Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Aspects
2.2. Patient Selection
2.3. Study Protocol
2.4. Statistical Analysis
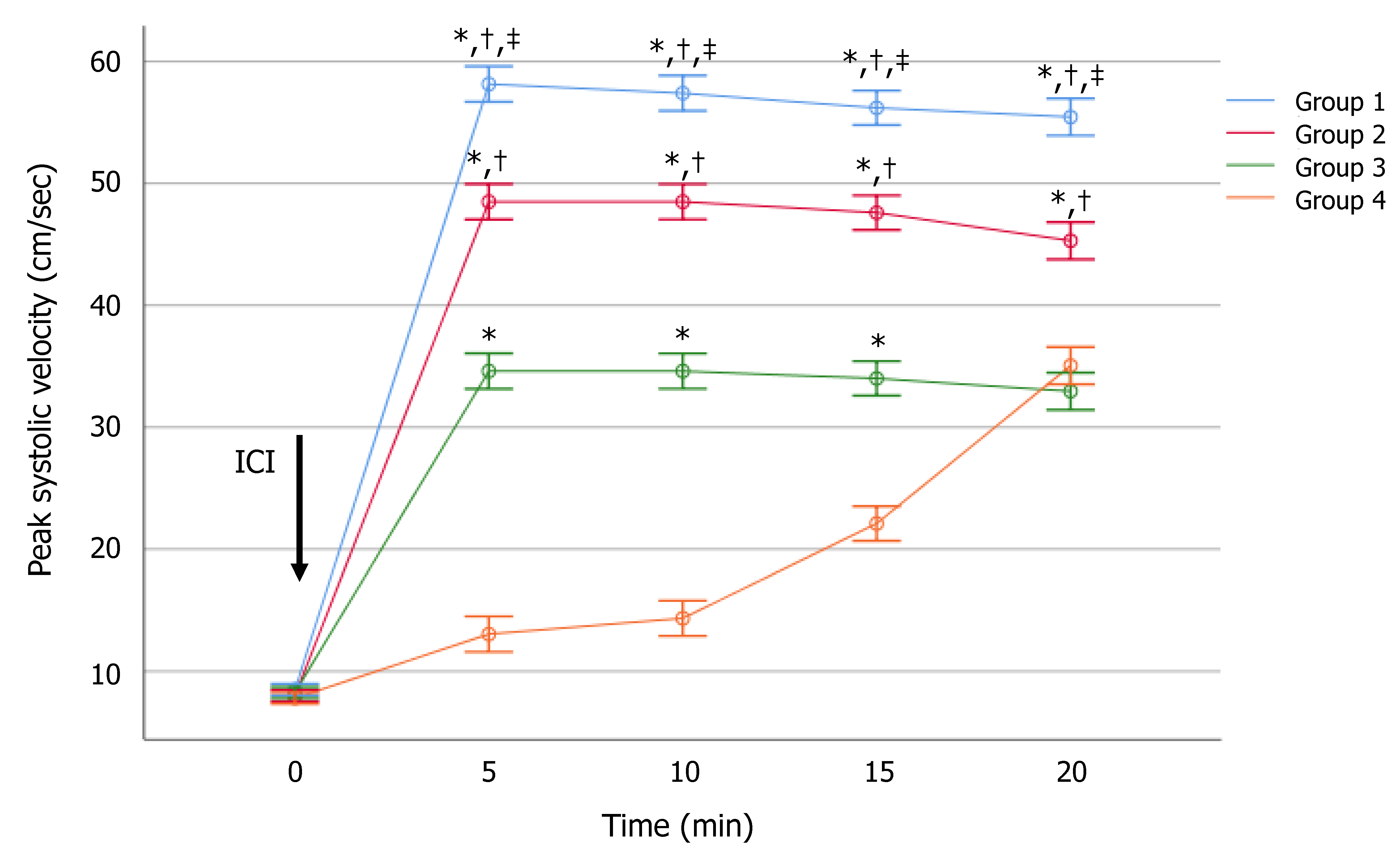
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993, 270, 83–90. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Epidemiology and Risk Factors of Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia and Erectile Dysfunction. Aging Male 2019, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia and Erectile Dysfunction: From Physiology to Clinical Aspects. Aging Male 2018, 21, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Aghighi, A.; Grigoryan, V.H.; Delavar, A. Psychological Determinants of Erectile Dysfunction among Middle-Aged Men. Int. J. Impot. Res. 2015, 27, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mourikis, I.; Antoniou, M.; Matsouka, E.; Vousoura, E.; Tzavara, C.; Ekizoglou, C.; Papadimitriou, G.N.; Vaidakis, N.; Zervas, I.M. Anxiety and depression among Greek men with primary erectile dysfunction and premature ejaculation. Ann. Gen. Psychiatry 2015, 14, 34. [Google Scholar] [CrossRef][Green Version]
- Liao, Z.C.; Li, X.C.; Tang, Y.X.; Li, D.J.; Tang, Z.Y. Is Milder Psychological Stress Responsible for More Severe Erectile Dysfunction? Andrologia 2010, 52, e13550. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Treatment of Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia and Erectile Dysfunction. Aging Male 2018, 21, 272–280. [Google Scholar] [CrossRef]
- Yafi, F.A.; Libby, R.P.; McCaslin, I.R.; Sangkum, P.; Sikka, S.C.; Hellstrom, W.J. Failure to attain stretched penile length after intracavernosal injection of a vasodilator agent is predictive of veno-occlusive dysfunction on penile duplex Doppler ultrasonography. Andrology 2015, 3, 919–923. [Google Scholar] [CrossRef]
- Pagano, M.J.; Stahl, P.J. Variation in penile hemodynamics by anatomic location of cavernosal artery imaging in penile duplex Doppler ultrasound. J. Sex. Med. 2015, 12, 1911–1919. [Google Scholar] [CrossRef]
- Aversa, A.; Sarteschi, L.M. The Role of Penile Color-Duplex Ultrasound for the Evaluation of Erectile Dysfunction. J. Sex. Med. 2007, 4, 1437–1447. [Google Scholar] [CrossRef]
- Slob, A.K.; Rowland, D.L.; Blom, J.H.; van der Werff ten Bosch, J.J. Psychologic factors affect erectile response to papaverine. Urology 1991, 38, 294–295. [Google Scholar] [CrossRef]
- Aversa, A.; Rocchietti-March, M.; Caprio, M.; Giannini, D.; Isidori, A.; Fabbri, A. Anxiety-induced Failure in Erectile Response to Intracorporeal prostaglandin-E1 in Non-Organic Male Impotence: A New Diagnostic Approach. Int. J. Androl. 1996, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The International Index of Erectile Function (IIEF): A Multidimensional Scale for Assessment of Erectile Dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Sikka, S.C.; Hellstrom, W.J.; Brock, G.; Morales, A.M. Standardization of Vascular Assessment of Erectile Dysfunction: Standard Operating Procedures for Duplex Ultrasound. J. Sex. Med. 2013, 10, 120–129. [Google Scholar] [CrossRef]
