A Simple Risk Score Based on Routine Clinical Parameters Can Predict Frailty in Hospitalized Heart Failure Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analyzed Parameters
2.3. Diagnosing Frailty Syndrome
2.4. Statistical Analysis and Model Development
3. Results
3.1. Baseline Characteristics
3.2. Frail and Non-Frail Subgroups
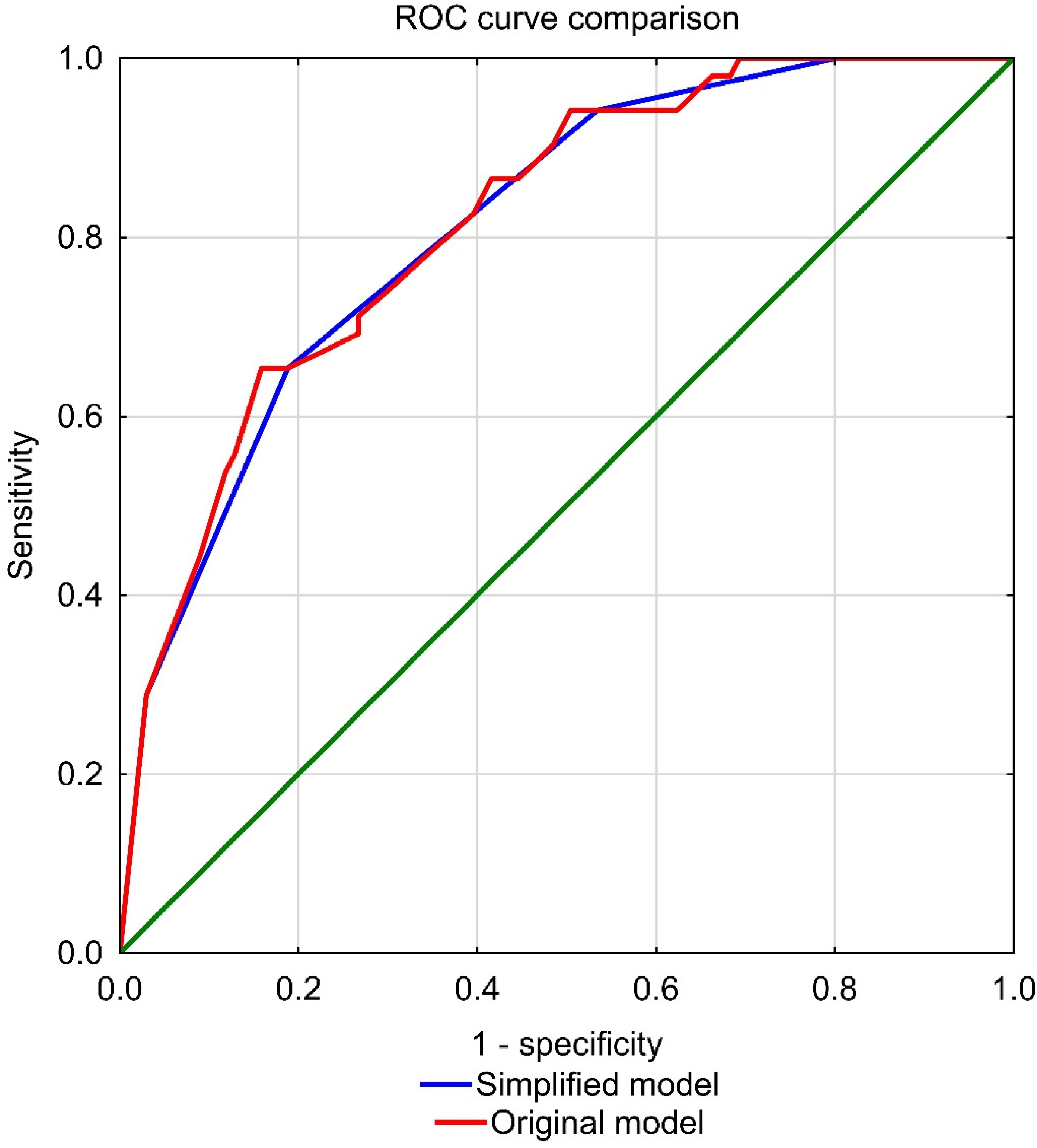
3.3. ROC Curve Analysis
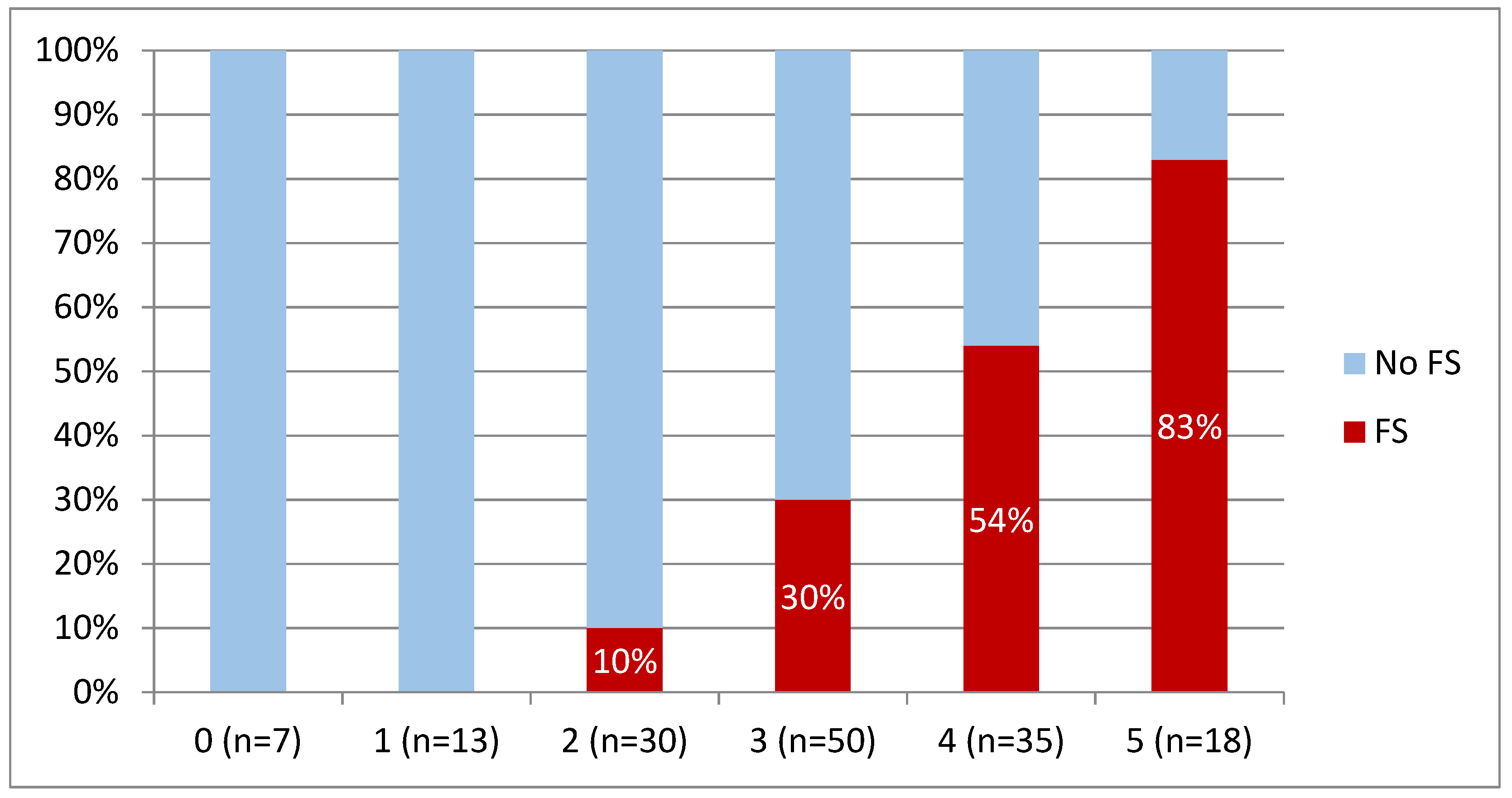
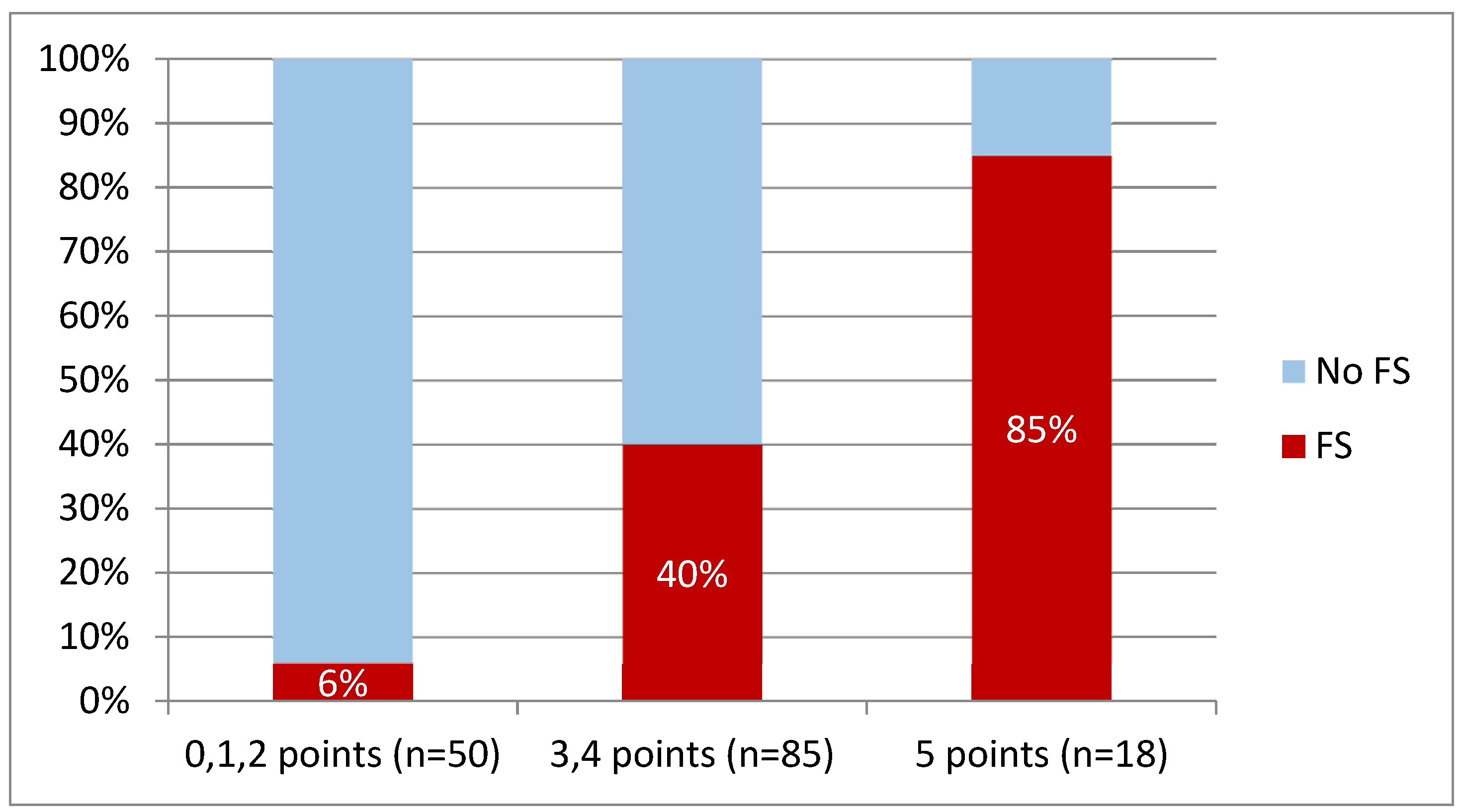
3.4. Regression Analysis and Model Development
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart Failure: Preventing Disease and Death Worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.U.; Tsai, V.; Klein, L.; Heidenreich, P.A. Characteristics and Outcomes of Very Elderly Patients after First Hospitalization for Heart Failure. Circ. Heart Fail. 2011, 4, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Bergman, H.; Ferrucci, L.; Guralnik, J.; Hogan, D.B.; Hummel, S.; Karunananthan, S.; Wolfson, C. Frailty: An Emerging Research and Clinical Paradigm--Issues and Controversies. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2007, 62, 731–737. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Galluzzo, L.; Rodríguez-Laso, Á.; van der Heyden, J.; Ranhoff, A.H.; Lamprini-Koula, M.; Ciutan, M.; Samaniego, L.L.; Carcaillon-Bentata, L.; Kennelly, S.; et al. Prevalence of Frailty at Population Level in European ADVANTAGE Joint Action Member States: A Systematic Review and Meta-Analysis. Ann. Dell’istituto Super. Sanita 2018, 54, 226–238. [Google Scholar] [CrossRef]
- Robinson, T.N.; Wu, D.S.; Stiegmann, G.v.; Moss, M. Frailty Predicts Increased Hospital and Six-Month Healthcare Cost Following Colorectal Surgery in Older Adults. Am. J. Surg. 2011, 202, 511–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchelli, A.; Vetrano, D.L.; Marengoni, A.; Grande, G.; Romanelli, G.; Calderón-Larrañaga, A.; Fratiglioni, L.; Rizzuto, D. Frailty Predicts Short-Term Survival Even in Older Adults without Multimorbidity. Eur. J. Intern. Med. 2018, 56, 53–56. [Google Scholar] [CrossRef]
- Veronese, N.; Sigeirsdottir, K.; Eiriksdottir, G.; Marques, E.A.; Chalhoub, D.; Phillips, C.L.; Launer, L.J.; Maggi, S.; Gudnason, V.; Harris, T.B. Frailty and Risk of Cardiovascular Diseases in Older Persons: The Age, Gene/Environment Susceptibility-Reykjavik Study. Rejuvenation Res. 2017, 20, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, J.; Martin, L.; Ferguson, C.; Jha, S.R.; Macdonald, P.S.; Davidson, P.M.; Newton, P.J. Frailty Assessment Instruments in Heart Failure: A Systematic Review. Eur. J. Cardiovasc. Nurs. J. Work. Group Cardiovasc. Nurs. Eur. Soc. Cardiol. 2018, 17, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Vitale, C.; Uchmanowicz, I. Frailty in Patients with Heart Failure. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2019, 21, L12. [Google Scholar] [CrossRef] [Green Version]
