Brain Metastases from Adult Sarcomas: A Retrospective Cohort Study from the Hellenic Group of Sarcomas and Rare Cancers (HGSRC)
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Patients Selection and Data Collection
2.3. Statistical Analysis
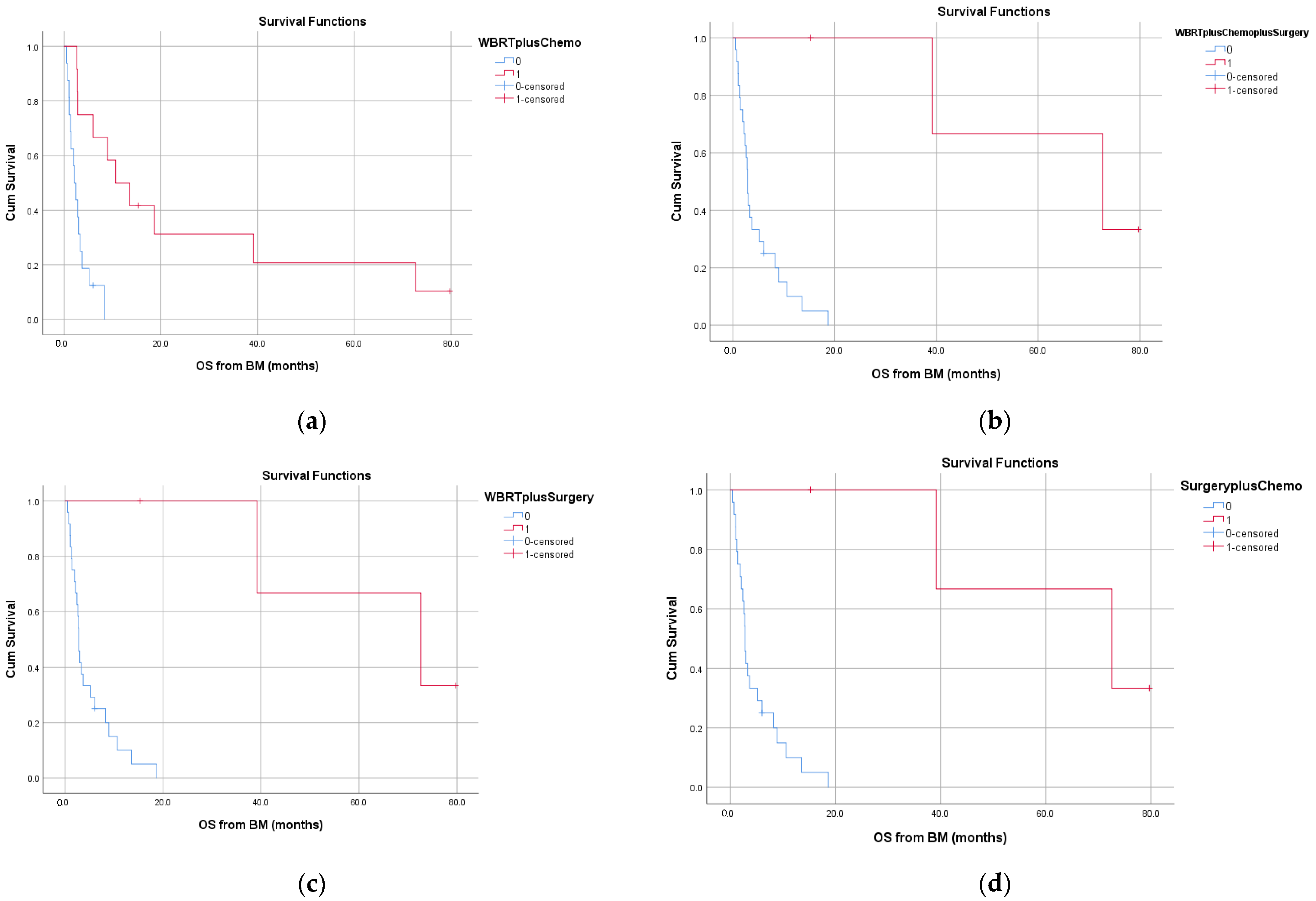
3. Results
3.1. Clinicopathological Characteristics
3.2. Treatment Modalities for Brain m3.2. Treatment Modalities for Brain Metastases and Outcome
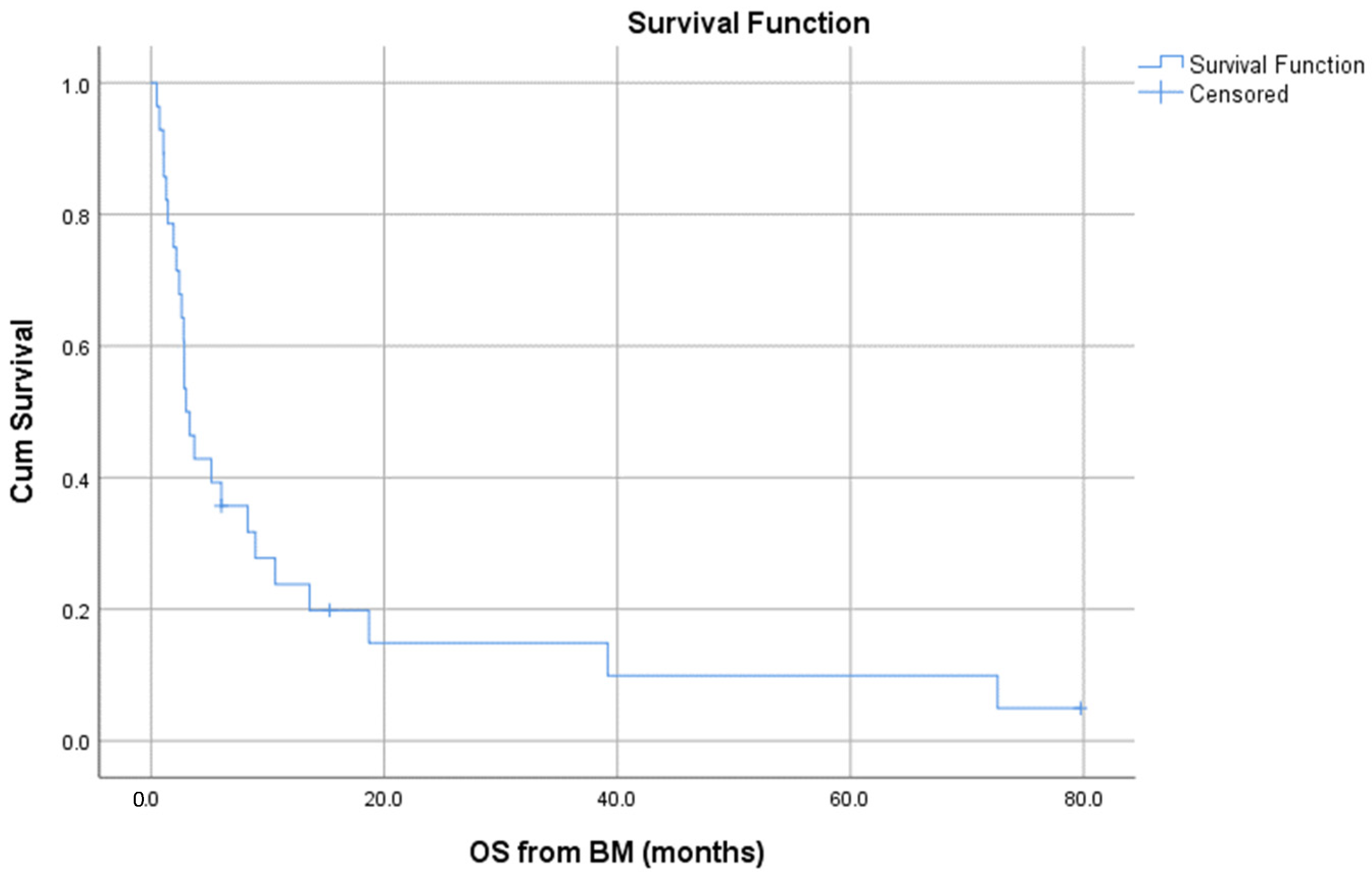
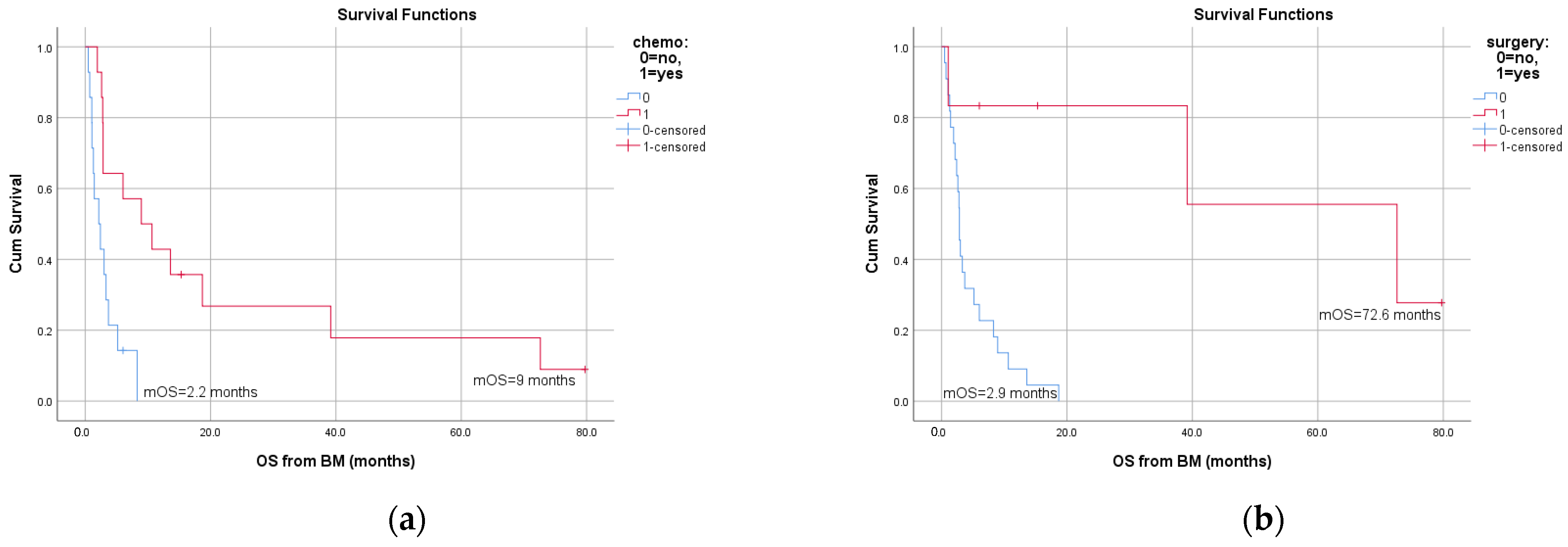
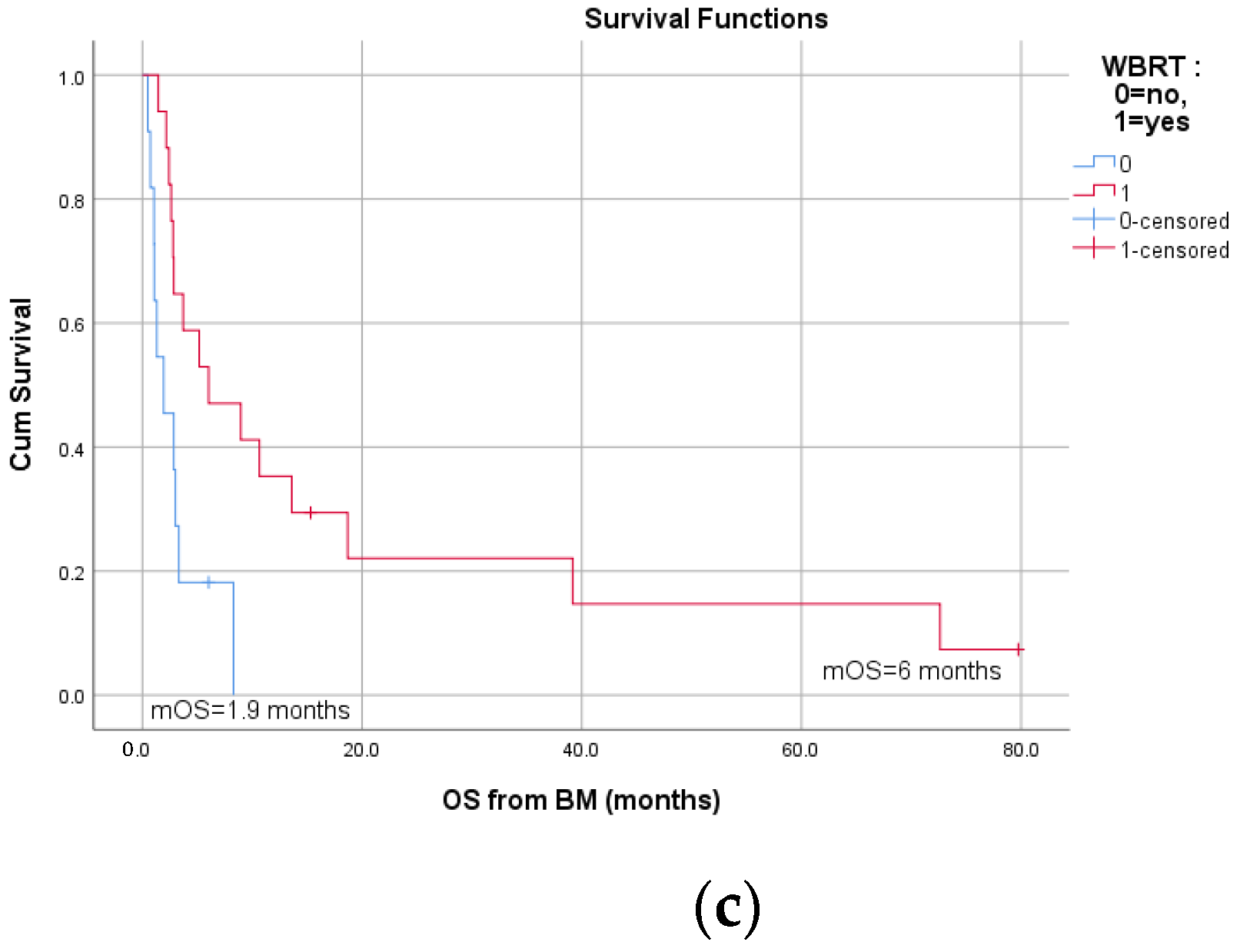
3.3. Survival and Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espat, N.J.; Bilsky, M.; Lewis, J.J.; Leung, D.; Brennan, M.F. Soft Tissue Sarcoma Brain Metastases. Prevalence in a Cohort of 3829 Patients. Cancer 2002, 94, 2706–2711. [Google Scholar] [CrossRef]
- Gusho, C.A.; Blank, A.T.; Batus, M. Outcomes of Brain Metastasis in High-Grade Bone and Soft Tissue Sarcoma: An Analysis of Clinicopathological Characteristics and Survival Data. Rare Tumors 2021, 13, 20363613211026150. [Google Scholar] [CrossRef]
- Gercovich, F.G.; Luna, M.A.; Gottlieb, J.A. Increased Incidence of Cerebral Metastases in Sarcoma Patients with Prolonged Survival from Chemotherapy. Report of Cases of Leiomysarcoma and Chondrosarcoma. Cancer 1975, 36, 1843–1851. [Google Scholar] [CrossRef]
