop PDAC of 11–55%. Level of evidence: moderate/low Strength of recommendation: strong |
| The recommendations for pancreatic surveillance of patients with PJS of the International CAPS Consortium are endorsed and should be followed. Level of evidence: moderate/low Strength of recommendation: weak |
| Prevailing regional pancreatic cyst surveillance guidelines should be carried out for cyst follow-up and management in PJS patients. Level of evidence: moderate/low Strength of recommendation: weak |
| Any significant abnormal finding during surveillance should be discussed in a multidisciplinary panel. Level of evidence: low Strength of recommendation: strong |
| According to the recent recommendations of the International CAPS Consortium, a (partial) pancreatectomy should be performed in case of detection of: (i) a solid lesion ≥ 10 mm (except biopsy-proven or highly suspicious to be neuroendocrine, autoimmune, or other benign conditions); (ii) IPMN in case of a mural nodule, an enhanced solid component, symptoms (including pancreatitis, jaundice, pain), thickened/enhanced cyst walls, abrupt change in pancreatic duct with distal pancreatic atrophy, or a main pancreatic duct ≥ 10 mm. Level of evidence: moderate/low Strength of recommendation: strong |
| Due to its significant morbidity and potential mortality even in experienced hands, a total pancreatectomy is not recommended for a localized lesion. Level of evidence: low Strength of recommendation: strong |
| Prophylactic pancreatectomy is not recommended because of the significant associated morbidity and potential mortality, even in experienced hands. Level of evidence: low Strength of recommendation: strong |
4.5. Breast Management
| The following breast surveillance is recommended in female PJS patients: Raising awareness at age 18 years e.g., by starting breast self-examination; Clinical breast exam every 6–12 months starting at age of 25 years; Annual breast contrast MRI screening (or breast ultrasound if MRI contraindication or unavailability) at age 25–30 years; Annual mammogram with consideration of tomosynthesis and ultrasound for dense breast and annual breast contrast MRI at age 30–50 years; Annual mammogram with consideration of annual breast contrast MRI for dense breast pattern at age 50–75 years; Management should be considered on an individual basis from age > 75 years. Level of evidence: low Strength of recommendation: moderate |
| The optimal breast surveillance strategy in female PJS patients remains debated and the benefits of surveillance remain to be established. Therefore, it is recommended that surveillance is conducted at centers of expertise in the framework of a study or registry. Level of evidence: low Strength of recommendation: strong |
| As evidence for its benefit is lacking, prophylactic mastectomy is currently not recommended for female PJS patients. Risk reducing mastectomy should be discussed in a multidisciplinary setting also taking into account family history and other clinical factors. Level of evidence: low Strength of recommendation: moderate |
4.6. Gynecological Management
| Expert gynecological surveillance should be offered to female patients with PJS, irrespective of their family history of gynecological cancer, because of an estimated lifetime risk of specific gynecological tumors of 18–50%. Level of evidence: low Strength of recommendation: moderate |
| It is recommended that female PJS patients are counseled regarding specific gynecological cancer risks, red flag symptoms, contraceptive choices, and family planning by a PJS specialist at 18–20 years of age. Level of evidence: low Strength of recommendation: moderate |