- Tse, G.; Gong, M.; Wong, S.H.; Wu, W.K.K.; Bazoukis, G.; Lampropoulos, K.; Wong, W.T.; Xia, Y.; Wong, M.C.S.; Liu, T.; et al. Frailty and Clinical Outcomes in Advanced Heart Failure Patients Undergoing Left Ventricular Assist Device Implantation: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2018, 19, 255–261.e1. [Google Scholar] [CrossRef]
- Uchmanowicz, I.; Kuśnierz, M.; Wleklik, M.; Jankowska-Polańska, B.; Jaroch, J.; Łoboz-Grudzień, K. Frailty Syndrome and Rehospitalizations in Elderly Heart Failure Patients. Aging Clin. Exp. Res. 2018, 30, 617–623. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart FailureDeveloped by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Hua Poi, P.J.; et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Walsh, C.D.; Lawlor, B.A.; Kenny, R.A. A Frailty Instrument for Primary Care: Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterstad, J.E. Measuring Left Ventricular Volume and Ejection Fraction with the Biplane Simpson’s Method. Heart 2002, 88, 559. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk-Nielsen, K.; Molholm Hansen, J.; Kampmann, J.; Kristensen, M. Rapid Evaluation of Creatinine Clearance. Lancet 1971, 1, 1133–1134. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461. [Google Scholar] [CrossRef]
- Muszalik, M.; Borowiak, E.; Kotarba, A.; Puto, G.; Doroszkiewicz, H.; Kędziora-Kornatowska, K. Adaptation and Reliability Testing of the Share-Fi Instrument for the Assessment of Risk of Frailty Syndrome among Older Polish Patients. Fam. Med. Prim. Care Rev. 2018, 20, 36–40. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Cuénoud, P.; Spagnoli, J.; Junod, J. Prevalence of Frailty in Middle-Aged and Older Community-Dwelling Europeans Living in 10 Countries. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, C.; Jankowska, E.; Hill, L.; Piepoli, M.; Doehner, W.; Anker, S.D.; Lainscak, M.; Jaarsma, T.; Ponikowski, P.; Rosano, G.M.C.; et al. Heart Failure Association/European Society of Cardiology Position Paper on Frailty in Patients with Heart Failure. Eur. J. Heart Fail. 2019, 21, 1299–1305. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The Prevalence of Frailty in Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Młynarska, A.; Lisiak, M.; Kałuzna-Oleksy, M.; Wleklik, M.; Chudiak, A.; Dudek, M.; Migaj, J.; Hinterbuchner, L.; Gobbens, R. Heart Failure and Problems with Frailty Syndrome: Why It Is Time to Care About Frailty Syndrome in Heart Failure. Card. Fail. Rev. 2019, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsue, Y.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Konishi, M.; Kitai, T.; Iwata, K.; Jujo, K.; et al. Prevalence and Prognostic Impact of the Coexistence of Multiple Frailty Domains in Elderly Patients with Heart Failure: The FRAGILE-HF Cohort Study. Eur. J. Heart Fail. 2020, 22, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.M.; Theou, O.; Mitnitski, A.; Howlett, S.E.; Rockwood, K. Associations between a Laboratory Frailty Index and Adverse Health Outcomes across Age and Sex. Aging Med. 2019, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwood, K.; Howlett, S.E.; MacKnight, C.; Beattie, B.L.; Bergman, H.; Hébert, R.; Hogan, D.B.; Wolfson, C.; McDowell, I. Prevalence, Attributes, and Outcomes of Fitness and Frailty in Community-Dwelling Older Adults: Report from the Canadian Study of Health and Aging. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2004, 59, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Panchangam, C.; White, D.A.; Goudar, S.; Birnbaum, B.; Malloy-Walton, L.; Gross-Toalson, J.; Reid, K.J.; Shirali, G.; Parthiban, A. Translation of the Frailty Paradigm from Older Adults to Children with Cardiac Disease. Pediatric Cardiol. 2020, 41, 1031–1041. [Google Scholar] [CrossRef]
- Sunaga, A.; Hikoso, S.; Yamada, T.; Yasumura, Y.; Uematsu, M.; Tamaki, S.; Abe, H.; Nakagawa, Y.; Higuchi, Y.; Fuji, H.; et al. Prognostic Impact of Clinical Frailty Scale in Patients with Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2021, 8, 3316. [Google Scholar] [CrossRef]
- Segar, M.W.; Singh, S.; Goyal, P.; Hummel, S.L.; Maurer, M.S.; Forman, D.E.; Butler, J.; Pandey, A. Prefrailty, Impairment in Physical Function, and Risk of Incident Heart Failure among Older Adults. J. Am. Geriatr. Soc. 2021, 69, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.C.; Lin, C.S.; Tarng, D.C.; Lin, S.Y. Physical Frailty and Long-Term Mortality in Older People with Chronic Heart Failure with Preserved and Reduced Ejection Fraction: A Retrospective Longitudinal Study. BMC Geriatr. 2021, 21, 92. [Google Scholar] [CrossRef]
- Lee, M.H.; Leda, M.; Buchan, T.; Malik, A.; Rigobon, A.; Liu, H.; Daza, J.F.; O’Brien, K.; Stein, M.; Hing, N.N.F.; et al. Prognostic Value of Blood Pressure in Ambulatory Heart Failure: A Meta-Analysis and Systematic Review. Ambulatory Blood Pressure Predicts Heart Failure Prognosis. Heart Fail. Rev. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- di Nora, C.; Cioffi, G.; Iorio, A.; Rivetti, L.; Poli, S.; Zambon, E.; Barbati, G.; Sinagra, G.; di Lenarda, A. Systolic Blood Pressure Target in Systemic Arterial Hypertension: Is Lower Ever Better? Results from a Community-Based Caucasian Cohort. Eur. J. Intern. Med. 2018, 48, 57–63. [Google Scholar] [CrossRef]
- Oga, E.A.; Eseyin, O.R. The Obesity Paradox and Heart Failure: A Systematic Review of a Decade of Evidence. J. Obes. 2016, 2016, 9040248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Antonini-Canterin, F.; di Nora, C.; Poli, S.; Sparacino, L.; Cosei, I.; Ravasel, A.; Popescu, A.; Popescu, B. Obesity, Cardiac Remodeling, and Metabolic Profile: Validation of a New Simple Index beyond Body Mass Index. J. Cardiovasc. Echogr. 2018, 28, 18. [Google Scholar] [CrossRef]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty Assessment in the Cardiovascular Care of Older Adults. J. Am. Coll. Cardiol. 2014, 63, 747. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Kitzman, D.; Reeves, G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019, 7, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ritt, M.; Jäger, J.; Ritt, J.I.; Sieber, C.C.; Gaßmann, K.G. Operationalizing a Frailty Index Using Routine Blood and Urine Tests. Clin. Interv. Aging 2017, 12, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Inoue, T.; Shinjo, T.; Miiji, A.; Tamashiro, M.; Oba, K.; Arima, H.; Arasaki, O. Cardiovascular Risk Profile and Frailty in Japanese Outpatients: The Nambu Cohort Study. Hypertens. Res. 2020, 43, 817–823. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Lehto, K.; Pedersen, N.L.; Williams, D.M.; Hägg, S. Genetically-Predicted Life-Long Lowering of Low-Density Lipoprotein Cholesterol Is Associated with Decreased Frailty: A Mendelian Randomization Study in UK Biobank. EBioMedicine 2019, 45, 487–494. [Google Scholar] [CrossRef]
- Wang, Y.; Han, H.R.; Yang, W.; Zhang, H.; Zhang, J.; Ruan, H.; Tang, N.; Ren, J.; Sun, X.; Li, C.; et al. Associations between Risk Factors for Cardiovascular Diseases and Frailty among Community-Dwelling Older Adults in Lanzhou, China. Int. J. Nurs. Sci. 2021, 8, 168–174. [Google Scholar] [CrossRef]
- Fernandes, J.; Dos Santos Gomes, C.; Guerra, R.O.; Pirkle, C.M.; Vafaei, A.; Curcio, C.L.; Dornelas de Andrade, A. Frailty Syndrome and Risk of Cardiovascular Disease: Analysis from the International Mobility in Aging Study. Arch. Gerontol. Geriatr. 2021, 92. [Google Scholar] [CrossRef]
- Pérez-Ros, P.; Vila-Candel, R.; López-Hernández, L.; Martínez-Arnau, F.M. Nutritional Status and Risk Factors for Frailty in Community-Dwelling Older People: A Cross-Sectional Study. Nutrients 2020, 12, 1041. [Google Scholar] [CrossRef]
- Arques, S. Human Serum Albumin in Cardiovascular Diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kanenawa, K.; Isotani, A.; Yamaji, K.; Nakamura, M.; Tanaka, Y.; Hirose-Inui, K.; Fujioka, S.; Mori, S.; Yano, M.; Ito, S.; et al. The Impact of Frailty According to Clinical Frailty Scale on Clinical Outcome in Patients with Heart Failure. ESC Heart Fail. 2021, 8, 1552. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef]
- Costa, D.; Aladio, M.; Girado, C.A.; Pérez de la Hoz, R.; Sara Berensztein, C. Frailty Is Independently Associated with 1-Year Mortality after Hospitalization for Acute Heart Failure. Int. J. Cardiol. Heart Vasc. 2018, 21, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Lee, C.S.; Vitale, C.; Manulik, S.; Denfeld, Q.E.; Uchmanowicz, B.; Rosińczuk, J.; Drozd, M.; Jaroch, J.; Jankowska, E.A. Frailty and the Risk of All-cause Mortality and Hospitalization in Chronic Heart Failure: A Meta-analysis. ESC Heart Fail. 2020, 7, 3427. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lupón, J.; Vidán, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayés-Genís, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef] [Green Version]
- Vidán, M.T.; Blaya-Novakova, V.; Sánchez, E.; Ortiz, J.; Serra-Rexach, J.A.; Bueno, H. Prevalence and Prognostic Impact of Frailty and Its Components in Non-Dependent Elderly Patients with Heart Failure. Eur. J. Heart Fail. 2016, 18, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Value ± SD |