- Chua, C.; Raaj, J.; Pan, S.; Farid, M.; Lee, J.F.M.; Ho, Z.C.; Sairi, A.; Sittampalam, K.; Tao, M.; Tay, K.; et al. Brain Metastasis in Sarcoma: Does Metastasectomy or Aggressive Multi-Disciplinary Treatment Improve Survival Outcomes. Asia Pac. J. Clin. Oncol. 2016, 12, e16–e22. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Liu, C.-Y.; Chen, W.-M.; Chen, T.-H.; Chen, P.C.-H.; Wu, H.-T.H.; Shiau, C.-Y.; Wu, Y.-C.; Liu, C.-L.; Chao, T.-C.; et al. Brain, the Last Fortress of Sarcoma: Similar Dismal Outcome but Discrepancy of Timing of Brain Metastasis in Bone and Soft Tissue Sarcoma. J. Surg. Oncol. 2011, 104, 765–770. [Google Scholar] [CrossRef]
- Wroński, M.; Arbit, E.; Burt, M.; Perino, G.; Galicich, J.H.; Brennan, M.F. Resection of Brain Metastases from Sarcoma. Ann. Surg. Oncol. 1995, 2, 392–399. [Google Scholar] [CrossRef]
- Malouf, G.G.; Beinse, G.; Adam, J.; Mir, O.; Chamseddine, A.N.; Terrier, P.; Honore, C.; Spano, J.-P.; Italiano, A.; Kurtz, J.-E.; et al. Brain Metastases and Place of Antiangiogenic Therapies in Alveolar Soft Part Sarcoma: A Retrospective Analysis of the French Sarcoma Group. Oncologist 2019, 24, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Rubino, F.; Eichberg, D.G.; Shah, A.H.; Luther, E.M.; Lu, V.M.; Saad, A.G.; Kahn, D.; Komotar, R.J.; Ivan, M.E. When “Peripheral” Becomes “Central”: Primary and Secondary Malignant Intracerebral Nerve Sheath Tumor: A Case Report and a Systematic Review. Neurosurgery 2021, 88, 1074–1087. [Google Scholar] [CrossRef]
- Al Sannaa, G.; Watson, K.L.; Olar, A.; Wang, W.-L.; Fuller, G.N.; McCutcheon, I.; Torres, K.E.; Lazar, A.J. Sarcoma Brain Metastases: 28 Years of Experience at a Single Institution. Ann. Surg. Oncol. 2016, 23, 962–967. [Google Scholar] [CrossRef]
