Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review
Abstract
:1. Introduction
2. Imaging Overview
3. TMVR: ViV, ViR, ViMAC
3.1. Procedural Description
3.2. Clinical Results and Published Evidence
3.3. Imaging Key Aspects
4. Valve in Native Mitral Valve Replacement
4.1. Procedural Description and Imaging Key Aspects
4.2. Clinical Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MR | mitral regurgitation |
| TEER | Transcatheter edge-to-edge mitral valve repair |
| TMVR | Transcatheter mitral valve replacement |
| ViV | Valve-in-valve |
| ViR | Valve-in-ring |
| ViMAC | Valve-in-mitral annulus calcification |
| LVOT | Left ventricular outflow tract |
| TTE | transthoracic echocardiography |
| TEE | transesophageal echocardiography |
| CCT | cardiac computed tomography |
| PVL | Paravalvular leak closure |
| LAMPOON | Intentional laceration of the anterior mitral leaflet to prevent LVOT obstruction |
| THV | transcatheter heart valve |
| PET | polyethylene terephthalate |
| SHV | surgical heart valve |
References
- Cahill, T.J.; Prothero, A.; Wilson, J.; Kennedy, A.; Brubert, J.; Masters, M.; Newton, J.D.; Dawkins, S.; Enriquez-Sarano, M.; Prendergast, B.D.; et al. Community prevalence, mechanisms and outcome of mitral or tricuspid regurgitation. Heart 2021, 107, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ielasi, A.; Oppizzi, M.; Faggiano, P.; Ferri, L.; Calabrese, A.; Vizzardi, E.; Alfieri, O.; Margonato, A. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 60, 727–800. [Google Scholar]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.-L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Sorajja, P.; Cavalcante, J.L.; Gössl, M. The need for transcatheter mitralvalve replacement. J. Am. Coll. Cardiol. 2019, 73, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Foster, E.; Glower, D.D.; Glower, D.G.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; et al. EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beigel, R.; Wunderlich, N.C.; Kar, S.; Siegel, R.J. The evolution of percutaneous mitral valve repair therapy: Lessons learned and implications for patient selection. J. Am. Coll. Cardiol. 2014, 64, 2688–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawish, E.; Schmidt, T.; Eitel, I.; Frerker, C. Current status of catheter-based mitral valve replacement. Curr. Cardiol. Rep. 2021, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Murdoch, D.J.; Boone, R.H.; Moss, R.; Attinger-Toller, A.; Blanke, P.; Cheung, A.; Hensey, M.; Leipsic, J.; Ong, K.; et al. Percutaneous transcatheter mitral valve replacement. J. Am. Coll. Cardiol. 2019, 73, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sayan, E.; Chen, T.; Khalique, O.K. Multimodality cardiac imaging for procedural planning and guidance of transcatheter mitral valve replacement and mitral paravalvular leak closure. Front. Cardiovasc. Med. 2021, 8, 582925. [Google Scholar] [CrossRef] [PubMed]
- Kargoli, F.; Pagnesi, M.; Rahgozar, K.; Goldberg, Y.; Ho, E.; Chau, M.; Colombo, A.; Latib, A. Current devices and complications related to transcatheter mitral valve replacement: The bumpy road to the top. Front. Cardiovasc. Med. 2021, 8, 639058. [Google Scholar] [CrossRef] [PubMed]
- Alperi, A.; Granada, J.F.; Bernier, M.; Dagenais, F.; Rodés-Cabau, J. Current status and future prospects of transcatheter mitral valve replacement: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 3058–3078. [Google Scholar] [CrossRef]