|---|---|
| Age (years) | 55.2 ± 11.6 |
| Men | 125 (81.7%) |
| BMI (kg/m2) | 28.7 ± 5.6 |
| HF of IHD etiology | 73 (48.0%) |
| HF exacerbation | 42 (27.8%) |
| SBP on admission (mmHg) | 112.9 ± 19.5 |
| DBP on admission (mmHg) | 73.8 ± 12.6 |
| HR on discharge (beats per minute) | 73.9 ± 12.4 |
| ICD | 76 (49.7%) |
| CRT (CRT-P or CRT-D) | 24 (15.7%) |
| Frailty status according to SHARE-FI | N (%) |
| Frail | 52 (34.0) |
| Pre-frail | 57 (37.2) |
| Non-frail | 44 (28.8) |
| Comorbidities | N (%) |
| DM | 42 (27.5) |
| COPD | 14 (9.2) |
| CKD | 26 (17.0) |
| Hypertension | 79 (51.6) |
| Persistent or permanent AF | 28 (18.3%) |
| NYHA class | N (%) |
| I | 4 (2.6) |
| II | 62 (40.5) |
| III | 71 (46.4) |
| IV | 16 (10.5) |
| I-II | 66 (43.1) |
| III-IV | 87 (56.9) |
| Biochemical parameters | Value ± SD |
| BNP (pg/mL) | 690.6 ± 704.1 |
| NT-proBNP (pg/mL) | 3733 ± 5614 |
| Uric acid (µmol/L) | 469.9 ± 134.9 |
| Creatinine (µmol/L) | 105.4 ± 36.8 |
| eGRF C-G (mL/min) | 93.8 ± 41.9 |
| eGFR MDRD (mL/min) | 72.3 ± 24.3 |
| Na+ (mmol/L) | 139.1 ± 3.5 |
| K+ (mmol/L) | 4.31 ± 0.43 |
| hsCRP (mg/L) | 6.8 ± 10.1 |
| Fasting glucose (mmol/L) | 6.30 ± 1.74 |
| Serum protein (g/L) | 71.2 ± 7.4 |
| Serum albumin (g/L) | 40.5 ± 5.1 |
| TBIL (µmol/L) | 20.1 ± 12.2 |
| ALT (U/L) | 36.8 ± 22.3 |
| GGT (U/L) | 96.3 ± 105.3 |
| CholT (mmol/L) | 4.28 ± 1.16 |
| TG (mmol/L) | 1.57 ± 0.84 |
| LDL (mmol/L) | 2.62 ± 0.93 |
| HDL (mmol/L) | 1.18 ± 0.33 |
| Hgb (mmol/L) | 8.9 ± 1.1 |
| Ferritin (μg/L) | 162.9 ± 166.6 |
| TIBC (μmol/L) | 63.7 ± 11.7 |
| TSAT (%) | 25.5 ± 12.3 |
| Serum iron (μmol/L) | 15.9 ± 7.3 |
| NLR | 3.65 ± 1.89 |
| Medications | N (%) |
| Loop diuretics | 142 (92.8) |
| Thiazides | 23 (15.0) |
| ß-blocker | 149 (97.4) |
| ACEI/ARB | 101 (66.0) |
| ARNI | 35 (22.9) |
| MRA | 131 (85.6) |
| Ca-blocker | 7 (4.6) |
| Statin | 93 (60.8) |
| Echocardiographic parameters | |
| LVEF (%) | 24.0 ± 8.0 |
| LVEDD (mm) | 70.2 ± 10.6 |
| RVD (mm) | 37.2 ± 7.7 |
| LAD (mm) | 52.1 ± 10.6 |
| IVS (mm) | 10.2 ± 1.8 |
| PW (mm) | 10.1 ± 1.7 |
| Ao (mm) | 33.6 ± 4.7 |
| Characteristics | Frail Syndrome (n = 52) | No Frail Syndrome (n = 101) | p |
|---|---|---|---|
| Age (years) | 59.1 ± 8.6 | 53.1 ± 12.4 | 0.002 * |
| Men | 41 (78.9%) | 84 (83.2%) | 0.51 |
| BMI (kg/m2) | 28.0 ± 5.5 | 29.1 ± 5.7 | 0.15 |
| IHD etiology | 28 (53.8%) | 46 (45.5%) | 0.33 |
| HF exacerbation | 21 (40.4%) | 21 (20.8%) | 0.01 |
| SBP on admission (mmHg) | 107.3 ± 19.8 | 115.7 ± 18.8 | 0.007 * |
| DBP on admission (mmHg) | 72.3 ± 12.0 | 74.6 ± 12.9 | 0.07 |
| HR on discharge (beats per minute) | 76.1 ± 13.6 | 72.7 ± 11.7 | 0.17 |
| ICD | 25 (48.1%) | 51 (50.5%) | 0.78 |
| CRT (CRT-P or CRT-D) | 10 (19.2%) | 14 (13.9%) | 0.38 |
| Comorbidities | |||
| DM | 12 (23.1%) | 30 (29.7%) | 0.38 |
| COPD | 4 (7.7%) | 10 (9.9%) | 0.88 |
| CKD | 14 (26.9%) | 12 (11.9%) | 0.02 * |