- Wilkins, C.J.; Sriprasad, S.; Sidhu, P.S. Colour Doppler Ultrasound of the Penis. Clin. Radiol. 2003, 58, 514–523. [Google Scholar] [CrossRef]
- Montorsi, P.; Ravagnani, P.M.; Galli, S.; Salonia, A.; Briganti, A.; Werba, J.P.; Montorsi, F. Association Between Erectile Dysfunction and Coronary Artery Disease: Matching the Right Target With the Right Test in the Right Patient. Eur. Urol. 2006, 50, 721–731. [Google Scholar] [CrossRef]
- Thompson, I.M.; Tangen, C.M.; Goodman, P.J.; Probstfield, J.L.; Moinpour, C.M.; Coltman, C.A. Erectile Dysfunction and Subsequent Cardiovascular Disease. JAMA 2005, 294, 2996–3002. [Google Scholar] [CrossRef]
- Slob, A.K.; Cornelissen, S.; Dohle, G.R.; Gijs, L.; van der Werff ten Bosch, J.J. The Limited Practical Value of Color Doppler Sonography in the Differential Diagnosis of Men with Erectile Dysfunction. Int. J. Impot. Res. 2002, 14, 201–203. [Google Scholar] [CrossRef]
- Aversa, A.; Bonifacio, V.; Moretti, C.; Frajese, G.; Fabbri, A. Re-dosing of Prostaglandin-E1 Versus Prostaglandin-E1 Plus Phentolamine in Male Erectile Dysfunction: A Dynamic Color Power Doppler Study. Int. J. Impot. Res. 2000, 12, 33–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corona, G.; Fagioli, G.; Mannucci, E.; Romeo, A.; Rossi, M.; Lotti, F.; Sforza, A.; Morittu, S.; Chiarini, V.; Casella, G.; et al. Penile Doppler Ultrasound in Patients With Erectile Dysfunction (ED): Role of Peak Systolic Velocity Measured in the Flaccid State in Predicting Arteriogenic ED and Silent Coronary Artery Disease. J. Sex. Med. 2008, 5, 2623–2634. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwon, D.D.; Oh, B.R.; Ryu, S.B.; Park, Y.I. Efficacy of virtual glasses in audio-visual sexual stimulation during penile color duplex Doppler ultrasonography. Eur. Urol. 2002, 41, 62–65. [Google Scholar] [CrossRef]

| Group 1 (n = 20) | Group 2 (n = 20) | Group 3 (n = 20) | Group 4 (n = 20) |
|---|---|---|---|
| 39.20 ± 7.20 *,† | 38.50 ± 7.70 *,† | 30.70 ± 7.10 | 35.20 ± 9.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Calogero, A.E.; Aversa, A.; Condorelli, R.A.; La Vignera, S. Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety. J. Clin. Med. 2021, 10, 402. https://doi.org/10.3390/jcm10030402
Cannarella R, Calogero AE, Aversa A, Condorelli RA, La Vignera S. Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety. Journal of Clinical Medicine. 2021; 10(3):402. https://doi.org/10.3390/jcm10030402
Chicago/Turabian StyleCannarella, Rossella, Aldo E. Calogero, Antonio Aversa, Rosita A. Condorelli, and Sandro La Vignera. 2021. "Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety" Journal of Clinical Medicine 10, no. 3: 402. https://doi.org/10.3390/jcm10030402
APA StyleCannarella, R., Calogero, A. E., Aversa, A., Condorelli, R. A., & La Vignera, S. (2021). Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety. Journal of Clinical Medicine, 10(3), 402. https://doi.org/10.3390/jcm10030402