- Niikura, H.; Gössl, M.; Kshettry, V.; Olson, S.; Sun, B.; Askew, J.; Stanberry, L.; Garberich, R.; Tang, L.; Lesser, J.; et al. Causes and clinical outcomes of patients who are ineligible for transcatheter mitral valve replacement. JACC Cardiovasc. Interv. 2019, 12, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Urena, M.; Himbert, D.; Brochet, E.; Carrasco, J.L.; Iung, B.; Nataf, P.; Vahanian, A. Transseptal Transcatheter Mitral Valve Replacement Using Balloon-Expandable Transcatheter Heart Valves: A Step-by-Step Approach. JACC Cardiovasc. Interv. 2017, 10, 1905–1919. [Google Scholar] [CrossRef]
- Little, S.H.; Bapat, V.; Blanke, P.; Guerrero, M.; Rajagopal, V.; Siegel, R. Imaging Guidance for Transcatheter Mitral Valve Intervention on Prosthetic Valves, Rings, and Annular Calcification. JACC Cardiovasc. Imaging 2021, 14, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Harloff, M.T.; Chowdhury, M.; Hirji, S.A.; Percy, E.D.; Yazdchi, F.; Shim, H.; Malarczyk, A.A.; Sobieszczyk, P.S.; Sabe, A.A.; Shah, P.B.; et al. A step-by-step guide to transseptal valve-in-valve transcatheter mitral valve replacement. Ann. Cardiothorac. Surg. 2021, 10, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pulerwitz, T.C.; Khalique, O.K.; Leb, J.; Hahn, R.T.; Nazif, T.; Leon, M.B.; George, I.; Vahl, T.P.; D’Souza, B.; Bapat, V.N.; et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc. Imaging 2020, 13, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Ben Zekry, S.; Turaga, M.; Tarazi, S.; Bax, J.J.; Wang, D.D.; Piazza, N.; Bapat, V.N.; Ihdayhid, A.R.; Cavalcante, J.L.; et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc. Imaging 2021, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Babaliaros, V.C.; Lederman, R.J.; Gleason, P.T.; Khan, J.M.; Kohli, K.; Sahu, A.; Rogers, T.; Bruce, C.G.; Paone, G.; Xie, J.X.; et al. The Art of SAPIEN 3 Transcatheter Mitral Valve Replacemente in Valve-in-Ring and Valve-in-Mitral-Annular-Calcification Procedures. Cardiovasc. Interv. 2021, 14, 2195–2214. [Google Scholar]
- Guerrero, M.; Dvir, D.; Himbert, D.; Urena, M.; Eleid, M.; Wang, D.D.; Greenbaum, A.; Mahadevan, V.S.; Holzhey, D.; O’Hair, D.; et al. Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease with Severe Mitral Annular Calcification: Results from the First Multicenter Global Registry. JACC Cardiovasc. Interv. 2016, 9, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Case, B.C.; Khan, J.M.; Satler, L.F.; Ben-Dor, I.; Lederman, R.; Babaliaros, V.C.; Greenbaum, A.B.; Waksman, R.; Rogers, T. Tip-to-Base LAMPOON to Prevent Left Ventricular Outflow Tract Obstruction in Valve-in-Valve Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Lisko, J.C.; Greenbaum, A.B.; Khan, J.M.; Kamioka, N.; Gleason, P.T.; Byku, I.; Condado, J.F.; Jadue, A.; Paone, G.; Grubb, K.J.; et al. Antegrade Intentional Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction: A Simplified Technique From Bench to Bedside. Circ. Cardiovasc. Interv. 2020, 13, e008903. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Taramasso, M.; Gökhan, G.; Maisano, F.; Mestres, C.A. Mitral anular calcification: Challenges and future perspectives. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Urena, M.; Vahanian, A.; Brochet, E.; Ducrocq, G.; Lung, B.; Himbert, D. Current Indications for Transcatheter Mitral Valve Replacement Using Transcatheter Aortic Valves. Circulation 2021, 143, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Salinger, M.; Pursnani, A.; Pearson, P.; Lampert, M.; Levisay, J.; Russell, H.; Feldman, T. Transseptal transcatheter mitral valve-in-valve: A step by step guide from preprocedural planning to postprocedural care. Catheter. Cardiovasc. Interv. 2018, 92, E185–E196. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Spillemaeker, H.; Kyheng, M.; Belin-Vincent, C.; Delhaye, C.; Piérache, A.; Denimal, T.; Verdier, B.; Debry, N.; Moussa, M.; et al. Ultrasound Guidance to Reduce Vascular and Bleeding Complications of Percutaneous Transfemoral Transcatheter Aortic Valve Replacement: A Propensity Score-Matched Comparison. J. Am. Heart Assoc. 2020, 9, e014916. [Google Scholar] [CrossRef]
- Alkhouli, M.; Rihal, C.S.; Holmes, D.R., Jr. Transseptal techniques for emerging structural heart interventions. J. Am. Coll. Cardiol. Interv. 2016, 9, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Bleiziffer, S.; Latib, A.; Eschenbach, L.; Ancona, M.; Vincent, F.; Kim, W.-K.; Unbehaum, A.; Asami, M.; Dhoble, A.; et al. Predictors of Left Ventricular Outflow Tract Obstruction after Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 182–193. [Google Scholar] [CrossRef]
- Yoon, S.H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprothesis, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, M.; Urena, M.; Himbert, D.; Wang, D.D.; Eleid, M.; Kodali, S.; George, I.; Chakravarty, T.; Mathur, M.; Holzhey, D.; et al. 1-Year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J. Am. Coll. Cardiol. 2018, 71, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Wang, D.D.; Eleid, M.F.; Pursnani, A.; Salinger, M.; Russell, H.M.; Kodali, S.K.; George, I.; Bapat, V.N.; Dangas, G.D.; et al. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC. MITRAL Trial 1-Year Outcomes. Cardiovasc. Interv. 2021, 14, 830–845. [Google Scholar]
- Blanke, P.; Dvir, D.; Cheung, A.; Ye, J.; Levine, R.A.; Precious, B.; Berger, A.; Stub, D.; Hague, C.; Murphy, D.; et al. A simplified Dshaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, M.; Wang, D.D.; Pursnani, A.; Eleid, M.; Khalique, O.; Urena, M.; Salinger, M.; Kodali, S.; Kaptzan, T.; Lewis, B.; et al. A cardiac computed tomography-based score to categorize mitral annular calcification severity and predict valve embolization. Cardiovasc. Imaging 2020, 13, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Gössl, M.; Babaliaros, V.; Rizik, D.; Conradi, L.; Bae, R.; Burke, R.F.; Schäfer, U.; Lisko, J.C.; Riley, R.D.; et al. Novel Transcatheter Mitral Valve Prothesis for Patients With Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2019, 74, 1431–1440. [Google Scholar] [CrossRef]
- Bapat, V.N.; Attia, R.; Thomas, M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: A concept of true internal diameter and its implications for the valve-invalve procedure. JACC Cardiovasc. Interv. 2014, 7, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Play.google.com. Mitral Valve-in-Valve APP. 2021. Available online: https://play.google.com/store/apps/details?id=com.ubqo.vivmitral&hl=es_AR&gl=US (accessed on 10 December 2021).
- App Store. Valve in Valve (Mitral). 2021. Available online: https://apps.apple.com/es/app/valve-in-valve-mitral/id703369667 (accessed on 10 December 2021).
