Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anesthesia
2.3. Cardiopulmonary Bypass
2.4. Microcirculatory Perfusion Measurements and Analysis
2.5. Hemodynamic and Laboratory Parameters
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
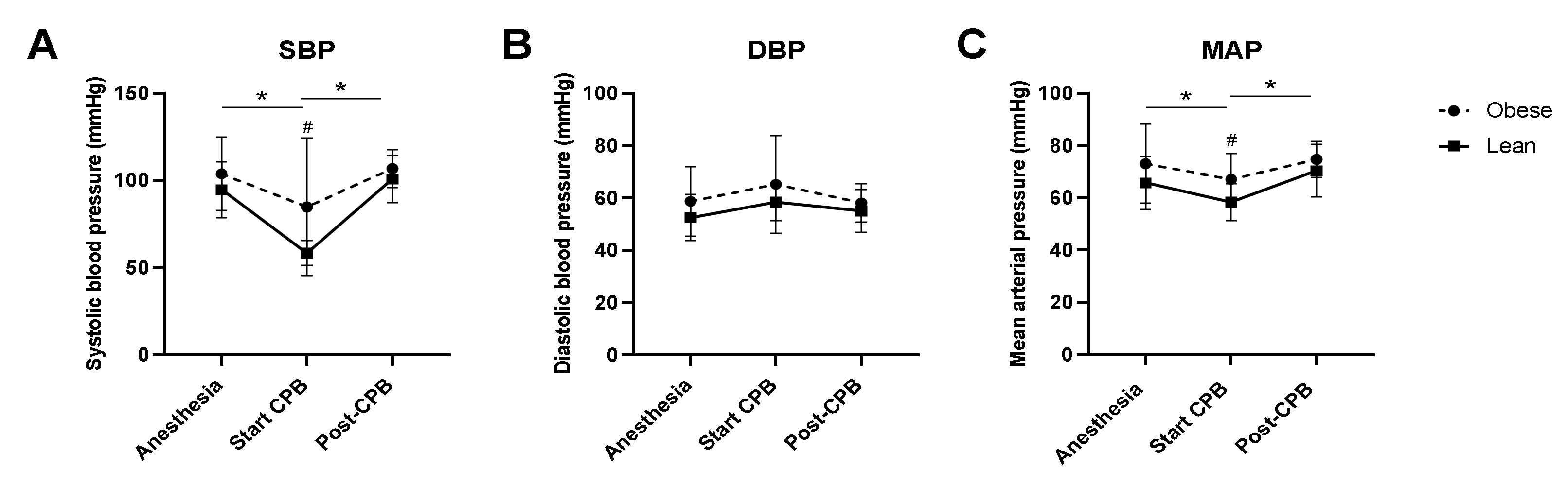
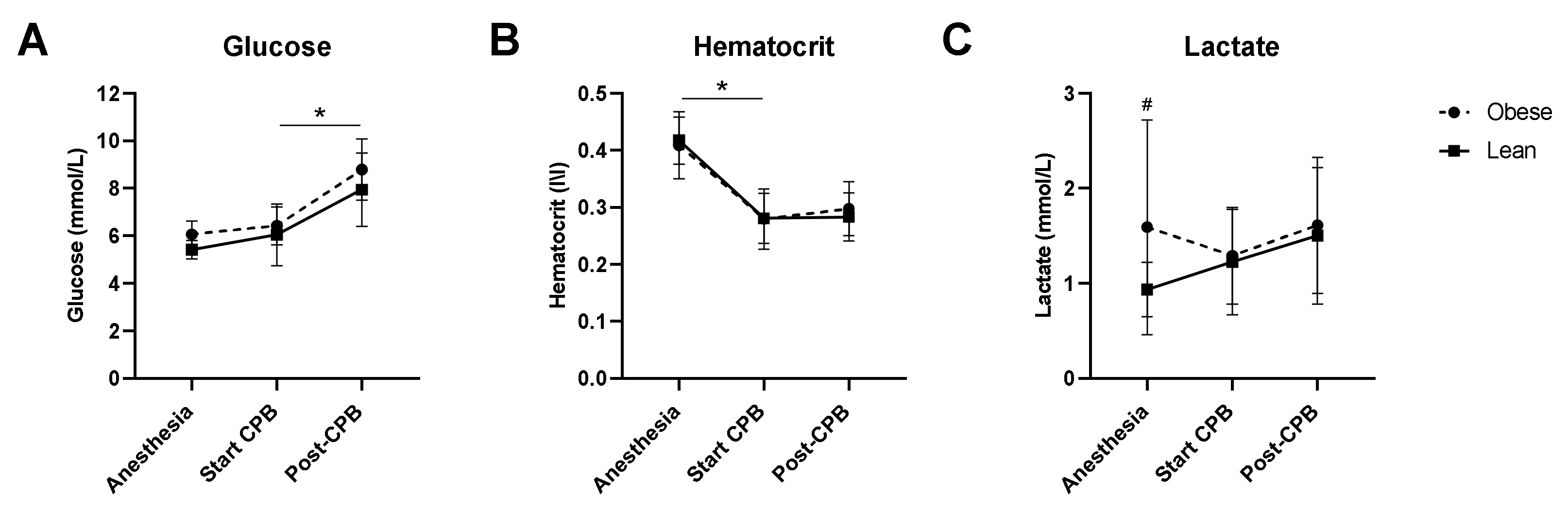
3.2. Intraoperative Hemodynamics, Temperature, and Lactate Levels
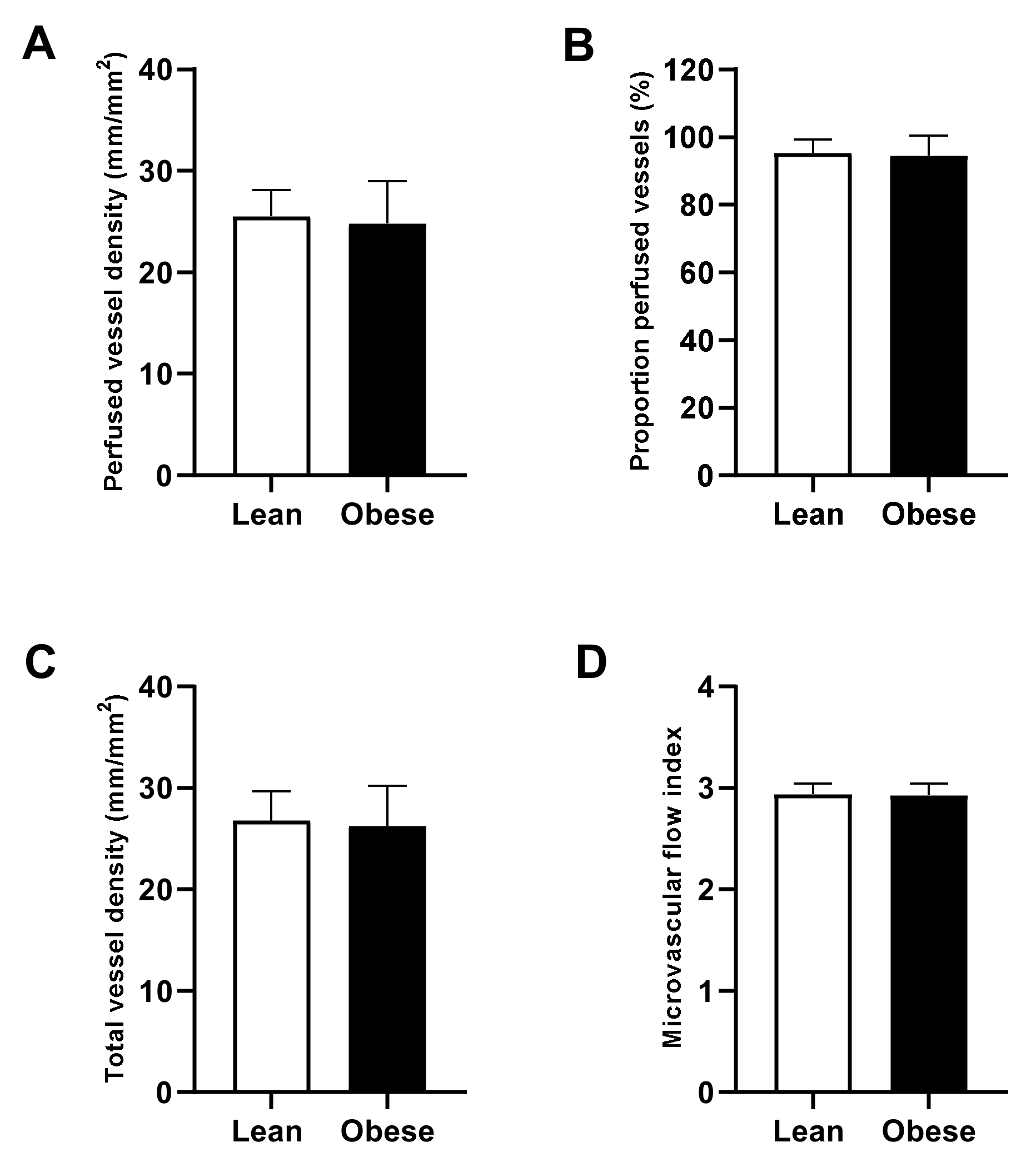