| Hypertension | 27 (51.9%) | 52 (51.5%) | 0.96 |
| Persistent or permanent AF | 15 (28.8%) | 13 (12.9%) | 0.015 * |
| Biochemical parameters | |||
| BNP (pg/mL) | 851.9 ± 698.7 | 604.2 ± 696.5 | 0.009 * |
| NT-proBNP (pg/mL) | 5216.0 ± 6314.9 | 3055.5 ± 5170.4 | 0.0006 * |
| Uric acid (µmol/L) | 472.8 ± 124.0 | 468.4 ± 141.0 | 0.77 |
| Creatinine (µmol/L) | 109.6 ± 39.3 | 103.2 ± 35.4 | 0.18 |
| eGFR C-G (mL/min) | 81.5 ± 35.4 | 100.2 ± 43.7 | 0.004 * |
| eGFR MDRD (mL/min) | 67.3 ± 22.7 | 74.9 ± 24.9 | 0.053 |
| Na+ (mmol/L) | 138.5 ± 3.5 | 139.4 ± 3.5 | 0.13 |
| K+ (mmol/L) | 4.24 ± 0.46 | 4.34 ± 0.40 | 0.21 |
| hsCRP (mg/L) | 7.5 ± 9.5 | 6.4 ± 10.4 | 0.24 |
| Fasting glucose (mmol/L) | 6.09 ± 1.05 | 6.42 ± 2.01 | 0.97 |
| Serum protein (g/L) | 69.0 ± 8.5 | 72.4 ± 6.5 | 0.02 * |
| Serum albumin (g/L) | 39.6 ± 5.8 | 41.0 ± 4.6 | 0.03 * |
| TBIL (µmol/L) | 24.0 ± 13.3 | 18.0 ± 11.0 | 0.0009 * |
| ALT (U/L) | 30.7 ± 17.4 | 39.9 ± 23.9 | 0.008 * |
| GGT (U/L) | 108.7 ± 92.1 | 89.8 ± 111.6 | 0.08 |
| CholT (mmol/L) | 3.76 ± 0.88 | 4.55 ± 1.20 | 0.0004 * |
| TG (mmol/L) | 1.32 ± 0.65 | 1.70 ± 0.90 | 0.006 * |
| LDL (mmol/L) | 2.29 ± 0.79 | 2.79 ± 0.96 | 0.004 * |
| HDL (mmol/L) | 1.11 ± 0.31 | 1.21 ± 0.33 | 0.15 |
| Hgb (mmol/L) | 8.75 ± 1.20 | 9.02 ± 1.07 | 0.17 |
| Ferritin (μg/L) | 127.1 ± 130.6 | 101.6 ± 180.5 | 0.004 * |
| TIBC (μmol/L) | 66.3 ± 13.2 | 62.3 ± 10.7 | 0.02 * |
| TSAT (%) | 21.3 ± 12.3 | 27.6 ± 11.8 | 0.002 * |
| Serum iron (μmol/L) | 14.0 ± 7.7 | 16.9 ± 7.0 | 0.009 * |
| NLR | 3.97 ± 2.14 | 3.48 ± 1.74 | 0.13 |
| Medications | |||
| Loop diuretics | 52 (100.0%) | 90 (89.1%) | 0.03 |
| Thiazides | 11 (21.2%) | 12 (11.9%) | 0.13 |
| ß-blocker | 51 (98.1%) | 98 (97.0%) | 0.88 |
| ACEI/ARB | 37 (71.2%) | 64 (63.4%) | 0.34 |
| ARNI | 9 (17.3%) | 26 (25.7%) | 0.33 |
| MRA | 48 (92.3%) | 83 (82.2%) | 0.15 |
| Ca-blocker | 2 (3.9%) | 5 (5.0%) | 0.92 |
| Statin | 34 (65.4%) | 59 (58.4%) | 0.40 |
| Echocardiographic parameters | |||
| LVEF (%) | 22.3 ± 8.1 | 24.9 ± 7.8 | 0.05 |
| LVEDD (mm) | 69.8 ± 11.6 | 70.4 ± 10.0 | 0.62 |
| RVD (mm) | 36.7 ± 6.9 | 37.4 ± 8.1 | 0.99 |
| LAD (mm) | 55.2 ± 10.2 | 50.5 ± 10.5 | 0.02 * |
| IVS (mm) | 10.4 ± 2.1 | 10.1 ± 1.6 | 0.60 |
| PW (mm) | 10.2 ± 2.2 | 10.0 ± 1.4 | 0.95 |
| Aorta (mm) | 33.1 ± 4.4 | 33.9 ± 4.9 | 0.32 |
| Characteristics | Cut-Off Points | Association with the FS Presence | Transformed Variable for Frailty Prediction |
|---|---|---|---|
| Continuous variables | |||
| Age (years) | 51 | Stimulus | age ≥ 51 |
| SBP on admission (mmHg) | 109 | De-stimulus | SBP ≤ 109 mmHg |
| BNP (pg/mL) | 209.6 | Stimulus | BNP ≥ 209.6 pg/mL |
| NT-proBNP (pg/mL) | 1623 | Stimulus | NT-proBNP ≥ 1623 pg/mL |
| TSAT (%) | 22.4 | De-stimulus | TSAT ≤ 22.4% |
| Serum iron (μmol/L) | 13.0 | De-stimulus | serum iron ≤ 13 qmol/L |
| Ferritin (ng/mL) | 132.3 | De-stimulus | ferritin ≤ 132.3 ng/mL |
| Serum protein (g/L) | 72.2 | De-stimulus | serum protein ≤ 72.2 g/L |
| Serum albumin (g/L) | 37.9 | De-stimulus | serum albumin ≤ 37.9 g/L |