- Chaigneau, L.; Patrikidou, A.; Ray-Coquard, I.; Valentin, T.; Linassier, C.; Bay, J.O.; Moureau Zabotto, L.; Bompas, E.; Piperno-Neumann, S.; Penel, N.; et al. Brain Metastases from Adult Sarcoma: Prognostic Factors and Impact of Treatment. A Retrospective Analysis from the French Sarcoma Group (GSF/GETO). Oncologist 2018, 23, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Bielack, S.S.; Kempf-Bielack, B.; Branscheid, D.; Carrle, D.; Friedel, G.; Helmke, K.; Kevric, M.; Jundt, G.; Kühne, T.; Maas, R.; et al. Second and Subsequent Recurrences of Osteosarcoma: Presentation, Treatment, and Outcomes of 249 Consecutive Cooperative Osteosarcoma Study Group Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 557–565. [Google Scholar] [CrossRef]
- Salvati, M.; D’Elia, A.; Frati, A.; Santoro, A. Sarcoma Metastatic to the Brain: A Series of 35 Cases and Considerations from 27 Years of Experience. J. Neurooncol. 2010, 98, 373–377. [Google Scholar] [CrossRef]
- Hoiczyk, M.; Herbrik, M.; Grabellus, F.; Podleska, L.; Pöttgen, C.; Schwindenhammer, B.; Bekas, V.; Schuler, M.H.; Bauer, S. Brain Metastases in Sarcoma Patients: Incidence and Outcome. J. Clin. Oncol. 2014, 32, 10591. [Google Scholar] [CrossRef]
- Shweikeh, F.; Bukavina, L.; Saeed, K.; Sarkis, R.; Suneja, A.; Sweiss, F.; Drazin, D. Brain Metastasis in Bone and Soft Tissue Cancers: A Review of Incidence, Interventions, and Outcomes. Sarcoma 2014, 2014, 475175. [Google Scholar] [CrossRef]