3.3. Preoperative Microcirculatory Perfusion
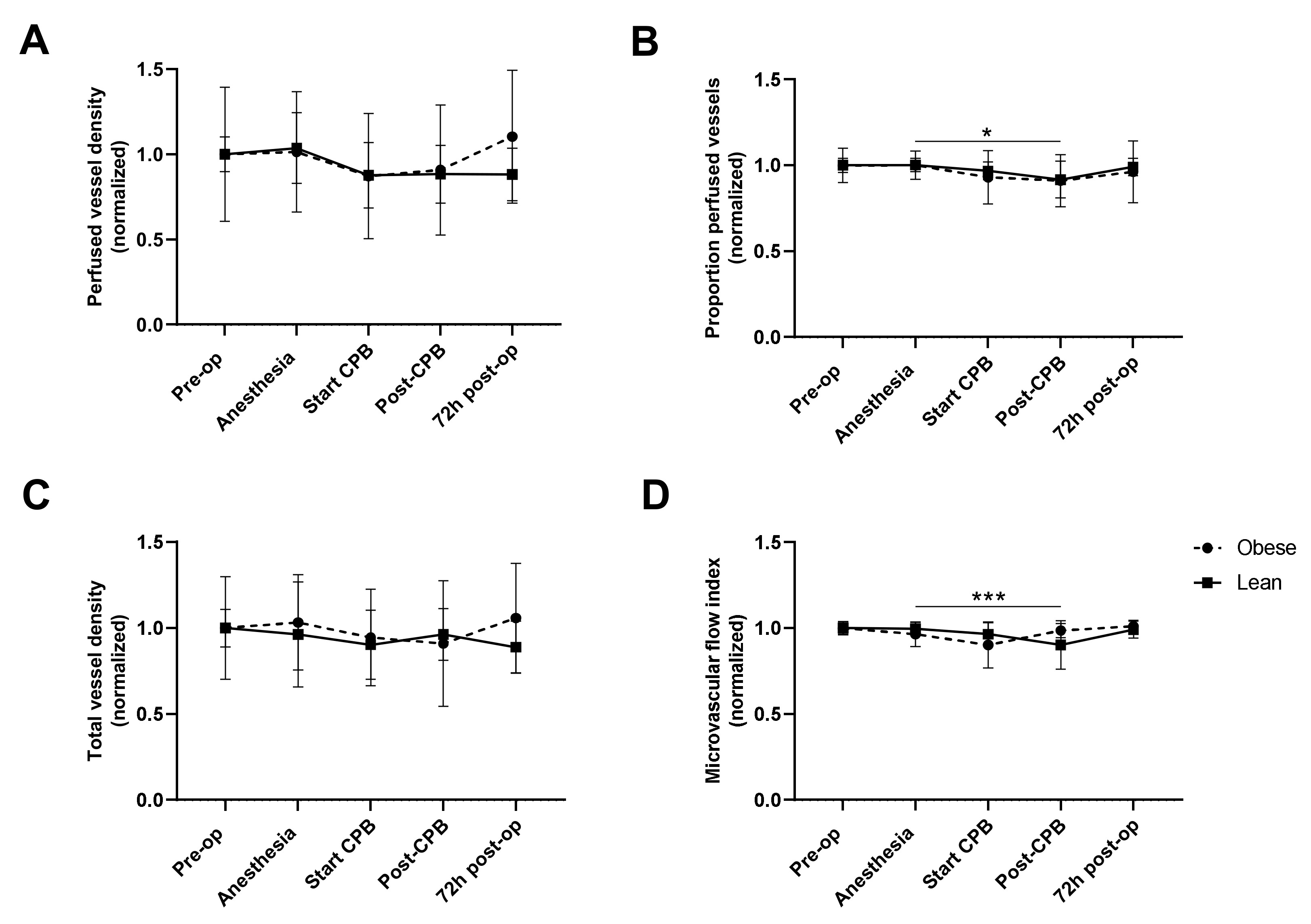
3.4. Intra- and Postoperative Microcirculatory Perfusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 December 2020).