| It is recommended that female PJS patients have annual gynecological examinations from the age of 25 years. In addition to cervical screening as performed in population-based screening programs that run in many countries, gynecological surveillance in female PJS patients should be focused on the detection of cervical adenocarcinomas, in particularly minimal deviation adenocarcinoma, and rare non-epithelial ovarian tumors. Surveillance for cervical adenocarcinomas should involve speculum examination and cervical screening ("Pap smear") including cytology even in an HPV-negative sample. Surveillance for non-epithelial ovarian cancers should involve bimanual pelvic examination with a transvaginal ultrasound in case of suspicion of a pelvic mass. CA125 testing is not indicated. Level of evidence: low Strength of recommendation: moderate |
| The optimal gynecological surveillance strategy in female PJS patients remains debated and the benefits of surveillance remain to be established. Therefore, it is recommended that surveillance is conducted by a gynecologist who is experienced in the particular cancer risks that PJS patients face in the framework of a study or registry. Level of evidence: low Strength of recommendation: strong |
| Peutz-Jeghers syndrome can be an indication for Prenatal Genetic Diagnosis (PND) and Preimplantation Genetic Diagnosis (PGD) and these options should be discussed with PJS patients in whom a STK11 pathogenic variant has been identified. Level of evidence: low Strength of recommendation: strong |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peutz, J.L.A. Over een zeer merkwaardige, gecombineerde familiaire polyposis van de slijmliezen van den tractus intestinalis met die van de neuskeelholte en gepaard met eigenaardige pigmentaties van huid-en slijmvliezen. Nederl Maandschr Geneesk 1921, 10, 134–146. [Google Scholar]
- Jeghers, H.; Mc, K.V.; Katz, K.H. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N. Engl. J. Med. 1949, 241, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.; Houlston, R.S. Peutz-Jeghers syndrome. J. Med. Genet. 1997, 34, 1007–1011. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Jarvinen, H.; Gruber, S.B.; Billaud, M.; Jass, J.R. Tumours of the small intestine: Peutz-Jeghers syndrome. In World Health Organization Classification of Tumours: Pathology and Genetics. Tumours of the Digestive System; IARC Press: Lyon, France, 2000. [Google Scholar]
- Beggs, A.D.; Latchford, A.R.; Vasen, H.F.; Moslein, G.; Alonso, A.; Aretz, S.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; et al. Peutz-Jeghers syndrome: A systematic review and recommendations for management. Gut 2010, 59, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.T.; Winship, I. The role of STK11 gene testing in individuals with oral pigmentation. Australas. J. Dermatol. 2017, 58, 135–138. [Google Scholar] [CrossRef]
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Höglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Linstone, H.A.; Turoff, M. The Delphi Method: Techniques and Applications; Addison-Wesley Pub. Co: Boston, MA, USA, 2002. [Google Scholar]
- Likert, R. Technique for the Measurement of Attitudes; The Science Press: New York, NY, USA, 1932. [Google Scholar]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef]
- Hearle, N.; Schumacher, V.; Menko, F.H.; Olschwang, S.; Boardman, L.A.; Gille, J.J.; Keller, J.J.; Westerman, A.M.; Scott, R.J.; Lim, W.; et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin. Cancer Res. 2006, 12, 3209–3215. [Google Scholar] [CrossRef]
