Surgical Management of Upper Urinary Tract Urothelial Cell Carcinoma with Venous Tumor Thrombus: A Liver Transplant-Based Approach
Abstract
1. Introduction
2. Materials and Methods
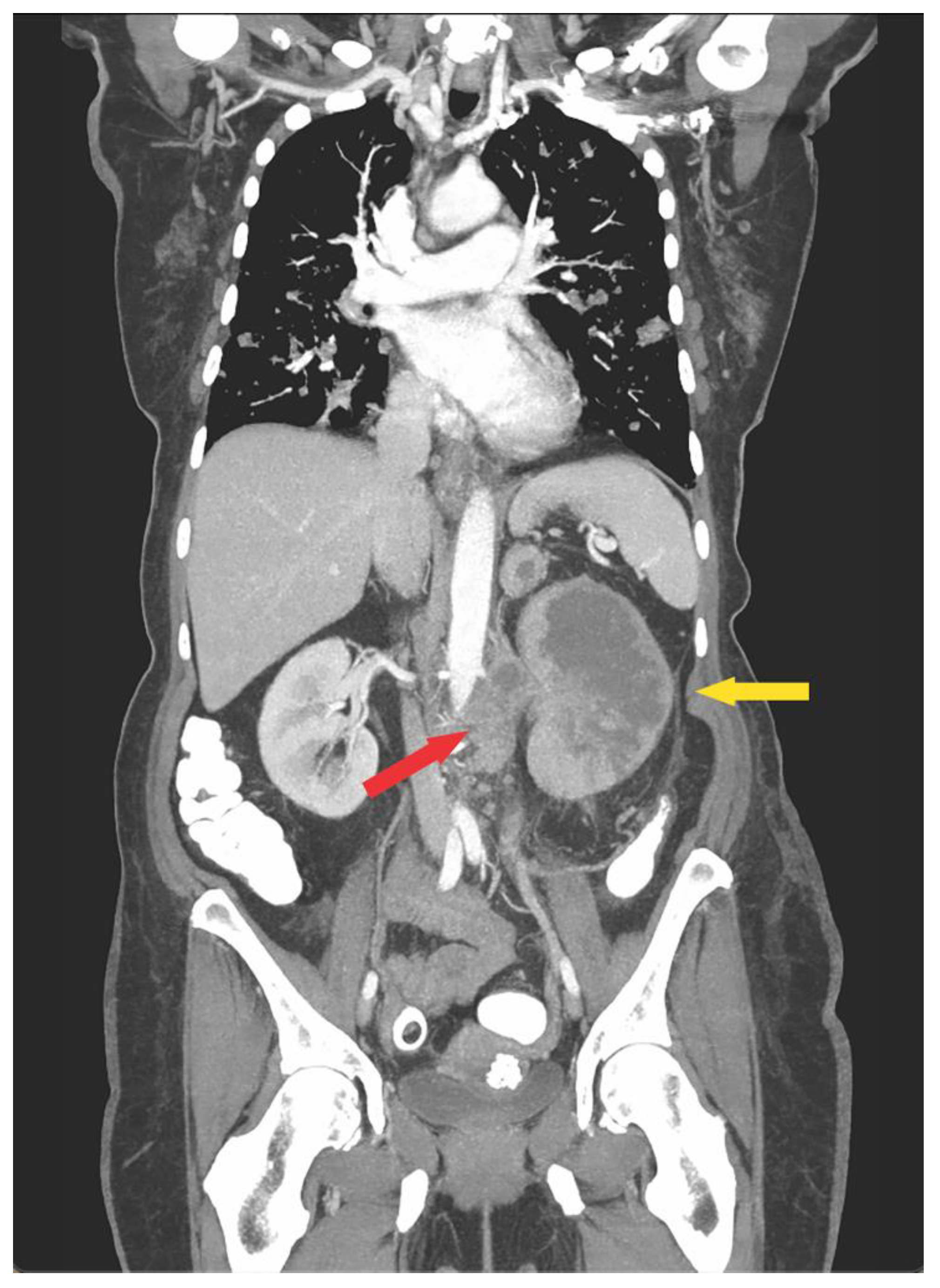
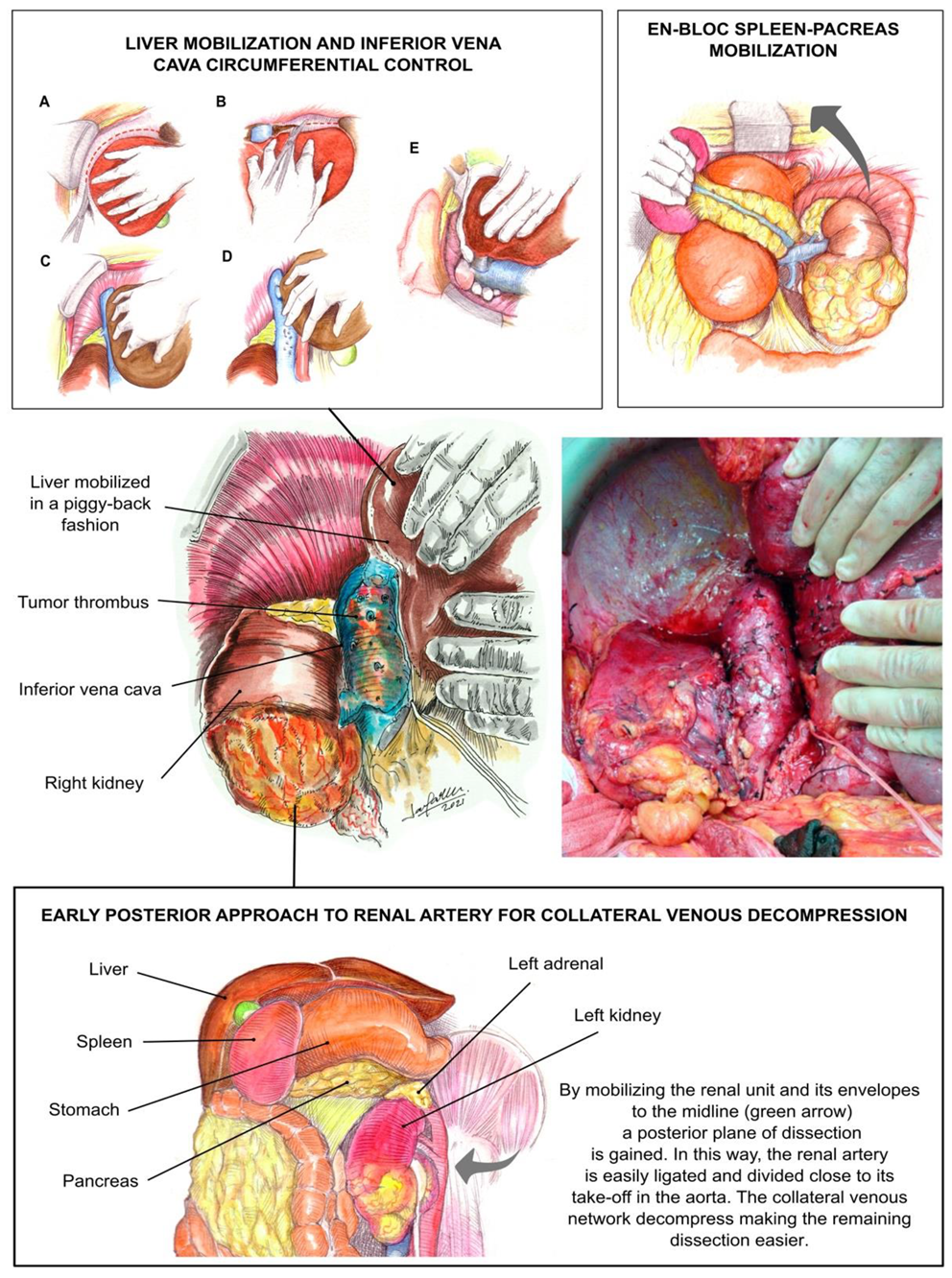
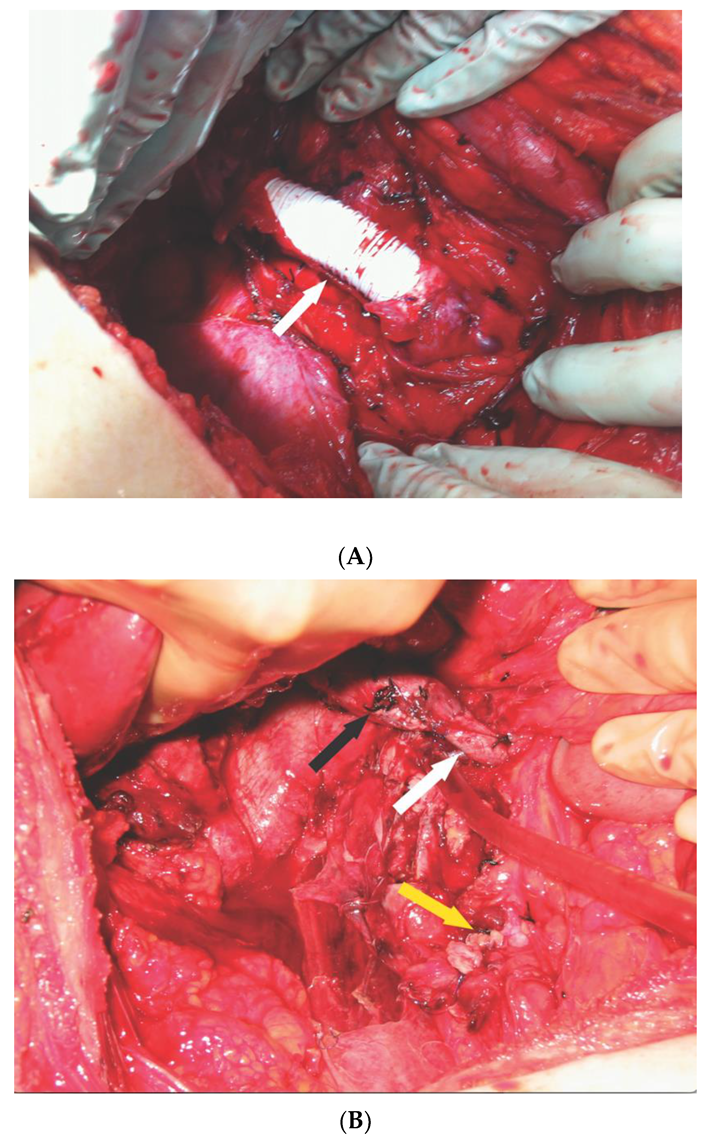
2.1. Operative Technique
2.2. Chemotherapy
2.3. Intraoperative and Postoperative Variables
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González, J.; Gaynor, J.J.; Martínez-Salamanca, J.I.; Capitanio, U.; Tilki, D.; Carballido, J.A.; Chantada, V.; Daneshmand, S.; Evans, C.P.; Gasch, C.; et al. Association of an organ transplant-based approach with a dramatic reduction in postoperative complications following radical nephrectomy and tumor thrombectomy in renal cell carcinoma. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 1983–1992. [Google Scholar] [CrossRef]
- Tian, X.; Hong, P.; Tang, S.; Liu, Z.; Yang, F.; Zhang, S.; Wang, G.; He, H.; Ma, L. Urothelial carcinoma of the renal pelvis with renal vein and inferior vena cava tumor thrombus: Case series and literature review. Transl. Androl. Urol. 2021, 10, 2879–2888. [Google Scholar] [CrossRef]
- Baard, J.; Cormio, L.; Cavadas, V.; Alcaraz, A.; Shariat, S.F.; de la Rosette, J.; Laguna, M.P. Contemporary patterns of presentation, diagnostics and management of upper tract urothelial cancer in 101 centres: The Clinical Research Office of the Endourological Society Global upper tract urothelial carcinoma registry. Curr. Opin. Urol. 2021, 31, 354–362. [Google Scholar] [CrossRef]
- Huber, J.; Teber, D.; Hatiboglu, G.; Popeneciu, V.; Jakobi, H.; Hallscheidt, P.; Pahernik, S.; Hohenfellner, M. Does a venous tumor thrombus exclude renal transitional cell carcinoma? Implications for neo-adjuvant treatment strategies. Anticancer Res. 2014, 34, 1031–1035. [Google Scholar]