- Lopez-Delgado, J.C.; Esteve, F.; Manez, R.; Torrado, H.; Carrio, M.L.; Rodríguez-Castro, D.; Farrero, E.; Javierre, C.; Skaltsa, K.; Ventura, J.L. The influence of body mass index on outcomes in patients undergoing cardiac surgery: Does the obesity paradox really exist? PLoS ONE 2015, 10, e0118858. [Google Scholar]
- Demir, A.; Aydınlı, B.; Güçlü, Ç.Y.; Yazıcıoğlu, H.; Saraç, A.; Elhan, A.H.; Erdemli, Ö. Obesity and postoperative early complications in open heart surgery. J. Anesth. 2012, 26, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D.; Grunwald, G.K.; Rumsfeld, J.S.; Hill, J.O.; Ho, P.M.; Wyatt, H.R.; Shroyer, A.L. Relationship of body mass index with outcomes after coronary artery bypass graft surgery. Ann. Thorac. Surg. 2007, 84, 10–11. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Dubois, M.J.; Schmartz, D.; Koch, M.; Ducart, A.; Barvais, L.; Vincent, J.L. Microcirculatory alterations in cardiac surgery: Effects of cardiopulmonary bypass and anesthesia. Ann. Thorac. Surg. 2009, 88, 1396–1403. [Google Scholar] [CrossRef]
- Koning, N.J.; Atasever, B.; Vonk, A.B.; Boer, C. Changes in microcirculatory perfusion and oxygenation during cardiac surgery with or without cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1331–1340. [Google Scholar] [CrossRef]
- Dekker, N.A.M.; Veerhoek, D.; Koning, N.J.; van Leeuwen, A.L.I.; Elbers, P.W.G.; van den Brom, C.E.; Vonk, A.B.A.; Boer, C. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019, 74, 609–618. [Google Scholar] [CrossRef]
- den Os, M.M.; van den Brom, C.E.; van Leeuwen, A.L.I.; Dekker, N.A.M. Microcirculatory perfusion disturbances following cardiopulmonary bypass: A systematic review. Crit. Care 2020, 24, 218. [Google Scholar] [CrossRef]
- Greenwood, J.C.; Jang, D.H.; Hallisey, S.D.; Gutsche, J.T.; Horak, J.; Acker, M.A.; Bermudez, C.A.; Zhou, V.L.; Chatterjee, S.; Shofer, F.S.; et al. Severe Impairment of Microcirculatory Perfused Vessel Density Is Associated With Postoperative Lactate and Acute Organ Injury After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 106–115. [Google Scholar] [CrossRef]
- Vellinga, N.A.; Ince, C.; Boerma, E.C. Microvascular dysfunction in the surgical patient. Curr. Opin. Crit. Care 2010, 16, 377–383. [Google Scholar] [CrossRef]
- Koch, M.; De Backer, D.; Vincent, J.L.; Barvais, L.; Hennart, D.; Schmartz, D. Effects of propofol on human microcirculation. Br. J. Anaesth. 2008, 101, 473–478. [Google Scholar] [CrossRef]
- Bulte, C.S.; Slikkerveer, J.; Kamp, O.; Heymans, M.W.; Loer, S.A.; de Marchi, S.F.; Vogel, R.; Boer, C.; Bouwman, R.A. General anesthesia with sevoflurane decreases myocardial blood volume and hyperemic blood flow in healthy humans. Anesth. Analg. 2013, 116, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.J.; de Lange, F.; Vonk, A.B.; Ahmed, Y.; van den Brom, C.E.; Bogaards, S.; van Meurs, M.; Jongman, R.M.; Schalkwijk, C.G.; Begieneman, M.P.; et al. Impaired microcirculatory perfusion in a rat model of cardiopulmonary bypass: The role of hemodilution. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H550–H558. [Google Scholar] [CrossRef] [PubMed]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.J.; Overmars, M.A.; van den Brom, C.E.; van Bezu, J.; Simon, L.E.; Vonk, A.B.; Girbes, A.R.; van Nieuw Amerongen, G.P.; Boer, C. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br. J. Anaesth. 2016, 116, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dekker, N.A.M.; van Leeuwen, A.L.I.; van Strien, W.W.J.; Majolée, J.; Szulcek, R.; Vonk, A.B.A.; Hordijk, P.L.; Boer, C.; van den Brom, C.E. Microcirculatory perfusion disturbances following cardiac surgery with cardiopulmonary bypass are associated with in vitro endothelial hyperpermeability and increased angiopoietin-2 levels. Crit. Care 2019, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Dekker, N.A.M.; van Meurs, M.; van Leeuwen, A.L.I.; Hofland, H.M.; van Slyke, P.; Vonk, A.B.A.; Boer, C.; van den Brom, C.E. Vasculotide, an angiopoietin-1 mimetic, reduces pulmonary vascular leakage and preserves microcirculatory perfusion during cardiopulmonary bypass in rats. Br. J. Anaesth. 2018, 121, 1041–1051. [Google Scholar] [CrossRef]
- Samad, F.; Ruf, W. Inflammation, obesity, and thrombosis. Blood 2013, 122, 3415–3422. [Google Scholar] [CrossRef]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef]
- Sorop, O.; Olver, T.D.; van de Wouw, J.; Heinonen, I.; van Duin, R.W.; Duncker, D.J.; Merkus, D. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 2017, 113, 1035–1045. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, R.T.; Serné, E.H.; IJzerman, R.G.; de Vries, G.; Stehouwer, C.D. Impaired microvascular function in obesity: Implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 2004, 109, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Eringa, E.; Stehouwer, C.D. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef]
- Le-Bert, G.; Santana, O.; Pineda, A.M.; Zamora, C.; Lamas, G.A.; Lamelas, J. The obesity paradox in elderly obese patients undergoing coronary artery bypass surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Forgie, K.; Bozso, S.J.; Hong, Y.; Norris, C.M.; Ishaque, A.; Gill, R.S.; Freed, D.H.; Moon, M.C.; Nagendran, J.; Nagendran, J. The effects of body mass index on outcomes for patients undergoing surgical aortic valve replacement. BMC Cardiovasc. Disord. 2020, 20, 255. [Google Scholar] [CrossRef]
- Gruberg, L.; Weissman, N.J.; Waksman, R.; Fuchs, S.; Deible, R.; Pinnow, E.E.; Ahmed, L.M.; Kent, K.M.; Pichard, A.D.; Suddath, W.O.; et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: The obesity paradox? J. Am. Coll. Cardiol. 2002, 39, 578–584. [Google Scholar] [CrossRef]
- De Backer, D.; Hollenberg, S.; Boerma, C.; Goedhart, P.; Büchele, G.; Ospina-Tascon, G.; Dobbe, I.; Ince, C. How to evaluate the microcirculation: Report of a round table conference. Crit Care 2007, 11, R101. [Google Scholar] [CrossRef]
- de Jongh, R.T.; Serné, E.H.; IJzerman, R.G.; Jørstad, H.T.; Stehouwer, C.D. Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc. Res. 2008, 75, 256–262. [Google Scholar] [CrossRef]
- Van Guilder, G.P.; Hoetzer, G.L.; Dengel, D.R.; Stauffer, B.L.; DeSouza, C.A. Impaired endothelium-dependent vasodilation in normotensive and normoglycemic obese adult humans. J. Cardiovasc. Pharmacol. 2006, 47, 310–313. [Google Scholar] [CrossRef]