- El Sabbagh, A.; Al-Hijji, M.; Wang, D.D.; Eleid, M.; Urena, M.; Himbert, D.; Chakravarty, T.; Holzhey, D.; Pershad, A.; Fang, H.K.; et al. Predictors of Left Ventricular Outflow Tract Obstruction after Transcatheter Mitral Valve Replacement in Severe Mitral Annular Calcification: An Analysis of the Transcatheter Mitral Valve Replacement in Mitral Annular Calcification Global Registry. Circ. Cardiovasc. Interv. 2021, 14, e010854. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Gennari, M.; Gavazzoni, M.; Pedicino, D.; Pozzoli, A.; Taramasso, M.; Maisano, F. Transcatheter Mitral Valve Implantation: Current Status and Future Perspectives. Circ. Cardiovasc. Interv. 2021, 14, e010628. [Google Scholar] [CrossRef] [PubMed]
- Hensey, M.; Brown, R.A.; Lal, S.; Sathananthan, J.; Ye, J.; Cheung, A.; Blanke, P.; Leipsic, J.; Moss, R.; Boone, R.; et al. Transcatheter Mitral Valve Replacement: An Update on Current Techniques, Technologies, and Future Directions. JACC Cardiovasc. Interv. 2021, 14, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Lutter, G.; Lozonschi, L.; Ebner, A.; Gallo, S.; Kall, C.M.Y.; Missov, E.; de Marchena, E. First-in-human off-pump transcatheter mitral valve replacement. JACC Cardiovasc. Interv. 2014, 7, 1077–1078. [Google Scholar] [CrossRef]
- Duncan, A.; Daqa, A.; Yeh, J.; Davies, S.; Uebing, A.; Quarto, C.; Moat, N.; Alison, D.; Anan, D.; James, Y.; et al. Transcatheter mitral valve replacement: Long-term outcomes of first-in-man experience with an apically tethered device—A case series from a single centre. EuroIntervention 2017, 13, e1047–e1057. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial feasibility study of a new transcatheter mitral prosthesis: The first 100 patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Lisko, J.C.; Greenbaum, A.B.; Guyton, R.A.; Kamioka, N.; Grubb, K.J.; Gleason, P.T.; Byku, I.; Condado, J.F.; Jadue, A.; Paone, G.; et al. Electrosurgical Detachment of MitraClips From the Anterior Mitral Leaflet Prior to Transcatheter Mitral Valve Implantation. JACC Cardiovasc. Interv. 2020, 13, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Zahr, F.; Song, H.K.; Chadderdon, S.M.