- Ciancio, G.; Livingstone, A.S.; Soloway, M. Surgical Management of Renal Cell Carcinoma with Tumor Thrombus in the Renal and Inferior Vena Cava: The University of Miami Experience in Using Liver Transplantation Techniques. Eur. Urol. 2007, 51, 988–995. [Google Scholar] [CrossRef]
- Cerwinka, W.H.; Manoharan, M.; Soloway, M.S.; Ciancio, G. The role of liver transplantation techniques in the surgical management of advanced renal urothelial carcinoma with or without inferior vena cava thrombus. Int. Braz. J. Urol 2009, 35, 19–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciancio, G.; Manoharan, M.; Katkoori, D.; Santos, R.D.L.; Soloway, M.S. Long-term Survival in Patients Undergoing Radical Nephrectomy and Inferior Vena Cava Thrombectomy: Single-Center Experience. Eur. Urol. 2010, 57, 667–672. [Google Scholar] [CrossRef]
- Ciancio, G.; Vaidya, A.; Shirodkar, S.; Manoharan, M.; Hakky, T.; Soloway, M. En Bloc Mobilization of the Pancreas and Spleen to Facilitate Resection of Large Tumors, Primarily Renal and Adrenal, in the Left Upper Quadrant of the Abdomen: Techniques Derived from Multivisceral Transplantation. Eur. Urol. 2009, 55, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.; Zincke, H. Surgical Treatment of Renal Cancer with Vena Cava Extension. BJU Int. 1987, 59, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Vaidya, A.; Savoie, M.; Soloway, M. Management of renal cell carcinoma with level III thrombus in the inferior vena cava. J. Urol. 2002, 168 Pt 1, 1374–1377. [Google Scholar] [CrossRef]
- Fukazawa, K.; Gologorsky, E.; Naguit, K.; Pretto, E.A.; Salerno, T.A.; Arianayagam, M.; Silverman, R.; Barron, M.E.; Ciancio, G. Invasive Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus: Cardiac Anesthesia in Liver Transplant Settings. J. Cardiothorac. Vasc. Anesth. 2014, 28, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.T.; Butterfield, M.; Fisher, J.; Torrent, D.; Bloomston, M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am. J. Cancer Res. 2013, 3, 518–522. [Google Scholar]