- Bindal, R.K.; Sawaya, R.E.; Leavens, M.E.; Taylor, S.H.; Guinee, V.F. Sarcoma Metastatic to the Brain: Results of Surgical Treatment. Neurosurgery 1994, 35, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Fox, B.D.; Patel, A.; Suki, D.; Rao, G. Surgical Management of Metastatic Sarcoma to the Brain. J. Neurosurg. 2009, 110, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Fan, G.; Cao, L.; Zhu, H.; Wu, S.; Zhao, J.; Zhou, G. Survival Outcomes of Patients with Brain Metastasis of Osteosarcoma Can Be Improved by Aggressive Multi-Disciplinary Interventions Including Chemotherapy. Br. J. Neurosurg. 2021, 1–6. [Google Scholar] [CrossRef]
- Patrikidou, A.; Chaigneau, L.; Isambert, N.; Kitikidou, K.; Shanley, R.; Ray-Coquard, I.; Valentin, T.; Malivoir, B.; Laigre, M.; Bay, J.-O.; et al. Development of a Disease-Specific Graded Prognostic Assessment Index for the Management of Sarcoma Patients with Brain Metastases (Sarcoma-GPA). BMC Cancer 2020, 20, 117. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer Oxf. Engl. 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kebudi, R.; Ayan, I.; Görgün, O.; Ağaoğlu, F.Y.; Vural, S.; Darendeliler, E. Brain Metastasis in Pediatric Extracranial Solid Tumors: Survey and Literature Review. J. Neurooncol. 2005, 71, 43–48. [Google Scholar] [CrossRef]
- Portera, C.A.; Ho, V.; Patel, S.R.; Hunt, K.K.; Feig, B.W.; Respondek, P.M.; Yasko, A.W.; Benjamin, R.S.; Pollock, R.E.; Pisters, P.W. Alveolar Soft Part Sarcoma: Clinical Course and Patterns of Metastasis in 70 Patients Treated at a Single Institution. Cancer 2001, 91, 585–591. [Google Scholar] [CrossRef]