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Bajwa, T.; Weiss, E.; Kodali, S.; et al. Thirty-Day Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc. Interv. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
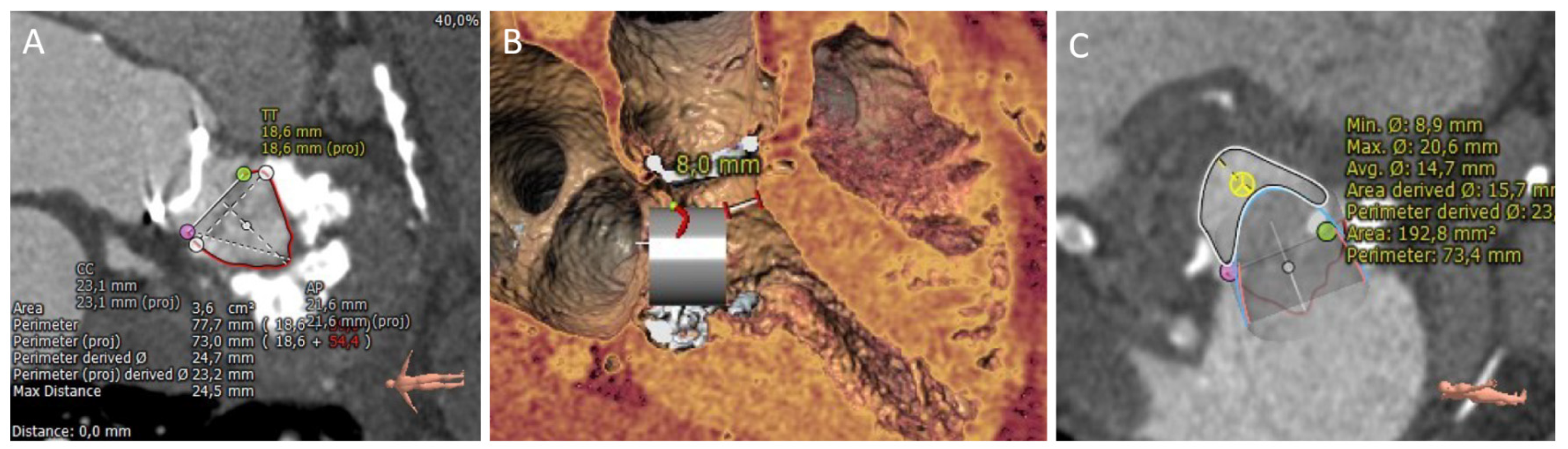
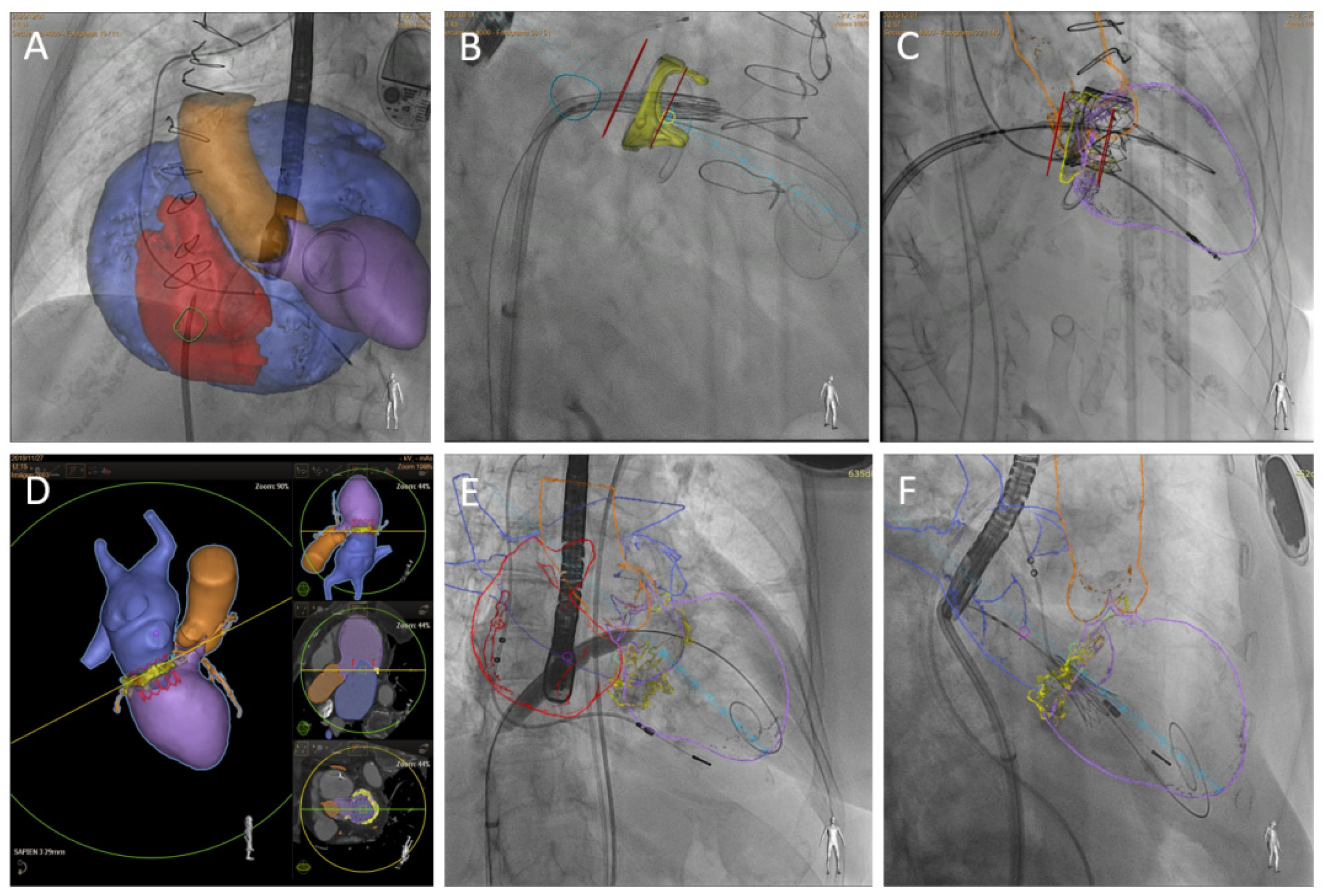
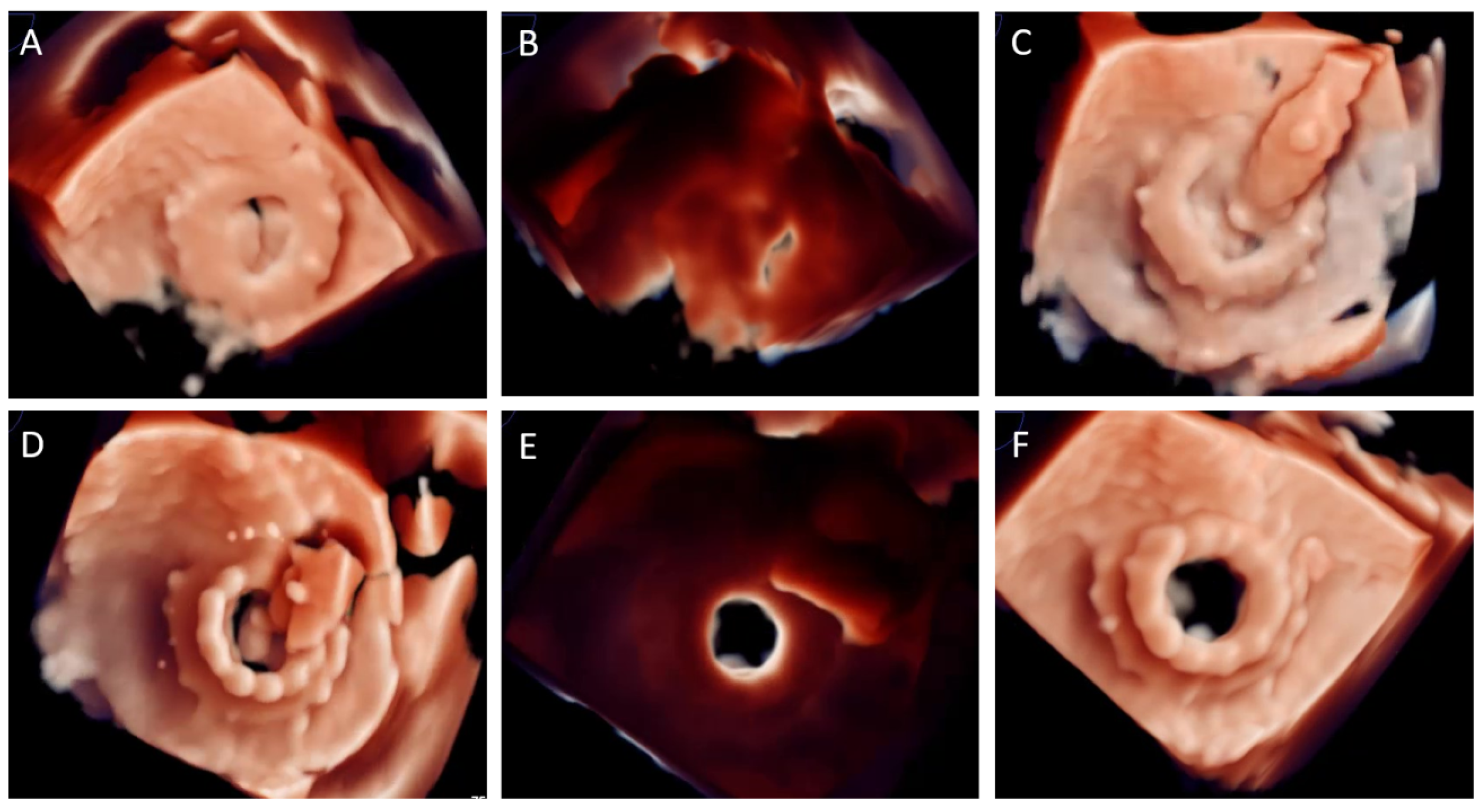
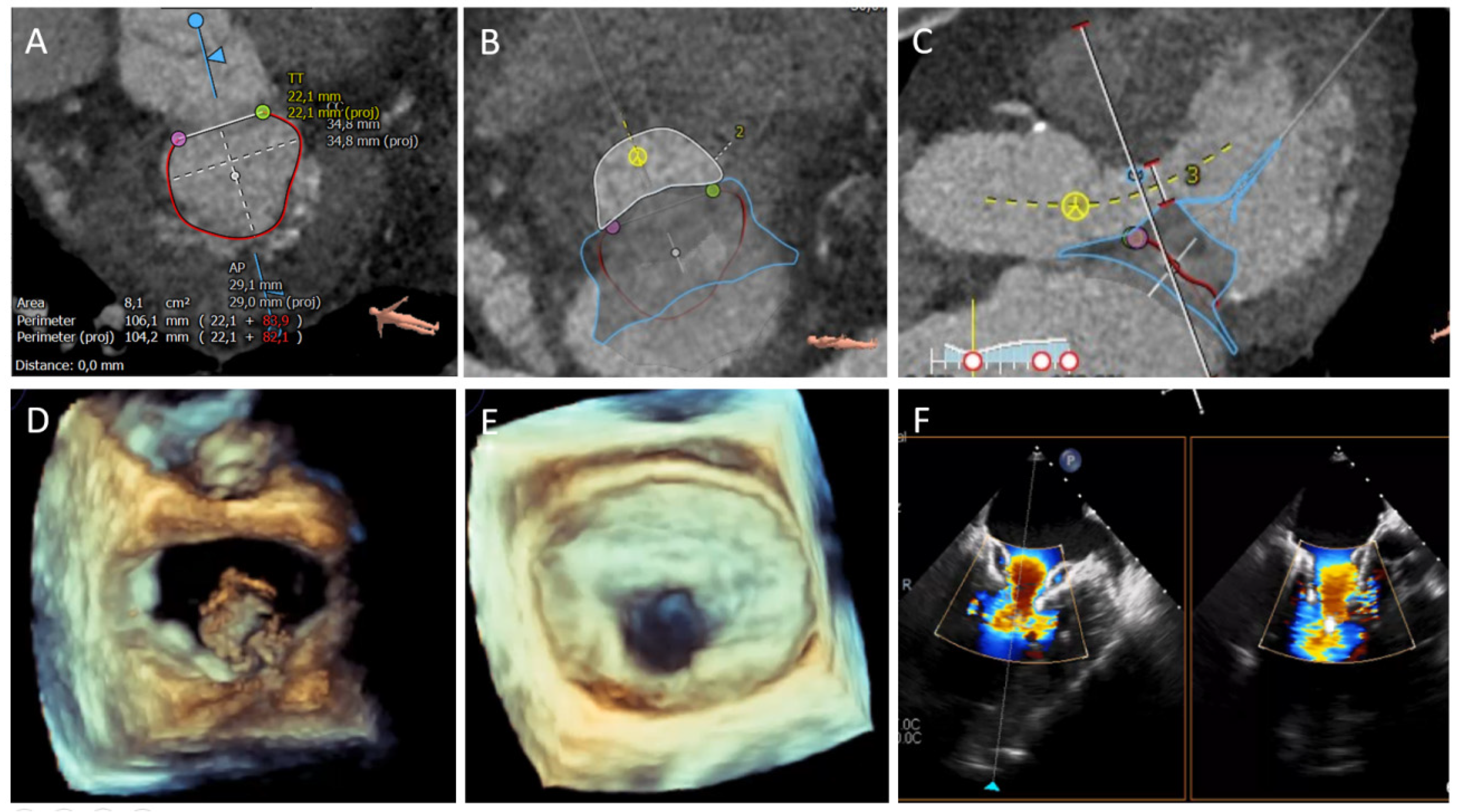
| Assessment Steps | TEE | CT |
|---|---|---|
| Screening | ||
| Valve disease mechanism | +++ | + |
| Chambers size | +++ | ++ |
| LV/RV function and pulmonary hypertension | +++ | + |
| Valve disease grading | +++ | - |
| Calcification extension | + | +++ |
| Contra-indications assessment | ||
| Endocarditis | +++ | + |
| Thrombus | +++ | +++ |
| Severe patient-prosthesis mismatch | +++ | - |
| Peri-intervention | ||
| Vascular access | - | +++ |
| Annulus sizing | ++ | +++ |
| Fusion imaging | ++ | +++ |
| Interatrial septum assessment/transeptal punction planning | +++ | +++ |
| Fluoroscopic projection estimation | - | +++ |
| Neo-LVOT size estimation | + | +++ |
| 3D simulation/printing | + | +++ |
