Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Frailty and Cognitive Assessment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Population Characteristics and Cognitive Function
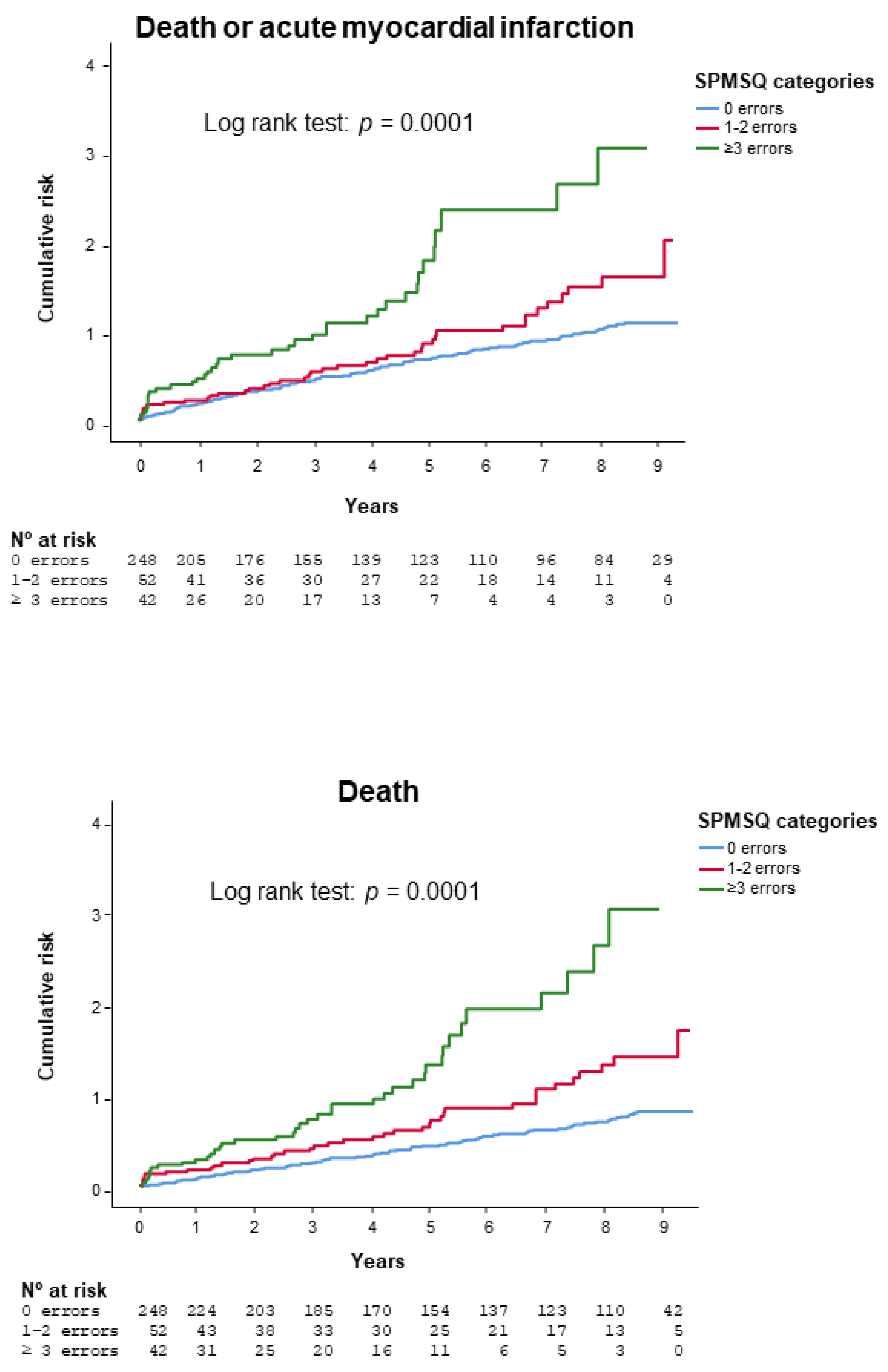
3.2. Outcomes
4. Discussion
4.1. Cognitive Function in Older Adults with Acute Coronary Syndrome
4.2. Prognosis Impact
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Alexander, K.; Roger, V.L.; Rihal, C.S.; Whitson, H.E.; Lerman, A.; Jahangir, A.; Nair, K.S. Frailty and its potential relevance to cardiovascular care. Mayo Clin. Proc. 2008, 83, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Soler, M.; Núñez, J.; Ruiz, V.; Bonanad, C.; Formiga, F.; Valero, E.; Martínez-Sellés, M.; Marín, F.; Ruescas, A.; et al. Comorbidity assessment for mortality risk stratification in elderly patients with acute coronary syndrome. Eur. J. Intern. Med. 2019, 62, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hovanesyan, A.; Rich, M.W. Outcomes of acute myocardial infarction in nonagenarians. Am. J. Cardiol. 2008, 101, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Trogdon, J.G.; Curtis, L.H.; Schulman, K.A. The effect of dementia on outcomes and process of care for Medicare beneficiaries admitted with acute myocardial infarction. J. Am. Geriatr. Soc. 2004, 52, 173–181. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Reid, K.J.; Arnold, S.V.; Spertus, J.; Rich, M.W.; Pellikka, P.A.; Singh, M.; Holsinger, T.; Krumholz, H.M.; Peterson, E.D.; et al. Cognitive impairment and outcomes in older adult survivors of acute myocardial infarction: Findings from the translational research investigating underlying disparities in acute myocardial infarction patients’ health status registry. Am. Heart J. 2011, 162, 860–869. [Google Scholar] [CrossRef]
- Bagai, A.; Chen, A.Y.; Udell, J.A.; Dodson, J.A.; McManus, D.D.; Maurer, M.S.; Enriquez, J.R.; Hochman, J.; Goyal, A.; Henry, T.D.; et al. Association of Cognitive Impairment With Treatment and Outcomes in Older Myocardial Infarction Patients: A Report From the NCDR Chest Pain-MI Registry. J. Am. Heart Assoc. 2019, 8, e012929. [Google Scholar] [CrossRef]
- Gu, S.Z.; Beska, B.; Chan, D.; Neely, D.; Batty, J.A.; Adams-Hall, J.; Mossop, H.; Qiu, W.; Kunadian, V. Cognitive Decline in Older Patients With Non- ST Elevation Acute Coronary Syndrome. J. Am. Heart Assoc. 2019, 8, e011218. [Google Scholar] [CrossRef]
- Zhao, E.; Lowres, N.; Woolaston, A.; Naismith, S.L.; Gallagher, R. Prevalence and patterns of cognitive impairment in acute coronary syndrome patients: A systematic review. Eur. J. Prev. Cardiol. 2020, 27, 284–293. [Google Scholar] [CrossRef]