- Mehenni, H.; Resta, N.; Park, J.G.; Miyaki, M.; Guanti, G.; Costanza, M.C. Cancer risks in LKB1 germline mutation carriers. Gut 2006, 55, 984–990. [Google Scholar] [CrossRef]
- Van Lier, M.G.; Westerman, A.M.; Wagner, A.; Looman, C.W.; Wilson, J.H.; de Rooij, F.W.; Lemmens, V.E.; Kuipers, E.J.; Mathus-Vliegen, E.M.; van Leerdam, M.E. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut 2011, 60, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.; Biermann, K.; Offerhaus, G.J.; Krak, N.; Looman, C.W.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: A large cohort study and implications for surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Resta, N.; Pierannunzio, D.; Lenato, G.M.; Stella, A.; Capocaccia, R.; Bagnulo, R.; Lastella, P.; Susca, F.C.; Bozzao, C.; Loconte, D.C.; et al. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: Results of an Italian multicenter study. Dig. Liver Dis. 2013, 45, 606–611. [Google Scholar] [CrossRef]
- Ishida, H.; Tajima, Y.; Gonda, T.; Kumamoto, K.; Ishibashi, K.; Iwama, T. Update on our investigation of malignant tumors associated with Peutz-Jeghers syndrome in Japan. Surg. Today 2016, 46, 1231–1242. [Google Scholar] [CrossRef]
- Chen, H.Y.; Jin, X.W.; Li, B.R.; Zhu, M.; Li, J.; Mao, G.P.; Zhang, Y.F.; Ning, S.B. Cancer risk in patients with Peutz-Jeghers syndrome: A retrospective cohort study of 336 cases. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Mehenni, H.; Blouin, J.L.; Radhakrishna, U.; Bhardwaj, S.S.; Bhardwaj, K.; Dixit, V.B.; Richards, K.F.; Bermejo-Fenoll, A.; Leal, A.S.; Raval, R.C.; et al. Peutz-Jeghers syndrome: Confirmation of linkage to chromosome 19p13.3 and identification of a potential second locus, on 19q13.4. Am. J. Hum. Genet. 1997, 61, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Amos, C.I.; Bali, D.; Thiel, T.J.; Anderson, J.P.; Gourley, I.; Frazier, M.L.; Lynch, P.M.; Luchtefeld, M.A.; Young, A.; McGarrity, T.J.; et al. Fine mapping of a genetic locus for Peutz-Jeghers syndrome on chromosome 19p. Cancer Res. 1997, 57, 3653–3656. [Google Scholar] [PubMed]
- Boardman, L.A.; Couch, F.J.; Burgart, L.J.; Schwartz, D.; Berry, R.; McDonnell, S.K.; Schaid, D.J.; Hartmann, L.C.; Schroeder, J.J.; Stratakis, C.A.; et al. Genetic heterogeneity in Peutz-Jeghers syndrome. Hum. Mutat. 2000, 16, 23–30. [Google Scholar] [CrossRef]
- Olschwang, S.; Boisson, C.; Thomas, G. Peutz-Jeghers families unlinked to STK11/LKB1 gene mutations are highly predisposed to primitive biliary adenocarcinoma. J. Med. Genet. 2001, 38, 356–360. [Google Scholar] [CrossRef]
- Scott, R.J.; Crooks, R.; Meldrum, C.J.; Thomas, L.; Smith, C.J.; Mowat, D.; McPhillips, M.; Spigelman, A.D. Mutation analysis of the STK11/LKB1 gene and clinical characteristics of an Australian series of Peutz-Jeghers syndrome patients. Clin. Genet. 2002, 62, 282–287. [Google Scholar] [CrossRef]
- Lim, W.; Hearle, N.; Shah, B.; Murday, V.; Hodgson, S.V.; Lucassen, A.; Eccles, D.; Talbot, I.; Neale, K.; Lim, A.G.; et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br. J. Cancer 2003, 89, 308–313. [Google Scholar] [CrossRef]
- Amos, C.I.; Keitheri-Cheteri, M.B.; Sabripour, M.; Wei, C.; McGarrity, T.J.; Seldin, M.F.; Nations, L.; Lynch, P.M.; Fidder, H.H.; Friedman, E.; et al. Genotype-Phenotype correlations in Peutz-Jeghers syndrome. J. Med. Genet. 2004, 41, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Aretz, S.; Stienen, D.; Uhlhaas, S.; Loff, S.; Back, W.; Pagenstecher, C.; McLeod, D.R.; Graham, G.E.; Mangold, E.; Santer, R.; et al. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum. Mutat. 2005, 26, 513–519. [Google Scholar] [CrossRef]