| Procedural guidance/Device deployment | +++ | - |
| Post-procedural | ||
| Prosthetic valve function | +++ | + |
| Paravalvular leak | +++ | ++ |
| Vascular complications | - | +++ |
| Device | Intrepid | Tendyne | Tiara | EVOQUE | HighLife | SAPIEN M3 |
|---|---|---|---|---|---|---|
| Patients, n | 50 | 109 | 79 | 14 | 15 | 45 |
| Etiology of MR | ||||||
| Organic | 16 | 11 | 8.9 | 28.6 | 27 | 55.6 |
| Functional | 72 | 89 | 62 | 21.4 | 73 | 35.6 |
| Mixed | 12 | 29.1 | 50 | 8.9 | ||
| LVEF, % | 43 ± 12 | 47.2 | 37 ± 9 | 54 | 38 | 44 |
| Approach | TA | TA | TA | TF | TA | TF |
| Device implant success | 98 | 97.2 | 92.4 | 92.9 | 72.7 | 88.9 |
| 30-day mortality | 14 (n = 7) | 5.5 (n = 6) | 11.3 (n = 8) | 7.1 (n = 1) | 20 (n = 3) | 2.2 (n = 1) |
| Residual MR | ||||||
| None/mild | 100 | 99 | 92.5 | 93 | 100 | 92.7 |
| Moderate/severe | 0 | 1 | 7.5 | 7 | 0 | 7.3 |
| LVOT obstruction | 0 | 0 | 0 | 7.1 (n = 1) | 6.6 (n = 1) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Perez, M.; Caneiro-Queija, B.; Puga, L.; Gonzalez-Ferreiro, R.; Alarcon, R.; Parada, J.A.; Iñiguez-Romo, A.; Estevez-Loureiro, R. Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review. J. Clin. Med. 2021, 10, 5973. https://doi.org/10.3390/jcm10245973
Barreiro-Perez M, Caneiro-Queija B, Puga L, Gonzalez-Ferreiro R, Alarcon R, Parada JA, Iñiguez-Romo A, Estevez-Loureiro R. Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review. Journal of Clinical Medicine. 2021; 10(24):5973. https://doi.org/10.3390/jcm10245973
Chicago/Turabian StyleBarreiro-Perez, Manuel, Berenice Caneiro-Queija, Luis Puga, Rocío Gonzalez-Ferreiro, Robert Alarcon, Jose Antonio Parada, Andrés Iñiguez-Romo, and Rodrigo Estevez-Loureiro. 2021. "Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review" Journal of Clinical Medicine 10, no. 24: 5973. https://doi.org/10.3390/jcm10245973
APA StyleBarreiro-Perez, M., Caneiro-Queija, B., Puga, L., Gonzalez-Ferreiro, R., Alarcon, R., Parada, J. A., Iñiguez-Romo, A., & Estevez-Loureiro, R. (2021). Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review. Journal of Clinical Medicine, 10(24), 5973. https://doi.org/10.3390/jcm10245973