- Borges, M.K.; Canevelli, M.; Cesari, M.; Aprahamian, I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2019, 6, 26. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Cabanas-Sánchez, V.; Higueras-Fresnillo, S.; Ortega, F.B.; Kramer, A.F.; Rodriguez-Artalejo, F.; Martinez-Gomez, D. Cognitive Frailty and Mortality in a National Cohort of Older Adults: The Role of Physical Activity. Mayo Clin. Proc. 2019, 94, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, M.J.R.; Cenzer, I.S.; Smith, A.K.; Lee, S.J.; Yaffe, K.; Covinsky, K.E. Assessing Risk for Adverse Outcomes in Older Adults: The Need to Include Both Physical Frailty and Cognition. J. Am. Geriatr. Soc. 2019, 67, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Bonanad, C.; Ruiz, V.; Fernández, J.; García-Blas, S.; Mainar, L.; Ventura, S.; Rodríguez-Borja, E.; Chorro, F.J.; Hermenegildo, C.; et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am. Heart J. 2014, 168, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Ruiz, V.; Bonanad, C.; Valero, E.; Ruescas-Nicolau, M.A.; Ezzatvar, Y.; Sastre, C.; García-Blas, S.; Mollar, A.; Bertomeu-González, V.; et al. Prognostic Value of Geriatric Conditions Beyond Age After Acute Coronary Syndrome. Mayo Clin. Proc. 2017, 92, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Sanchis, J.; Ruiz, V.; Sastre, C.; Bonanad, C.; Ruescas, A.; Fernández-Cisnal, A.; Mollar, A.; Valero, E.; Blas, S.G.; González, J.; et al. Frailty tools for assessment of long-term prognosis after acute coronary syndrome. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Erkinjuntti, T.; Sulkava, R.; Wikström, J.; Autio, L. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J. Am. Geriatr. Soc. 1987, 35, 412–416. [Google Scholar] [CrossRef]
- Fillenbaum, G.; Heyman, A.; Williams, K.; Prosnitz, B.; Burchett, B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly black and white community residents. J. Clin. Epidemiol. 1990, 43, 651–660. [Google Scholar] [CrossRef]
- Martínez de la Iglesia, J.; Dueñas Herrero, R.; Onís Vilches, M.C.; Aguado Taberné, C.; Albert Colomer, C.; Luque Luque, R. Cross-cultural adaptation and validation of Pfeiffer’s test (Short Portable Mental Status Questionnaire [SPMSQ]) to screen cognitive impairment in general population aged 65 or older. Med. Clin. (Barc) 2001, 117, 129–134. [Google Scholar]
- CRAN-Package rms. Available online: https://CRAN.R-project.org/package=rms (accessed on 24 November 2020).