- Chow, E.; Meldrum, C.J.; Crooks, R.; Macrae, F.; Spigelman, A.D.; Scott, R.J. An updated mutation spectrum in an Australian series of PJS patients provides further evidence for only one gene locus. Clin. Genet. 2006, 70, 409–414. [Google Scholar] [CrossRef]
- Thakur, N.; Reddy, D.N.; Rao, G.V.; Mohankrishna, P.; Singh, L.; Chandak, G.R. A novel mutation in STK11 gene is associated with Peutz-Jeghers Syndrome in Indian patients. BMC Med. Genet. 2006, 7, 73. [Google Scholar] [CrossRef]
- Volikos, E.; Robinson, J.; Aittomäki, K.; Mecklin, J.P.; Järvinen, H.; Westerman, A.M.; de Rooji, F.W.; Vogel, T.; Moeslein, G.; Launonen, V.; et al. LKB1 exonic and whole gene deletions are a common cause of Peutz-Jeghers syndrome. J. Med. Genet. 2006, 43, e18. [Google Scholar] [CrossRef]
- De Leng, W.W.; Jansen, M.; Carvalho, R.; Polak, M.; Musler, A.R.; Milne, A.N.; Keller, J.J.; Menko, F.H.; de Rooij, F.W.; Iacobuzio-Donahue, C.A.; et al. Genetic defects underlying Peutz-Jeghers syndrome (PJS) and exclusion of the polarity-associated MARK/Par1 gene family as potential PJS candidates. Clin. Genet. 2007, 72, 568–573. [Google Scholar] [CrossRef]
- Salloch, H.; Reinacher-Schick, A.; Schulmann, K.; Pox, C.; Willert, J.; Tannapfel, A.; Heringlake, S.; Goecke, T.O.; Aretz, S.; Stemmler, S.; et al. Truncating mutations in Peutz-Jeghers syndrome are associated with more polyps, surgical interventions and cancers. Int. J. Colorectal. Dis. 2010, 25, 97–107. [Google Scholar] [CrossRef]
- Papp, J.; Kovacs, M.E.; Solyom, S.; Kasler, M.; Børresen-Dale, A.L.; Olah, E. High prevalence of germline STK11 mutations in Hungarian Peutz-Jeghers Syndrome patients. BMC Med. Genet. 2010, 11, 169. [Google Scholar] [CrossRef]
- Yang, H.R.; Ko, J.S.; Seo, J.K. Germline mutation analysis of STK11 gene using direct sequencing and multiplex ligation-dependent probe amplification assay in Korean children with Peutz-Jeghers syndrome. Dig. Dis. Sci. 2010, 55, 3458–3465. [Google Scholar] [CrossRef]
- Borun, P.; Bartkowiak, A.; Banasiewicz, T.; Nedoszytko, B.; Nowakowska, D.; Teisseyre, M.; Limon, J.; Lubinski, J.; Kubaszewski, L.; Walkowiak, J.; et al. High Resolution Melting analysis as a rapid and efficient method of screening for small mutations in the STK11 gene in patients with Peutz-Jeghers syndrome. BMC Med. Genet. 2013, 14, 58. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, B.; Mosig, R.A.; Chen, Y.; Ye, F.; Zhang, Y.; Gong, W.; Gong, L.; Huang, F.; Wang, X.; et al. STK11 domain XI mutations: Candidate genetic drivers leading to the development of dysplastic polyps in Peutz-Jeghers syndrome. Hum. Mutat. 2014, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Miao, S.; Wang, L.; Zhang, P.; Wu, B.; Wu, J.; Huang, Y. Clinical characteristics and STK11 gene mutations in Chinese children with Peutz-Jeghers syndrome. BMC Gastroenterol. 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Jelsig, A.M.; Qvist, N.; Sunde, L.; Brusgaard, K.; Hansen, T.; Wikman, F.P.; Nielsen, C.B.; Nielsen, I.K.; Gerdes, A.M.; Bojesen, A.; et al. Disease pattern in Danish patients with Peutz-Jeghers syndrome. Int. J. Colorectal. Dis. 2016, 31, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.M.; Chen, T.C. Clinical manifestations and STK11 germline mutations in Taiwanese patients with Peutz-Jeghers syndrome. Asian J. Surg. 2018, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zhao, Z.Y.; Li, B.R.; Wang, H.; Yu, E.D.; Ning, S.B. STK11 gene analysis reveals a significant number of splice mutations in Chinese PJS patients. Cancer Genet. 2019, 230, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Yang, Y.J.; Duan, J.Q.; Ouyang, H.J.; Liu, L.; Yi, L.C.; Xiao, Z.H.; Zheng, Y.; Peng, L.; Attard, T.M.; et al. Clinical and genetic study of children with Peutz-Jeghers syndrome identifies a high frequency of STK11 de novo mutation. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 199–206. [Google Scholar] [CrossRef]