- Ciancio, G.; Gonzalez, J.; Shirodkar, S.P.; Angulo, J.; Soloway, M.S. Liver Transplantation Techniques for the Surgical Management of Renal Cell Carcinoma with Tumor Thrombus in the Inferior Vena Cava: Step-by-Step Description. Eur. Urol. 2011, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Vaidya, A.; Soloway, M. Early ligation of the renal artery using the posterior approach: A basic surgical concept reinforced during resection of large hypervascular renal cell carcinoma with or without inferior vena cava thrombus. BJU Int. 2003, 92, 488–489. [Google Scholar] [CrossRef]
- Tzakis, A.; Todo, S.; Starzl, T.E. Orthotopic Liver Transplantation with Preservation of the Inferior Vena Cava. Ann. Surg. 1989, 210, 649–652. [Google Scholar] [CrossRef]
- Brown, G.A.; Busby, J.E.; Wood, C.G.; Pisters, L.L.; Dinney, C.P.; Swanson, D.A.; Grossman, H.B.; Pettaway, C.A.; Munsell, M.F.; Kamat, A.M.; et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: Time to change the treatment paradigm? BJU Int. 2006, 98, 1176–1180. [Google Scholar] [CrossRef]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Territo, A.; Gallioli, A.; Meneghetti, I.; Fontana, M.; Huguet, J.; Palou, J.; Breda, A. Diagnostic ureteroscopy for upper tract urothelial carcinoma: Friend or foe? Arab. J. Urol. 2021, 19, 46–58. [Google Scholar] [CrossRef]
- Herr, H.W. Extravesical tumor relapse in patients with superficial bladder tumors. J. Clin. Oncol. 1998, 16, 1099–1102. [Google Scholar] [CrossRef]