- Daigeler, A.; Kuhnen, C.; Hauser, J.; Goertz, O.; Tilkorn, D.; Steinstraesser, L.; Steinau, H.-U.; Lehnhardt, M. Alveolar Soft Part Sarcoma: Clinicopathological Findings in a Series of 11 Cases. World J. Surg. Oncol. 2008, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Doval, D.C.; Chacko, M.; Sinha, R.; Choudhury, K.D.; Sharma, A.; Rao, A.; Jaggi, R.S.; Mehta, A. A Rare Case of Brain Metastasis in a Patient with Osteosarcoma. South Asian J. Cancer 2017, 6, 36–37. [Google Scholar] [CrossRef]
- Badiu, D.C.; Manea, C.A.; Porojan, V.; Paraschiv, M.; Mehedintu, C.; Coman, I.S.; Grigorean, V.T. A Rare Cause of Bowel Obstruction: Peritoneal Metastases in Osteosarcoma at the Tibia in a Young Female Patient with Brain Metastasis. Case Report. Chir. Buchar. Rom. 2016, 111, 274–278. [Google Scholar]
- Rabah, F.; Al-Mashaikhi, N.; Beshlawi, I.; Bhuyan, D.; Al-Hinai, M.; Al-Balushi, S.; El-Banna, N. Brain Is Not Always the Last Fortress; Osteosarcoma with Large Brain Metastasis. J. Pediatr. Hematol. Oncol. 2013, 35, e91–e93. [Google Scholar] [CrossRef]
- Kokkali, S.; Andriotis, E.; Katsarou, E.; Theocharis, A.; Drizou, M.; Magou, E.; Tzovaras, A.; Ardavanis, A. Cerebral Metastasis from Osteosarcoma: “Bone” in the Brain. Radiol. Case Rep. 2020, 15, 780–783. [Google Scholar] [CrossRef]
- Merinsky, A.; Schmidt, M.; Heidner, K.; Koelbl, H. Cerebral Metastases of an Endometrial Stromal Sarcoma: Report of the First Case. Onkologie. 2012, 35, 272–274. [Google Scholar] [CrossRef]