- Vogels, R.L.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Worrall-Carter, L.; Page, K.; Riegel, B.; Lo, S.K.; Stewart, S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur. J. Heart Fail. 2010, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Silbert, B.S.; Scott, D.A.; Evered, L.A.; Lewis, M.S.; Maruff, P.T. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007, 104, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Fu, B.; Snitz, B.E.; Hughes, T.F.; Chang, C.C. Mild cognitive impairment: Incidence and vascular risk factors in a population-based cohort. Neurology 2013, 80, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Vittinghoff, E.; Pletcher, M.J.; Hoang, T.D.; Launer, L.J.; Whitmer, R.A.; Coker, L.H.; Sidney, S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014, 129, 1560–1567. [Google Scholar] [CrossRef]
- Volonghi, I.; Pendlebury, S.T.; Welch, S.J.; Mehta, Z.; Rothwell, P.M. Cognitive outcomes after acute coronary syndrome: A population based comparison with transient ischaemic attack and minor stroke. Heart 2013, 99, 1509–1514. [Google Scholar] [CrossRef]
- Mone, P.; Pansini, A. Gait speed test and cognitive decline in frail women with acute myocardial infarction. Am. J. Med. Sci. 2020, 360, 484–488. [Google Scholar] [CrossRef]
- Sanchis, J.; Sastre, C.; Ruescas, A.; Ruiz, V.; Valero, E.; Bonanad, C.; García-Blas, S.; Fernández-Cisnal, A.; González, J.; Miñana, G.; et al. Randomized comparison of exercise intervention versus usual care in older adult patients with frailty after acute myocardial infarction. Am. J. Med. 2020. [Google Scholar] [CrossRef]
- Sanchis, J.; Acuña, J.M.; Raposeiras, S.; Barrabés, J.A.; Cordero, A.; Martínez-Sellés, M.; Bardají, A.; Díez-Villanueva, P.; Marín, F.; Ruiz-Nodar, J.M.; et al. Comorbidity burden and revascularization benefit in elderly patients with acute coronary syndrome. Rev. Esp. Cardiol. 2020. [Google Scholar] [CrossRef]
- Strandberg, T.E.; Pitkala, K.H.; Tilvis, R.S. Predictors of mortality in home-dwelling patients with cardiovascular disease aged 75 and older. J. Am. Geriatr. Soc. 2009, 57, 279–284. [Google Scholar] [CrossRef]
- Sokoreli, I.; Pauws, S.C.; Steyerberg, E.W.; de Vries, G.J.; Riistama, J.M.; Tesanovic, A.; Kazmi, S.; Pellicori, P.; Cleland, J.G.; Clark, A.L. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: Insights from the OPERA-HF study. Eur. J. Heart Fail. 2018, 20, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Konishi, M.; Kitai, T.; Iwata, K.; Jujo, K.; et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur. J. Heart Fail. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zheng, P.; Liang, Y.; Wan, Y.; Sun, N.; Luo, Y.; Yang, J.; Wang, H. Predicting non-elective hospital readmission or death using a composite assessment of cognitive and physical frailty in elderly inpatients with cardiovascular disease. BMC Geriatr. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M. Post-hospital syndrome—An acquired, transient condition of generalized risk. N. Engl. J. Med. 2013, 368, 100–102. [Google Scholar] [CrossRef]
| Fried Score [15] (0 to 5 Points) | Points | |
|---|---|---|
| Weight loss | Self-reported unintentional weight loss of greater than 4.5 Kg in the preceding year | 1 |
| Physical activity | Minnesota Leisure Time Activity questionnaire. Men < 383 Kcal per week, women < 270 Kcal per week | 1 |
| Walk time | Time to walk 4.57 m: ≥7 s for height ≤ 173 cm in men or ≤ 159 cm in women, ≥6 s for height > 173 cm in men or >159 cm in women | 1 |
| Grip strength | Lowest 20% (by gender, body mass index) using a hand-held isometric dynamometer (Kg) | 1 |
| Exhaustion | Self-reported based on 2 questions from the Center for Epidemiological Studies—Depression scale: “I felt that everything I did was an effort” and “I could not get going,” with answers graded from 0 to 3. If 2 or 3 = exhaustion | 1 |
| Questions |
|---|
| 1. What is the date today? Correct only when the month, date, and year are all correct. |
| 2. What day of the week is it? Correct only when the day is correct. |
| 3. What is the name of this place? Correct if any of the descriptions of the location is given. “My home,” the correct city/town, or the correct name of the hospital/institution are all acceptable. |
| 4. What is your telephone number? Correct when the number can be verified or the subject can repeat the same number at a later time in the interview. |
| 4a. What is your street address? Ask only if the subject does not have a telephone. |
| 5. How old are you? Correct when the stated age corresponds to the date of birth. |
| 6. When were you born? Correct only when the month, date, and year are correct. |
| 7. Who is the president of your country now? Requires only the correct last name. |
| 8. Who was president just before him? Requires only the correct last name. |
| 9. What was your mother’s maiden name? Needs no verification; it only requires a female first name plus a last name other than the subjects. |
| 10. Subtract 3 from 20 and keep subtracting 3 from each new number, all the way down. The entire series must be performed correctly to be scored as correct. Any error in the series—or an unwillingness to attempt the series—is scored as incorrect. |