- Dong, F.; Fu, H.; Shi, X.; Shen, Y.; Xu, T.; Gao, F.; Wang, X.; Zhong, S.; Ding, Q.; Shen, Z.; et al. How do organ-specific metastases affect prognosis and surgical treatment for patients with metastatic upper tract urothelial carcinoma: First evidence from population based data. Clin. Exp. Metastasis 2017, 34, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hashimoto, Y.; Kobayashi, H.; Iizuka, J.; Nakazawa, H.; Ito, F.; Tanabe, K. Template-based lymphadenectomy in urothelial carcinoma of the upper urinary tract: Impact on patient survival. Int. J. Urol. 2010, 17, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G. Inferior Vena Cava Reconstruction Using a Ringed Polytetrafluoroethylene Interposition Graft and Inferior Vena Cava Filter Placement Following Resection of Renal Cell Carcinoma With a Tumor Thrombus Directly Infiltrating the Inferior Vena Cava. Vasc. Endovasc. Surg. 2021, 56, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ayyathurai, R.; Garcia-Roig, M.; Gorin, M.A.; González, J.; Manoharan, M.; Kava, B.R.; Soloway, M.S.; Ciancio, G. Bland thrombus association with tumour thrombus in renal cell carcinoma: Analysis of surgical significance and role of inferior vena caval interruption. BJU Int. 2012, 110 Pt B, E449–E455. [Google Scholar] [CrossRef] [PubMed]
- Psutka, S.P.; Leibovich, B.C. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther. Adv. Urol. 2015, 7, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Hatakeyama, S.; Tanaka, T.; Fujita, N.; Iwamura, H.; Mikami, J.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: A multicenter study. Oncotarget 2017, 8, 101500–101508. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in Renal Function Following Nephroureterectomy May Affect the Use of Perioperative Chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lucca, I.; Leow, J.J.; Shariat, S.F.; Chang, S.L. Diagnosis and Management of Upper Tract Urothelial Carcinoma. Hematol. Clin. North Am. 2015, 29, 271–288. [Google Scholar] [CrossRef]