- Khanal, S.; Singh, Y.P.; Bhandari, A.; Sharma, R. Malignant Phyllodes Tumor with Metastases to Lung, Adrenal and Brain: A Rare Case Report. Ann. Med. Surg. 2018, 36, 113–117. [Google Scholar] [CrossRef]
- Singh, J.; Majumdar, K.; Gupta, R.; Batra, V.V. Cerebellar Metastases of Recurrent Phyllodes Tumor Breast; a Rare Phenomenon Reflecting the Unpredictable Outcome. J. Cancer Res. Ther. 2015, 11, 1035. [Google Scholar] [CrossRef]
- Guillou, L.; Coindre, J.M.; Bonichon, F.; Nguyen, B.B.; Terrier, P.; Collin, F.; Vilain, M.O.; Mandard, A.M.; Le Doussal, V.; Leroux, A.; et al. Comparative Study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group Grading Systems in a Population of 410 Adult Patients with Soft Tissue Sarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 350–362. [Google Scholar] [CrossRef]
- Schroeder, T.; Bittrich, P.; Kuhne, J.F.; Noebel, C.; Leischner, H.; Fiehler, J.; Schroeder, J.; Schoen, G.; Gellißen, S. Mapping Distribution of Brain Metastases: Does the Primary Tumor Matter? J. Neurooncol. 2020, 147, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Niazi, T.N.; Forester, C.; Afify, Z.; Riva-Cambrin, J. Osteosarcoma Presenting as Hemorrhagic Cerebellar Metastasis. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2009, 25, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Pratt, C.B.; Shema, S.J.; Brooks, T.; Rao, B.; Meyer, W.H. Brain Metastases in Osteosarcoma. Report of a Long-Term Survivor and Review of the St. Jude Children’s Research Hospital Experience. Cancer 1993, 71, 3656–3660. [Google Scholar] [CrossRef]