| 0 Errors n = 248 | 1–2 Errors n = 52 | ≥3 Errors n = 42 | p | |
|---|---|---|---|---|
| Age (years) | 75.6 ± 6.4 | 80.3 ± 6.9 | 84.1 ± 6.6 | 0.0001 |
| Female | 90 (36%) | 30 (58%) | 26 (62%) | 0.0001 |
| Smoking | 26 (11%) | 4 (7.7%) | 4 (9.5%) | 0.826 |
| Hypertension | 205 (83%) | 43 (83%) | 35 (83%) | 0.994 |
| Hypercholesterolemia | 149 (60%) | 31 (60%) | 20 (48%) | 0.312 |
| Diabetes | 100 (40%) | 27 (52%) | 17 (41%) | 0.297 |
| Prior myocardial infarction | 87 (35%) | 14 (27%) | 18 (43%) | 0.268 |
| Prior percutaneous coronary intervention | 53 (21%) | 5 (9.6%) | 8 (19%) | 0.148 |
| Prior coronary artery bypass graft | 18 (7.3%) | 6 (11.5%) | 3 (7.1%) | 0.571 |
| Prior admission for heart failure | 31 (13%) | 13 (25%) | 8 (19%) | 0.056 |
| Prior stroke | 26 (11%) | 9 (17%) | 9 (21%) | 0.086 |
| Peripheral artery disease | 20 (8.1%) | 7 (14%) | 6 (14%) | 0.270 |
| Chronic lung disease | 44 (18%) | 7 (14%) | 7 (17%) | 0.755 |
| Admission systolic blood pressure (mmHg) | 142 ± 32 | 137 ± 34 | 146 ± 34 | 0.438 |
| Admission heart rate (beats/minute) | 80 ± 20 | 85 ± 24 | 86 ± 26 | 0.154 |
| Admission Killip class ≥ 2 | 61 (25%) | 20 (39%) | 20 (48%) | 0.003 |
| ST-segment elevation myocardial infarction | 49 (20%) | 12 (23%) | 10 (24%) | 0.756 |
| Left bundle branch block | 16 (6.5%) | 3 (5.8%) | 6 (14%) | 0.177 |
| Admission atrial fibrillation | 31 (13%) | 6 (12%) | 6 (14.3) | 0.921 |
| Troponin elevation | 226 (91%) | 48 (92%) | 40 (95%) | 0.661 |
| Admission hemoglobin (g/dL) | 12.7 ± 1.9 | 12.2 ± 1.8 | 12.0 ± 1.4 | 0.033 |
| Admission glomerular filtration rate (mL/min/1.73 m²) | 53 ± 14 | 49 ± 15 | 46 ± 16 | 0.007 |
| Left ventricular ejection fraction | 54 ± 13 | 54 ± 14 | 50 ± 14 | 0.094 |
| GRACE score | 132 ± 22 | 148 ± 27 | 153 ±26 | 0.0001 |
| Fried score | 1.8 ± 1.1 | 2.4 ± 0.89 | 2.7 ± 0.7 | 0.0001 |
| In-hospital revascularization | 118 (48%) | 22 (42%) | 121 (29%) | 0.07 |
| Aspirin | 225 (91%) | 43 (84%) | 37 (88% | 0.380 |
| Clopidogrel | 186 (77%) | 38 (75%) | 32 (76%) | 0.981 |
| Oral anticoagulants | 30 (12%) | 9 (18%) | 3 (7%) | 0.302 |
| Statins | 233 (94%) | 48 (94%) | 40 (95%) | 0.948 |
| Beta-blockers | 218 (88%) | 43 (84%) | 36 (86%) | 0.753 |
| ACE inhibitors | 216 (87%) | 39 (77%) | 38 (91%) | 0.09 |
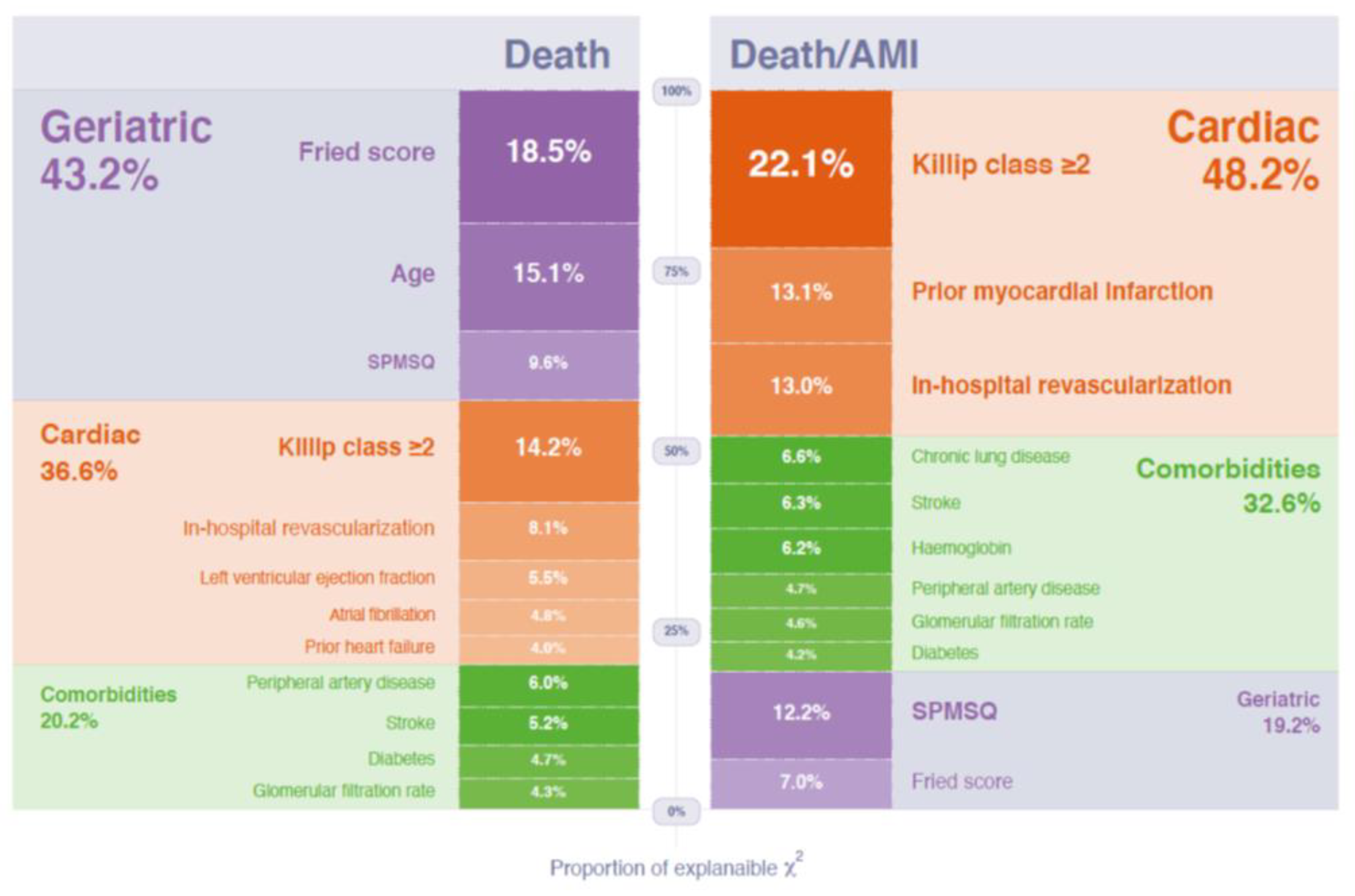
| HR | 95% CI | p | Relative Importance (Proportion of Overall χ2) | |
|---|---|---|---|---|
| Admission Killip ≥ 2 | 1.80 | 1.36 to 2.36 | 0.0001 | 0.140 |
| Prior myocardial infarction | 1.56 | 1.19 to 2.04 | 0.001 | 0.083 |
| In-hospital revascularization | 0.65 | 0.50 to 0.85 | 0.001 | 0.082 |