- Marneros, A.G.; Mehenni, H.; Reichenberger, E.; Antonarakis, S.E.; Krieg, T.; Olsen, B.R. Gene for the human transmembrane-type protein tyrosine phosphatase H (PTPRH): Genomic structure, fine-mapping and its exclusion as a candidate for Peutz-Jeghers syndrome. Cytogenet. Cell Genet. 2001, 92, 213–216. [Google Scholar] [CrossRef]
- Buchet-Poyau, K.; Mehenni, H.; Radhakrishna, U.; Antonarakis, S.E. Search for the second Peutz-Jeghers syndrome locus: Exclusion of the STK13, PRKCG, KLK10, and PSCD2 genes on chromosome 19 and the STK11IP gene on chromosome 2. Cytogenet. Genome. Res. 2002, 97, 171–178. [Google Scholar] [CrossRef]
- Woodford-Richens, K.L.; Halford, S.; Rowan, A.; Bevan, S.; Aaltonen, L.A.; Wasan, H.; Bicknell, D.; Bodmer, W.F.; Houlston, R.S.; Tomlinson, I.P. CDX2 mutations do not account for juvenile polyposis or Peutz-Jeghers syndrome and occur infrequently in sporadic colorectal cancers. Br. J. Cancer 2001, 84, 1314–1316. [Google Scholar] [CrossRef][Green Version]
- Alhopuro, P.; Katajisto, P.; Lehtonen, R.; Ylisaukko-Oja, S.K.; Näätsaari, L.; Karhu, A.; Westerman, A.M.; Wilson, J.H.; de Rooij, F.W.; Vogel, T.; et al. Mutation analysis of three genes encoding novel LKB1-interacting proteins, BRG1, STRADalpha, and MO25alpha, in Peutz-Jeghers syndrome. Br. J. Cancer 2005, 92, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Butel-Simoes, G.I.; Spigelman, A.D.; Scott, R.J.; Vilain, R.E. Low-Level parental mosaicism in an apparent de novo case of Peutz-Jeghers syndrome. Fam. Cancer 2019, 18, 109–112. [Google Scholar] [CrossRef] [PubMed]
- McKay, V.; Cairns, D.; Gokhale, D.; Mountford, R.; Greenhalgh, L. First report of somatic mosaicism for mutations in STK11 in four patients with Peutz-Jeghers syndrome. Fam. Cancer 2016, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Latchford, A.; Cohen, S.; Auth, M.; Scaillon, M.; Viala, J.; Daniels, R.; Talbotec, C.; Attard, T.; Durno, C.; Hyer, W. Management of Peutz-Jeghers syndrome in children and adolescents: A Position paper from the ESPGHAN polyposis working group. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.C.; et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef]
- Oncel, M.; Remzi, F.H.; Church, J.M.; Goldblum, J.R.; Zutshi, M.; Fazio, V.W. Course and follow-up of solitary Peutz-Jeghers polyps: A case series. Int. J. Colorectal. Dis. 2003, 18, 33–35. [Google Scholar] [CrossRef]
- Iwamuro, M.; Aoyama, Y.; Suzuki, S.; Kobayashi, S.; Toyokawa, T.; Moritou, Y.; Hori, S.; Matsueda, K.; Yoshioka, M.; Tanaka, T.; et al. Long-Term outcome in patients with a solitary Peutz-Jeghers polyp. Gastroenterol. Res. Pract. 2019, 2019, 8159072. [Google Scholar] [CrossRef]
- Van Lier, M.G.; Mathus-Vliegen, E.M.; Wagner, A.; van Leerdam, M.E.; Kuipers, E.J. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: Time to update surveillance guidelines? Am. J. Gastroenterol. 2011, 106, 940–945. [Google Scholar] [CrossRef]
- Edwards, D.P.; Khosraviani, K.; Stafferton, R.; Phillips, R.K. Long-Term results of polyp clearance by intraoperative enteroscopy in the Peutz-Jeghers syndrome. Dis. Colon. Rectum. 2003, 46, 48–50. [Google Scholar] [CrossRef]