| TBIL (µmol/L) | 15.5 | Stimulus | TBIL ≥ 15.5µmol/L |
| ALT (U/L) | 34 | De-stimulus | ALT ≤ 34 U/L |
| CholT (mmol/L) | 4.85 | De-stimulus | CholT ≤ 4.85 mmol/L |
| TG (mmol/L) | 1.25 | De-stimulus | TG ≤ 1.25 mmol/L |
| LDL (mmol/L) | 2.68 | De-stimulus | LDL ≤ 2.68 mmol/L |
| LAD (mm) | 58 | Stimulus | LAD ≥ 58 mm |
| Categorical variables | |||
| CKD | - | Stimulus | - |
| AF (persistant or permanent) | - | Stimulus | - |
| Characteristics | Estimated β (95% CI) | Wald. | OR (95% CI) | p |
|---|---|---|---|---|
| Age ≥ 51 | 1.74 (0.73–2.74) | 11.4 | 5.67 (2.07–15.51) | 0.0007 * |
| SBP ≤ 109 mmHg | 1.08 (0.38–1.77) | 9.34 | 2.94 (1.47–5.87) | 0.002 * |
| BNP ≥ 209.6 pg/mL | 0.95 (0.24–1.66) | 6.94 | 2.59 (1.27–5.24) | 0.008 * |
| NT-proBNP ≥ 1623 pg/mL | 0.94 (0.25–1.63) | 7.08 | 2.56 (1.28–5.1) | 0.008 * |
| eGFR C-G ≤ 91.1 mL/min | 1.38 (0.63–2.14) | 12.9 | 3.99 (1.87–8.48) | 0.0003 * |
| TSAT ≤ 22.4% | 1.10 (0.40–1.80) | 9.64 | 3.00 (1.50–6.02) | 0.002 * |
| Serum iron ≤ 13 μmol/L | 0.86 (0.18–1.54) | 6.06 | 2.36 (1.19–4.67) | 0.01 * |
| Ferritin ≤ 132.3 ng/mL | 1.10 (0.37–1.82) | 8.76 | 3.00 (1.45–6.20) | 0.003 * |
| Serum protein ≤ 72.2 g/L | 0.44 (−0.28–1.16) | 1.42 | 1.55 (0.75–3.19) | 0.23 |
| Serum albumin ≤ 37.9 g/L | 0.65 (−0.02–1.33) | 3.58 | 1.92 (0.98–3.78) | 0.06 * |
| TBIL ≥ 15.5 µmol/L | 1.32 (0.60–2.04) | 13.0 | 3.76 (1.83–7.73) | 0.0003 * |
| ALT ≤ 34 U/L | 1.22 (0.47–1.98) | 10.1 | 3.40 (1.60–7.23) | 0.0015 * |
| CholT ≤ 4.85 mmol/L | 1.85 (0.75–2.95) | 10.9 | 6.36 (2.12–19.1) | 0.001 * |
| TG ≤ 1.25 mmol/L | 0.73 (0.05–1.41) | 4.41 | 2.68 (1.26–5.70) | 0.04 * |
| LDL ≤ 2.68 mmol/L | 0.98 (0.23–1.74) | 6.54 | 2.07 (1.05–4.10) | 0.01 * |
| LAD ≥ 58 mm | 1.15 (0.41–1.90) | 9.24 | 3.16 (1.51–6.65) | 0.02 * |
| CKD | 1.00 (0.15–1.86) | 5.25 | 2.732 (1.16–6.45) | 0.02 * |
| AF (persistent or permanent) | 1.01 (0.17–1.85) | 5.60 | 2.744 (1.19–6.33) | 0.02 * |
| Step | Parameters Included in the Model | Removed Parameter | p of Elimination |
|---|---|---|---|
| 1. | ALL | TSAT ≤ 22.4% | 0.81 |
| 2. | ALL except TSAT ≤ 22.4% | AF | 0.78 |
| 3. | ALL except TSAT ≤ 22.4%, AF | CKD | 0.69 |
| 4. | ALL except TSAT ≤ 22.4%, AF, CKD | NT-proBNP ≥ 1623 pg/mL | 0.70 |
| 5. | ALL except TSAT ≤ 22.4%, AF, CKD, NT-proBNP ≥ 1623 pg/mL | serum iron ≤ 13 qmol/L | 0.50 |
| 6. | ALL except TSAT ≤ 22.4%, AF, CKD, NT-proBNP ≥ 1623 pg/mL | LDL ≤ 2.68 mmol/L | 0.43 |
| 7. | ALL except TSAT ≤ 22.4%, AF, CKD, NT-proBNP ≥ 1623 pg/mL, LDL ≤ 2.68 mmol/L | TG ≤ 1.25 mmol/L | 0.51 |
| 8. | ALL except TSAT ≤ 22.4%, AF, CKD, NT-proBNP ≥ 1623 pg/mL, LDL ≤ 2.68 mmol/L, TG ≤ 1.25 mmol/L | LAD ≥ 58 mm | 0.33 |
| 9. | ALL except TSAT ≤ 22.4%, AF, CKD, NT-proBNP ≥ 1623 pg/mL, LDL ≤ 2.68 mmol/L, TG ≤ 1.25 mmol/L, LAD ≥ 58 mm | ferritin ≤ 132.3 ng/mL | 0.25 |
| 10. | age ≥ 51, SBP ≤ 109 mmHg, BNP ≥ 209.6 pg/mL, serum albumin ≤ 37.9 g/L, TBIL ≥ 15.5µmol/L, ALT ≤ 34 U/L, CholT ≤ 4.85 mmol/L | serum albumin ≤ 37.9 g/L | 0.13 |