- Teragawa, H.; Morita, K.; Shishido, H.; Otsuka, N.; Hirokawa, Y.; Chayama, K.; Tamaki, N.; Kihara, Y. Impaired myocardial blood flow reserve in subjects with metabolic syndrome analyzed using positron emission tomography and N-13 labeled ammonia. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 368–376. [Google Scholar] [CrossRef]
- Hall, M.E.; Brinkley, T.E.; Chughtai, H.; Morgan, T.M.; Hamilton, C.A.; Jordan, J.H.; Stacey, R.B.; Soots, S.; Hundley, W.G. Adiposity Is Associated with Gender-Specific Reductions in Left Ventricular Myocardial Perfusion during Dobutamine Stress. PLoS ONE 2016, 11, e0146519. [Google Scholar]
- Meijer, R.I.; Serné, E.H.; Korkmaz, H.I.; van der Peet, D.L.; de Boer, M.P.; Niessen, H.W.M.; van Hinsbergh, V.W.M.; Yudkin, J.S.; Smulders, Y.M.; Eringa, E.C. Insulin-induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 2015, 58, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Hiller, K.; Ruile, P.; Kraus, G.; Bauer, W.R.; Waller, C. Tissue ACE inhibition improves microcirculation in remote myocardium after coronary stenosis: MR imaging study in rats. Microvasc. Res. 2010, 80, 484–490. [Google Scholar] [CrossRef]
- Dogan, A.; Ozgul, M.; Ozaydin, M.; Aslan, S.M.; Gedikli, O.; Altinbas, A. Effect of clopidogrel plus aspirin on tissue perfusion and coronary flow in patients with ST-segment elevation myocardial infarction: A new reperfusion strategy. Am. Heart J. 2005, 149, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, V.; Favory, R.; Boulo, M.; Mathieu, D. Microcirculatory alterations induced by sedation in intensive care patients. Effects of midazolam alone and in association with sufentanil. Crit Care 2006, 10, R176. [Google Scholar] [CrossRef]
- Onorati, F.; Rubino, A.S.; Nucera, S.; Foti, D.; Sica, V.; Santini, F.; Gulletta, E.; Renzulli, A. Off-pump coronary artery bypass surgery versus standard linear or pulsatile cardiopulmonary bypass: Endothelial activation and inflammatory response. Eur. J. Cardiothorac. Surg. 2010, 37, 897–904. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015, 19, S8. [Google Scholar] [CrossRef]
- Sleeman, P.; Patel, N.N.; Lin, H.; Walkden, G.J.; Ray, P.; Welsh, G.I.; Satchell, S.C.; Murphy, G.J. High fat feeding promotes obesity and renal inflammation and protects against post cardiopulmonary bypass acute kidney injury in swine. Crit Care 2013, 17, R262. [Google Scholar] [CrossRef]
- Pasco, J.A.; Nicholson, G.C.; Brennan, S.L.; Kotowicz, M.A. Prevalence of obesity and the relationship between the body mass index and body fat: Cross-sectional, population-based data. PLoS ONE 2012, 7, e29580. [Google Scholar]
- Farb, M.G.; Ganley-Leal, L.; Mott, M.; Liang, Y.; Ercan, B.; Widlansky, M.E.; Bigornia, S.J.; Fiscale, A.J.; Apovian, C.M.; Carmine, B.; et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 467–473. [Google Scholar] [CrossRef]
| Lean (n = 22) | Obese (n = 14) | p-Value | |
|---|---|---|---|
| Gender (male/female) | 18/4 | 12/2 | 0.99 |
| Age (years) | 72 ± 9 | 67 ± 11 | 0.14 |
| Length (cm) | 177 ± 9 | 173 ± 7 | 0.11 |
| Weight (kg) | 73 ± 9 | 104 ± 14 | <0.0001 |
| BMI (kg/m2) | 23.2 ± 1.4 | 34.8 ± 2.8 | <0.0001 |
| Smoker (yes/no) | 3/19 | 1/13 | 0.99 |
| Preoperative creatinine (µmol/L) | 86.2 ± 13.2 | 98.1 ± 24.9 | 0.07 |