- Crona, D.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Yonou, H.; Sugaya, K.; Koyama, Y.; Hatano, T.; Ogawa, Y. Transitional cell carcinoma of the renal pelvis forming tumor thrombus in the vena cava. Int. J. Urol. 2001, 8, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; George, A.J.P.; Singh, J.C.; Devasia, A. Transitional cell carcinoma of the renal pelvis with venous tumor thrombus. Rev. Urol. 2017, 19, 145–148. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciancio, G.; Tabbara, M.M.; Martucci, M.; Gaynor, J.J.; Morsi, M.; Gonzalez, J. Surgical Management of Upper Urinary Tract Urothelial Cell Carcinoma with Venous Tumor Thrombus: A Liver Transplant-Based Approach. J. Clin. Med. 2021, 10, 5964. https://doi.org/10.3390/jcm10245964
Ciancio G, Tabbara MM, Martucci M, Gaynor JJ, Morsi M, Gonzalez J. Surgical Management of Upper Urinary Tract Urothelial Cell Carcinoma with Venous Tumor Thrombus: A Liver Transplant-Based Approach. Journal of Clinical Medicine. 2021; 10(24):5964. https://doi.org/10.3390/jcm10245964
Chicago/Turabian StyleCiancio, Gaetano, Marina M. Tabbara, Melanie Martucci, Jeffrey J. Gaynor, Mahmoud Morsi, and Javier Gonzalez. 2021. "Surgical Management of Upper Urinary Tract Urothelial Cell Carcinoma with Venous Tumor Thrombus: A Liver Transplant-Based Approach" Journal of Clinical Medicine 10, no. 24: 5964. https://doi.org/10.3390/jcm10245964
APA StyleCiancio, G., Tabbara, M. M., Martucci, M., Gaynor, J. J., Morsi, M., & Gonzalez, J. (2021). Surgical Management of Upper Urinary Tract Urothelial Cell Carcinoma with Venous Tumor Thrombus: A Liver Transplant-Based Approach. Journal of Clinical Medicine, 10(24), 5964. https://doi.org/10.3390/jcm10245964