| 11. | age ≥ 51, SBP ≤ 109 mmHg, BNP ≥ 209.6 pg/mL, TBIL ≥ 15.5 µmol/L, ALT ≤ 34 U/L, CholT ≤ 4.85 mmol/L, | BNP ≥ 209.6 pg/mL | 0.06 |
| 12. | age ≥ 51, SBP ≤ 109 mmHg, TBIL ≥ 15.5 µmol/L, ALT ≤ 34 U/L, CholT ≤ 4.85 mmol/L | - | - |
| Characteristics | Estimated β (95% CI) | Wald. | OR (95% CI) | p |
|---|---|---|---|---|
| Intercept | −5.045 (−6.283–(−3.267)) | 30.927 | 0.006 (0.001–0.038) | <0.0001 |
| age ≥ 51 | 1.757 (0.641–2.873) | 9.517 | 5.794 (1.898–17.689) | 0.002 |
| SBP ≤ 109 mmHg | 1.026 (0.210–1.841) | 6.078 | 2.789 (1.234–6.303) | 0.014 |
| TBIL ≥ 15.5 µmol/L | 1.312 (0.470–2.155) | 9.135 | 3.715 (1.599–8.628) | 0.002 |
| ALT ≤ 34 U/L | 1.177 (0.300–2.054) | 6.923 | 3.245 (1.350–7.799) | 0.009 |
| CholT ≤ 4.85 mmol/L | 1.285 (0.046–2.523) | 4.134 | 3.613 (1.047–12.465) | 0.042 |
| Cut-Off Value (Value Higher or Equal Indicates FS Presence) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 5 points | 0.288 | 0.970 | 0.833 | 0.726 |
| 4 points | 0.654 | 0.812 | 0.642 | 0.820 |
| 3 points | 0.942 | 0.465 | 0.476 | 0.940 |
| 2 points | 1.000 | 0.198 | 0.391 | 1.000 |
| 1 point | 1.000 | 0.069 | 0.356 | 1.000 |
| Paramete | The Cut-Off Value for Predicting Frailty | Points |
|---|---|---|
| Age | higher or equal to 51 years | 1 |
| SBP on admission | lower or equal to 109 mmHg | 1 |
| CholT | lower or equal to 4.85 mmol/L | 1 |
| TBIL | higher or equal to 15.5 μmol/L | 1 |
| ALT | lower or equal to 34 U/L | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałużna-Oleksy, M.; Kukfisz, A.; Migaj, J.; Dudek, M.; Krysztofiak, H.; Sawczak, F.; Szczechla, M.; Przytarska, K.; Straburzyńska-Migaj, E.; Wleklik, M.; et al. A Simple Risk Score Based on Routine Clinical Parameters Can Predict Frailty in Hospitalized Heart Failure Patients. J. Clin. Med. 2021, 10, 5963. https://doi.org/10.3390/jcm10245963
Kałużna-Oleksy M, Kukfisz A, Migaj J, Dudek M, Krysztofiak H, Sawczak F, Szczechla M, Przytarska K, Straburzyńska-Migaj E, Wleklik M, et al. A Simple Risk Score Based on Routine Clinical Parameters Can Predict Frailty in Hospitalized Heart Failure Patients. Journal of Clinical Medicine. 2021; 10(24):5963. https://doi.org/10.3390/jcm10245963
Chicago/Turabian StyleKałużna-Oleksy, Marta, Agata Kukfisz, Jacek Migaj, Magdalena Dudek, Helena Krysztofiak, Filip Sawczak, Magdalena Szczechla, Katarzyna Przytarska, Ewa Straburzyńska-Migaj, Marta Wleklik, and et al. 2021. "A Simple Risk Score Based on Routine Clinical Parameters Can Predict Frailty in Hospitalized Heart Failure Patients" Journal of Clinical Medicine 10, no. 24: 5963. https://doi.org/10.3390/jcm10245963
APA StyleKałużna-Oleksy, M., Kukfisz, A., Migaj, J., Dudek, M., Krysztofiak, H., Sawczak, F., Szczechla, M., Przytarska, K., Straburzyńska-Migaj, E., Wleklik, M., & Uchmanowicz, I. (2021). A Simple Risk Score Based on Routine Clinical Parameters Can Predict Frailty in Hospitalized Heart Failure Patients. Journal of Clinical Medicine, 10(24), 5963. https://doi.org/10.3390/jcm10245963