- Latchford, A.R.; Neale, K.; Phillips, R.K.; Clark, S.K. Peutz-Jeghers syndrome: Intriguing suggestion of gastrointestinal cancer prevention from surveillance. Dis. Colon. Rectum. 2011, 54, 1547–1551. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Blackford, A.L.; Tamura, K.; Ford, M.; McCormick, P.; Chuidian, M.; Almario, J.A.; Borges, M.; Lennon, A.M.; Shin, E.J.; et al. Deleterious germline mutations are a risk factor for neoplastic progression among high-risk individuals undergoing pancreatic surveillance. J. Clin. Oncol. 2019, 37, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Bannon, S.A.; Montiel, M.F.; Goldstein, J.B.; Dong, W.; Mork, M.E.; Borras, E.; Hasanov, M.; Varadhachary, G.R.; Maitra, A.; Katz, M.H.; et al. High prevalence of hereditary cancer syndromes and outcomes in adults with early-onset pancreatic cancer. Cancer Prev. Res. 2018, 11, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.J. Pancreatic cancer screening in high-risk individuals: Ready for prime time? Gastrointest. Endosc. 2018, 87, 1451–1453. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012, 142, 796–804; quiz e714–e795. [Google Scholar] [CrossRef] [PubMed]
- Harinck, F.; Konings, I.C.; Kluijt, I.; Poley, J.W.; van Hooft, J.E.; van Dullemen, H.M.; Nio, C.Y.; Krak, N.C.; Hermans, J.J.; Aalfs, C.M.; et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016, 65, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, M.; Bruno, M.J.; Zerboni, G.; Poley, J.W.; Delle Fave, G.; Capurso, G. Results of surveillance in individuals at high-risk of pancreatic cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Konings, I.C.; Harinck, F.; Poley, J.W.; Aalfs, C.M.; van Rens, A.; Krak, N.C.; Wagner, A.; Nio, C.Y.; Sijmons, R.H.; van Dullemen, H.M.; et al. Prevalence and progression of pancreatic cystic precursor lesions differ between groups at high risk of developing pancreatic cancer. Pancreas 2017, 46, 28–34. [Google Scholar] [CrossRef]
- Barnes, C.A.; Krzywda, E.; Lahiff, S.; McDowell, D.; Christians, K.K.; Knechtges, P.; Tolat, P.; Hohenwalter, M.; Dua, K.; Khan, A.H.; et al. Development of a high risk pancreatic screening clinic using 3.0 T MRI. Fam. Cancer 2018, 17, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018, 155, 740–751.e742. [Google Scholar] [CrossRef]
- Rieder, H.; Bartsch, D.K. Familial pancreatic cancer. Fam. Cancer 2004, 3, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kekis, P.B.; Friess, H.; Kleeff, J.; Büchler, M.W. Timing and extent of surgical intervention in patients from hereditary pancreatic cancer kindreds. Pancreatology 2001, 1, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, K.P.; Brentnall, T.A.; Bronner, M.P.; Byrd, D.; Marsh, C. A new indication for pancreas transplantation: High grade pancreatic dysplasia. Clin. Transplant. 2004, 18, 105–107. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Zerbi, A.; Capurso, G.; Zamboni, G.; Maisonneuve, P.; Presciuttini, S.; Arcidiacono, P.G.; Calculli, L.; Falconi, M.; Italian Registry for Familial Pancreatic, C. Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: A position paper from the Italian Registry. Dig. Liver Dis. 2010, 42, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Rulyak, S.J.; Brentnall, T.A. Inherited pancreatic cancer: Surveillance and treatment strategies for affected families. Pancreatology 2001, 1, 477–485. [Google Scholar] [CrossRef]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Adsay, N.V.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar]
- Canto, M.I.; Goggins, M.; Hruban, R.H.; Petersen, G.M.; Giardiello, F.M.; Yeo, C.; Fishman, E.K.; Brune, K.; Axilbund, J.; Griffin, C.; et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin. Gastroenterol. Hepatol. 2006, 4, 766–781; quiz 665. [Google Scholar] [CrossRef]
- Paiella, S.; Salvia, R.; De Pastena, M.; Pollini, T.; Casetti, L.; Landoni, L.; Esposito, A.; Marchegiani, G.; Malleo, G.; De Marchi, G.; et al. Screening/Surveillance programs for pancreatic cancer in familial high-risk individuals: A systematic review and proportion meta-analysis of screening results. Pancreatology 2018, 18, 420–428. [Google Scholar] [CrossRef]