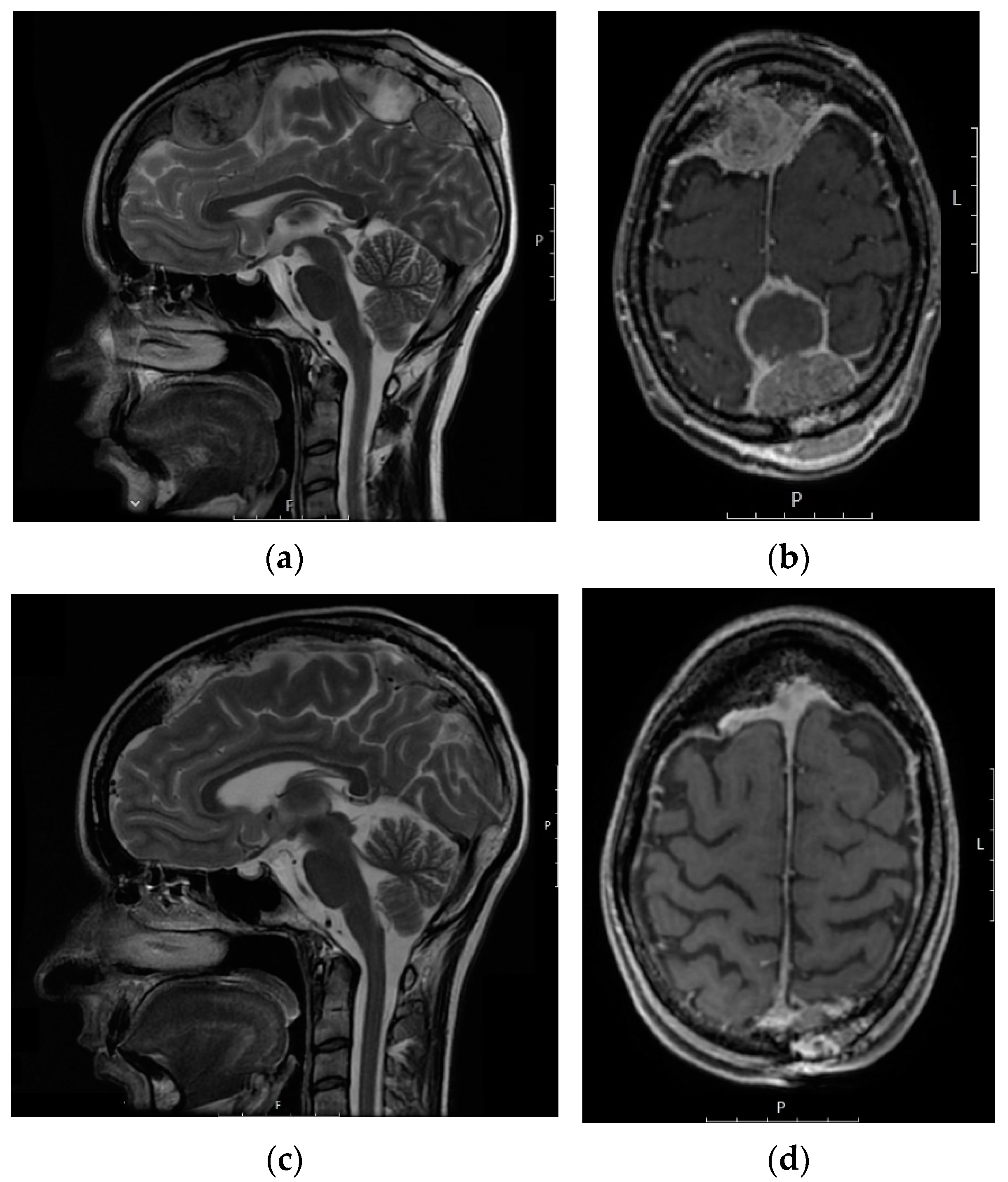
| Characteristics | No. (%) |
|---|---|
| Sarcoma origin | |
| Soft tissue | 27 (79.4) |
| Bone | 7 (20.6) |
| Localization of the primary | |
| Extremities | 18 (52.9) |
| Trunk | 3 (8.8) |
| Retroperitoneum | 1 (2.9) |
| Uterus | 5 (14.7) |
| Other | 7 (20.6) |
| Histological type | |
| Ewing/PNET | 7 (20.6) |
| Leiomyosarcoma-soft tissue | 4 (11.8) |
| Leiomyosarcoma-uterine | 4 (11.8) |
| Osteosarcoma | 3 (8.8) |
| Alveolar soft part sarcoma (ASPS) | 2 (5.9) |
| Undifferentiated pleomorphic sarcoma (UPS) | 3 (8.8) |
| Rhabdomyosarcoma | 2 (5.9) |
| Synovial sarcoma | 2 (5.9) |
| Malignant peripheral nerve sheath tumor | 2 (5.9) |
| Other 1 | 5 (14.7) |
| Grade | |
| 3 | 25 (73.5) |
| 2 | 7 (20.6) |
| Unknown | 2 (5.9) |
| Stage at initial presentation | |
| Localized | 9 (26.5) |
| Metastatic | 25 (73.5) |
| Characteristic | No. (%) |
|---|---|
| Brain metastases localization | |
| Supratentorial | 20 (58.8) |
| Infratentorial | 5 (14.7) |
| Both supratentorial and infratentorial | 5 (14.7) |
| Meningeal | 1 (2.9) |
| Unknown | 3 (8.8) |
| Number of brain metastases | |
| 1 | 13 (38.2) |
| 2–4 | 11 (32.4) |
| >4 | 7 (20.6) |
| Unknown | 3 (8.8) |
| Therapy | No. (%) |
|---|---|
| Surgery alone | 2 (5.9) |
| Systemic therapy (chemotherapy/targeted therapy) alone | 4 (11.8) |
| Chemotherapy (CTx) | 2 (5.9) |
| Targeted therapy | 2 (5.9) |
| Whole-brain radiation therapy (WBRT) alone | 8 (23.6) |