| SPMSQ (errors) | 1.11 | 1.04 to 1.19 | 0.002 | 0.078 |
| Fried score (points) | 1.19 | 1.03 to 1.37 | 0.019 | 0.044 |
| Chronic pulmonary disease | 1.46 | 1.06 to 2.02 | 0.022 | 0.042 |
| Prior stroke | 1.50 | 1.05 to 2.13 | 0.026 | 0.040 |
| Admission hemoglobin (g/dL) | 0.92 | 0.86 to 0.99 | 0.027 | 0.039 |
| Peripheral artery disease | 1.51 | 1.0 to 2.28 | 0.052 | 0.030 |
| Admission glomerular filtration rate (per 5 mL/min/1.73 m²) | 0.96 | 0.92 to 1.0 | 0.057 | 0.029 |
| Diabetes | 1.27 | 0.98 to 1.65 | 0.07 | 0.027 |
| HR | 95% CI | p | Relative Importance (Proportion of Overall χ2) | |
|---|---|---|---|---|
| Fried score (points) | 1.34 | 1.15–1.55 | 0.0001 | 0.089 |
| Age (years) | 1.04 | 1.02–1.07 | 0.001 | 0.073 |
| Admission Killip ≥ 2 | 1.72 | 1.25–2.37 | 0.001 | 0.069 |
| SPMSQ test (errors) | 1.11 | 1.03–1.20 | 0.007 | 0.046 |
| In-hospital revascularization | 0.69 | 0.52–0.92 | 0.012 | 0.039 |
| Peripheral artery disease | 1.60 | 1.04–2.47 | 0.032 | 0.029 |
| Left ventricular ejection fraction at discharge (per 5%) | 0.94 | 0.89–0.99 | 0.039 | 0.027 |
| Prior stroke | 1.47 | 1.01–2.12 | 0.044 | 0.025 |
| Atrial fibrillation at admission | 1.46 | 0.99–2.14 | 0.054 | 0.023 |
| Diabetes | 1.31 | 0.99–1.74 | 0.057 | 0.023 |
| Admission glomerular filtration rate (per 5 mL/min/1.73 m²) | 0.96 | 0.91–1.0 | 0.069 | 0.021 |
| Prior admission for heart failure | 1.39 | 0.96–2.0 | 0.080 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchis, J.; Bonanad, C.; García-Blas, S.; Ruiz, V.; Fernández-Cisnal, A.; Sastre, C.; Ruescas, A.; Valero, E.; González, J.; Mollar, A.; et al. Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 444. https://doi.org/10.3390/jcm10030444
Sanchis J, Bonanad C, García-Blas S, Ruiz V, Fernández-Cisnal A, Sastre C, Ruescas A, Valero E, González J, Mollar A, et al. Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome. Journal of Clinical Medicine. 2021; 10(3):444. https://doi.org/10.3390/jcm10030444
Chicago/Turabian StyleSanchis, Juan, Clara Bonanad, Sergio García-Blas, Vicent Ruiz, Agustín Fernández-Cisnal, Clara Sastre, Arancha Ruescas, Ernesto Valero, Jessika González, Anna Mollar, and et al. 2021. "Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome" Journal of Clinical Medicine 10, no. 3: 444. https://doi.org/10.3390/jcm10030444
APA StyleSanchis, J., Bonanad, C., García-Blas, S., Ruiz, V., Fernández-Cisnal, A., Sastre, C., Ruescas, A., Valero, E., González, J., Mollar, A., Miñana, G., & Núñez, J. (2021). Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome. Journal of Clinical Medicine, 10(3), 444. https://doi.org/10.3390/jcm10030444