- De Mestier, L.; Muller, M.; Cros, J.; Vullierme, M.P.; Vernerey, D.; Maire, F.; Dokmak, S.; Rebours, V.; Sauvanet, A.; Lévy, P.; et al. Appropriateness of pancreatic resection in high-risk individuals for familial pancreatic ductal adenocarcinoma: A patient-level meta-analysis and proposition of the Beaujon score. United Eur. Gastroenterol. J. 2019, 7, 358–368. [Google Scholar] [CrossRef]
- Konings, I.; Canto, M.I.; Almario, J.A.; Harinck, F.; Saxena, P.; Lucas, A.L.; Kastrinos, F.; Whitcomb, D.C.; Brand, R.E.; Lachter, J.; et al. Surveillance for pancreatic cancer in high-risk individuals. BJS Open 2019, 3, 656–665. [Google Scholar] [CrossRef]
- Canto, M.I.; Kerdsirichairat, T.; Yeo, C.J.; Hruban, R.H.; Shin, E.J.; Almario, J.A.; Blackford, A.; Ford, M.; Klein, A.P.; Javed, A.A.; et al. Surgical outcomes after pancreatic resection of screening-detected lesions in individuals at high risk for developing pancreatic cancer. J. Gastrointest. Surg. 2020, 24, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fostira, F.; Mollaki, V.; Lypas, G.; Alexandrakis, G.; Christianakis, E.; Tzouvala, M.; Zacharopoulou, E.; Kalfakakou, D.; Konstantopoulou, I.; Yannoukakos, D. Genetic analysis and clinical description of Greek patients with Peutz-Jeghers syndrome: Creation of a national registry. Cancer Genet. 2018, 220, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Lipsa, A.; Kowtal, P.; Sarin, R. Novel germline STK11 variants and breast cancer phenotype identified in an Indian cohort of Peutz-Jeghers syndrome. Hum. Mol. Genet. 2019, 28, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.A.; Thibodeau, S.N.; Schaid, D.J.; Lindor, N.M.; McDonnell, S.K.; Burgart, L.J.; Ahlquist, D.A.; Podratz, K.C.; Pittelkow, M.; Hartmann, L.C. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann. Intern. Med. 1998, 128, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Olschwang, S.; Keller, J.J.; Westerman, A.M.; Menko, F.H.; Boardman, L.A.; Scott, R.J.; Trimbath, J.; Giardiello, F.M.; Gruber, S.B.; et al. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology 2004, 126, 1788–1794. [Google Scholar] [CrossRef]
- Van Lier, M.G.; Wagner, A.; Mathus-Vliegen, E.M.; Kuipers, E.J.; Steyerberg, E.W.; van Leerdam, M.E. High cancer risk in Peutz-Jeghers syndrome: A systematic review and surveillance recommendations. Am. J. Gastroenterol. 2010, 105, 1258–1264; author reply 1265. [Google Scholar] [CrossRef]
- Vangala, D.B.; Cauchin, E.; Balmaña, J.; Wyrwicz, L.; van Cutsem, E.; Güller, U.; Castells, A.; Carneiro, F.; Hammel, P.; Ducreux, M.; et al. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur. J. Cancer 2018, 104, 91–103. [Google Scholar]
- Cobain, E.F.; Milliron, K.J.; Merajver, S.D. Updates on breast cancer genetics: Clinical implications of detecting syndromes of inherited increased susceptibility to breast cancer. Semin. Oncol. 2016, 43, 528–535. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Trimbath, J.D. Peutz-Jeghers syndrome and management recommendations. Clin. Gastroenterol. Hepatol. 2006, 4, 408–415. [Google Scholar] [CrossRef]