| Preoperative hemoglobin (mmol/L) | 8.8 ± 0.8 | 8.7 ± 1.0 | 0.82 |
| Preoperative HbA1c (mmol/L) | 39.2 ± 3.7 | 42.0 ± 4.4 | 0.09 |
| Hypertension (yes/no) | 8/15 | 9/5 | 0.10 |
| Antihypertensive medication (yes/no) | 9/13 | 12/2 | 0.01 |
| Beta-blocker (n) | 4 | 4 | |
| ACE inhibitor/ATII antagonist (n) | 1 | 2 | |
| Calcium blocker (n) | 0 | 1 | |
| Diuretics (n) | 1 | 0 | |
| Combination (n) | 3 | 5 | |
| Type of surgery (n) | CABG: 8 | CABG: 9 | |
| AVR: 8 | AVR: 4 | ||
| MVR: 4 | MVR: 0 | ||
| AVR/CABG: 2 | AVR/CABG: 1 | ||
| EuroSCORE II (%) | 1.3 (0.7–2.3) | 1.5 (1.1–3.3) | 0.39 |
| Anesthesia | Start CPB | Post-CPB | ||
|---|---|---|---|---|
| Hemoglobin (mmol/L) | Lean | 8.4 ± 0.8 | 5.6 ± 0.9 * | 5.7 ± 0.9 |
| Obese | 8.3 ± 1.2 | 5.6 ± 1.1 * | 6.0 ± 1.0 | |
| pH | Lean | 7.4 ± 0.04 | 7.4 ± 0.04 | 7.4 ± 0.04 |
| Obese | 7.4 ± 0.02 | 7.4 ± 0.02 | 7.4 ± 0.03 | |
| pCO2 (mmHg) | Lean | 5.25 ± 0.62 | 5.24 ± 0.41 | 5.19 ± 0.37 |
| Obese | 5.43 ± 0.46 | 5.31 ± 0.25 | 5.22 ± 0.44 | |
| Base excess (mEq/L) | Lean | 0.5 ± 1.8 | −0.1 ± 1.7 | −1.3 ± 1.7 * |
| Obese | 1.1 ± 1.4 | −0.04 ± 1.6 | −1.4 ± 1.3 | |
| HCO3− (mmol/L) | Lean | 24.5 ± 1.9 | 24.1 ± 1.4 | 23.0 ± 1.3 * |
| Obese | 24.9 ± 1.4 | 24.1 ± 1.6 | 23.0 ± 1.3 | |
| Temperature (°C) | Lean | 36.3 ± 0.4 | 35.0 ± 1.3 | 36.4 ± 0.3 |
| Obese | 36.4 ± 0.4 | 35.5 ± 0.6 | 36.5 ± 0.2 |
| Lean (n = 22) | Obese (n = 14) | p-Value | |
|---|---|---|---|
| Cross-clamp time (min) | 86 ± 42 | 75 ± 18 | 0.37 |
| Bypass time (min) | 118 ± 51 | 112 ± 23 | 0.68 |
| Surgery time (min) | 265 ± 89 | 248 ± 44 | 0.51 |
| Noradrenaline (yes/no) | 6/16 | 5/9 | 0.72 |
| Dopamine (yes/no) | 21/1 | 10/4 | 0.06 |
| Nadir hematocrit | 0.26 ± 0.03 | 0.28 ± 0.06 | 0.47 |
| Nadir hemoglobin (mmol/L) | 5.2 ± 0.7 | 5.5 ± 1.3 | 0.38 |
| Highest lactate (mmol/L) | 2.5 ± 0.8 | 2.5 ± 0.6 | 0.96 |
| Nadir pH | 7.3 ± 0.04 | 7.3 ± 0.04 | 0.83 |
| CPB outflow temperature (°C) | 34.4 ± 1.5 | 34.3 ± 0.7 | 0.42 |
| Total fluids (mL) | 3985 ± 1237 | 3052 ± 1921 | 0.08 |
| Atrial fibrillation (yes/no) | 3/18 | 2/12 | 0.99 |
| ICU stay (days) | 1 [1–1] | 1 [1–1] | 0.27 |
| Hospital stay (days) | 7 [5–9.5] | 5 [4–8] | 0.38 |
| In-hospital mortality (n) | 1 | 0 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boly, C.A.; Venhuizen, M.; Dekker, N.A.M.; Vonk, A.B.A.; Boer, C.; Brom, C.E.v.d. Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. J. Clin. Med. 2021, 10, 469. https://doi.org/10.3390/jcm10030469
Boly CA, Venhuizen M, Dekker NAM, Vonk ABA, Boer C, Brom CEvd. Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. Journal of Clinical Medicine. 2021; 10(3):469. https://doi.org/10.3390/jcm10030469
Chicago/Turabian StyleBoly, Chantal A., Margot Venhuizen, Nicole A. M. Dekker, Alexander B. A. Vonk, Christa Boer, and Charissa E. van den Brom. 2021. "Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass" Journal of Clinical Medicine 10, no. 3: 469. https://doi.org/10.3390/jcm10030469
APA StyleBoly, C. A., Venhuizen, M., Dekker, N. A. M., Vonk, A. B. A., Boer, C., & Brom, C. E. v. d. (2021). Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. Journal of Clinical Medicine, 10(3), 469. https://doi.org/10.3390/jcm10030469