| Surgery + WBRT | 0 |
| Surgery + systemic therapy | 1 (2.9) |
| Surgery + systemic therapy + WBRT | 6 (17.6) |
| WBRT + systemic therapy | 8 (23.6) |
| Stereotactic radiosurgery (+surgery, WBRT, CTx) Exclusive palliative therapy | 2 (5.9) 5 (14.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkali, S.; Vini, L.; Stergioula, A.; Kyriazoglou, A.; Vassos, N.; Boukovinas, I. Brain Metastases from Adult Sarcomas: A Retrospective Cohort Study from the Hellenic Group of Sarcomas and Rare Cancers (HGSRC). J. Clin. Med. 2021, 10, 5978. https://doi.org/10.3390/jcm10245978
Kokkali S, Vini L, Stergioula A, Kyriazoglou A, Vassos N, Boukovinas I. Brain Metastases from Adult Sarcomas: A Retrospective Cohort Study from the Hellenic Group of Sarcomas and Rare Cancers (HGSRC). Journal of Clinical Medicine. 2021; 10(24):5978. https://doi.org/10.3390/jcm10245978
Chicago/Turabian StyleKokkali, Stefania, Louiza Vini, Anastasia Stergioula, Anastasios Kyriazoglou, Nikolaos Vassos, and Ioannis Boukovinas. 2021. "Brain Metastases from Adult Sarcomas: A Retrospective Cohort Study from the Hellenic Group of Sarcomas and Rare Cancers (HGSRC)" Journal of Clinical Medicine 10, no. 24: 5978. https://doi.org/10.3390/jcm10245978
APA StyleKokkali, S., Vini, L., Stergioula, A., Kyriazoglou, A., Vassos, N., & Boukovinas, I. (2021). Brain Metastases from Adult Sarcomas: A Retrospective Cohort Study from the Hellenic Group of Sarcomas and Rare Cancers (HGSRC). Journal of Clinical Medicine, 10(24), 5978. https://doi.org/10.3390/jcm10245978