- Boetes, C. Update on screening breast MRI in high-risk women. Obstet. Gynecol. Clin. N. Am. 2011, 38, 149–158, viii–ix. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Liu, S.; Wang, Y.; Chen, J.; Wu, B. Prenatal diagnosis in a hereditary Peutz-Jeghers syndrome family with high cancer risk. BMC Med. Genet. 2018, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Sadana, A.; Mauger, D.T.; Baker, M.J.; Berk, T.; McGarrity, T.J. Psychosocial impact of Peutz-Jeghers Syndrome. Fam. Cancer 2009, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, M.G.; Korsse, S.E.; Mathus-Vliegen, E.M.; Kuipers, E.J.; van den Ouweland, A.M.; Vanheusden, K.; van Leerdam, M.E.; Wagner, A. Peutz-Jeghers syndrome and family planning: The attitude towards prenatal diagnosis and pre-implantation genetic diagnosis. Eur. J. Hum. Genet. 2012, 20, 236–239. [Google Scholar] [CrossRef] [PubMed]
| Tomlinson and Houlston 1997 [3] |
| 1: Two or more PJS polyps in the gastrointestinal tract or |
| 2: One PJS polyp in the gastrointestinal tract, together with either classical PJS pigmentation or a family history of PJS |
| WHO Criteria 2000 [4] |
| A: A positive family history of PJS and |
| 1: Any number of histologically confirmed PJS polyps or |
| 2: Characteristic prominent mucocutaneous pigmentation |
| B: A negative family history of PJS and |
| 1: Three histologically confirmed PJS polyps or |
| 2: Any number of histologically confirmed PJS polyps and characteristic prominent mucocutaneous pigmentation |
| Beggs et al. 2010 [5] |
| 1: Two or more histologically confirmed PJS polyps or |
| 2: Any number of PJS polyps in an individual who has a family history of PJS in close relative(s) or |
| 3: Characteristic mucocutaneous pigmentation in an individual who has a family history of PJS in close relative(s) or |
| 4: Any number of PJS polyps in an individual who also has characteristic mucocutaneous pigmentation |
| Study | N | gac | smbc | crc | Gastroint. Cancer | pac | bc | utc | ovc | cx | Gynecol. Cancer | All | At Age (Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gardielo et al., 2000 [12], meta-analysis | 210 | 29 | 13 | 39 | 36 | 54 | 9 | 21 | 10 | 93 | 64 | ||
| Hearle et al., 2006 [13], cohort study | 419 | 57 | 11 | 45 | 18 | 85 | 70 | ||||||
| Mehenni et al., 2006 [14], cohort study | 149 | 63 * | 18 # | 67 | 70 | ||||||||
| Van Lier et al., 2011 [15], cohort study | 133 | 51 ** | 76 | 70 | |||||||||
| Korsse et al., 2013 [16], cohort study | 144 | 26 | 70 | ||||||||||
| Resta et al., 2013 [17], cohort study | 119 | 12 | 55 | 24 | 23 | 89 | 60–65 | ||||||
| Ishida et al., 2016 [18], meta-analysis | 583 | 24 | 10–14 | 36 | 29 | 19 | 47 *** | 10 | 83 | 70 | |||
| Chen et al., 2017 [19], cohort study | 336 | 28 | 55 | 60 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, A.; Aretz, S.; Auranen, A.; Bruno, M.J.; Cavestro, G.M.; Crosbie, E.J.; Goverde, A.; Jelsig, A.M.; Latchford, A.R.; van Leerdam, M.E.; et al. The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J. Clin. Med. 2021, 10, 473. https://doi.org/10.3390/jcm10030473
Wagner A, Aretz S, Auranen A, Bruno MJ, Cavestro GM, Crosbie EJ, Goverde A, Jelsig AM, Latchford AR, van Leerdam ME, et al. The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. Journal of Clinical Medicine. 2021; 10(3):473. https://doi.org/10.3390/jcm10030473
Chicago/Turabian StyleWagner, Anja, Stefan Aretz, Annika Auranen, Marco J. Bruno, Giulia M. Cavestro, Emma J. Crosbie, Anne Goverde, Anne Marie Jelsig, Andrew R. Latchford, Monique E. van Leerdam, and et al. 2021. "The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline" Journal of Clinical Medicine 10, no. 3: 473. https://doi.org/10.3390/jcm10030473
APA StyleWagner, A., Aretz, S., Auranen, A., Bruno, M. J., Cavestro, G. M., Crosbie, E. J., Goverde, A., Jelsig, A. M., Latchford, A. R., van Leerdam, M. E., Lepisto, A. H., Puzzono, M., Winship, I., Zuber, V., & Möslein, G. (2021). The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. Journal of Clinical Medicine, 10(3), 473. https://doi.org/10.3390/jcm10030473







