HDL in COVID-19 Patients: Evidence from an Italian Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.1.1. Inclusion Criteria
2.1.2. Patients’ Comorbidities Included in Data Set and Outcome
2.2. Matched-Control Group
2.3. HDL Isolation
2.4. SAA Content in HDL Fraction
2.5. PON-1 Activity in HDL Fraction
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients and Matched-Controls
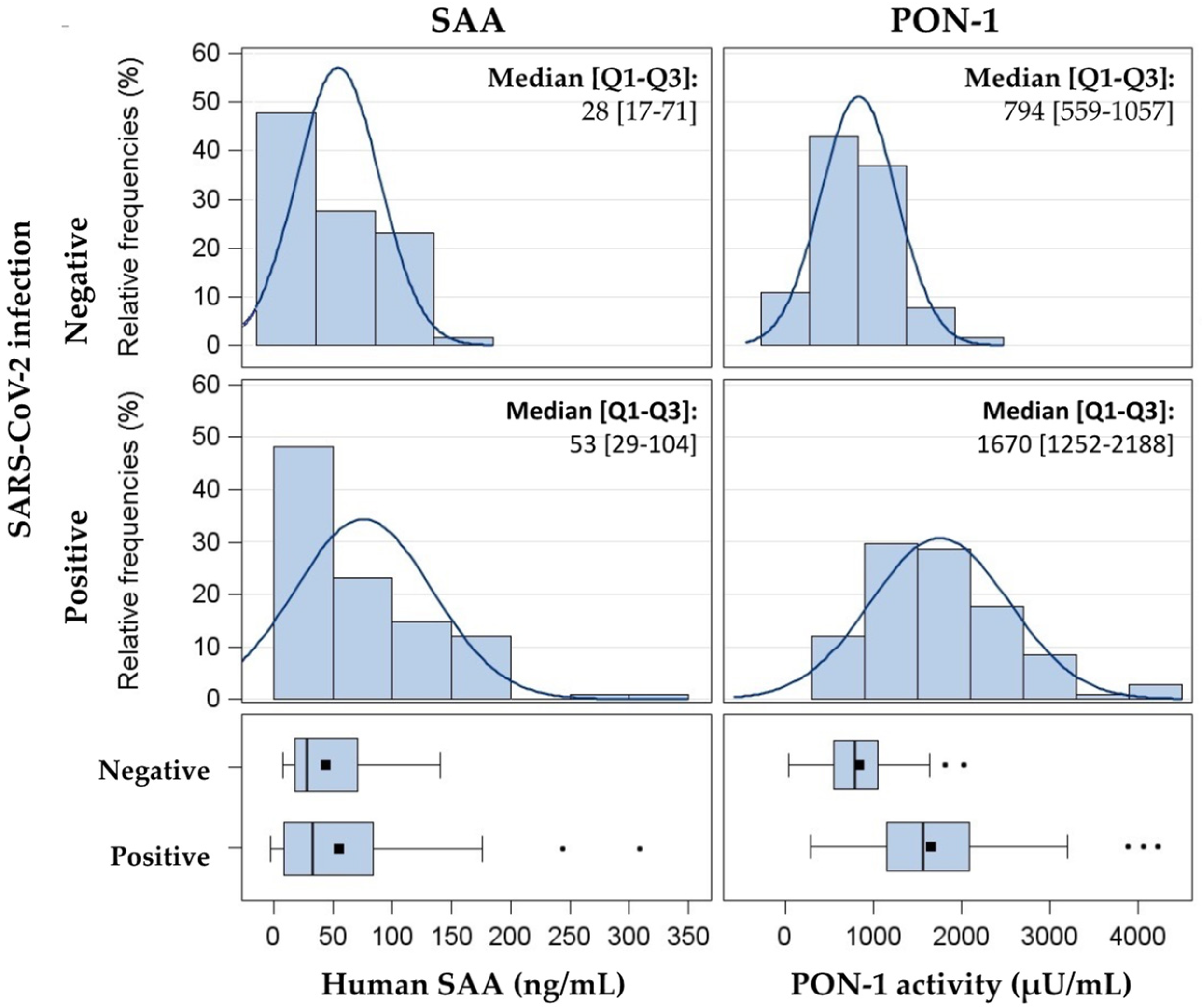
3.2. SARS-CoV-2 Infection Raises SAA Levels and PON-1 Activity
3.3. Survival Status, SAA Levels, and PON-1 Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Vicenzi, M.; Di Cosola, R.; Ruscica, M.; Ratti, A.; Rota, I.; Rota, F.; Bollati, V.; Aliberti, S.; Blasi, F. The liaison between respiratory failure and high blood pressure: Evidence from COVID-19 patients. Eur. Respir. J. 2020, 56, 2001157. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Iodice, S.; Tersalvi, G.; Rota, I.; Ghidini, S.; Terranova, L.; Valenti, L.; Amati, F.; Aliberti, S.; et al. Prognostic parameters of in-hospital mortality in COVID-19 patients-An Italian experience. Eur. J. Clin. Investig. 2021, 51, e13629. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Filippas-Ntekouan, S.; Liberopoulos, E.; Elisaf, M. Lipid testing in infectious diseases: Possible role in diagnosis and prognosis. Infection 2017, 45, 575–588. [Google Scholar] [CrossRef]
- Bernardi, S.; Marcuzzi, A.; Piscianz, E.; Tommasini, A.; Fabris, B. The Complex Interplay between Lipids, Immune System and Interleukins in Cardio-Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 4058. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.R. The bidirectional link between HDL and COVID-19 infections. J. Lipid Res. 2021, 62, 100067. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Zhao, X.; Dong, H.; Wu, C.; Wu, F.; Yu, B.; Lv, J.; Zhang, S.; Wu, G.; et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: An observational study. Lipids Health Dis. 2020, 19, 204. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Pagkali, A.; Zintzaras, E.; Rizos, E.C.; Ntzani, E.E. High-density lipoprotein cholesterol: A marker of COVID-19 infection severity? Atheroscler. Plus 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.; Feng, G.; Chen, M.; Wan, Q.; Lin, J.; Wu, L.; Nie, W.; Chen, S. Distinct lipid metabolic dysregulation in asymptomatic COVID-19. Iscience 2021, 24, 102974. [Google Scholar] [CrossRef]
- Thakkar, H.; Vincent, V.; Sen, A.; Singh, A.; Roy, A. Changing Perspectives on HDL: From Simple Quantity Measurements to Functional Quality Assessment. J. Lipids 2021, 2021, 5585521. [Google Scholar] [CrossRef] [PubMed]
- Zimetti, F.; De Vuono, S.; Gomaraschi, M.; Adorni, M.P.; Favari, E.; Ronda, N.; Ricci, M.A.; Veglia, F.; Calabresi, L.; Lupattelli, G. Plasma cholesterol homeostasis, HDL remodeling and function during the acute phase reaction. J. Lipid Res. 2017, 58, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Ronda, N.; Favari, E.; Borghi, M.O.; Ingegnoli, F.; Gerosa, M.; Chighizola, C.; Zimetti, F.; Adorni, M.P.; Bernini, F.; Meroni, P.L. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 2014, 73, 609–615. [Google Scholar] [CrossRef]
- Nazir, S.; Jankowski, V.; Bender, G.; Zewinger, S.; Rye, K.A.; van der Vorst, E.P.C. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv. Drug Deliv. Rev. 2020, 159, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Kotur-Stevuljevic, J.; Vekic, J.; Stefanovic, A.; Zeljkovic, A.; Ninic, A.; Ivanisevic, J.; Miljkovic, M.; Sopic, M.; Munjas, J.; Mihajlovic, M.; et al. Paraoxonase 1 and atherosclerosis-related diseases. Biofactors 2020, 46, 193–205. [Google Scholar] [CrossRef]
- Webb, N.R. High-Density Lipoproteins and Serum Amyloid A (SAA). Curr. Atheroscler. Rep. 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, H.J.; Heezius, E.C.; Dallinga, G.M.; van Strijp, J.A.; Verhoef, J.; van Kessel, K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003, 31, 1359–1366. [Google Scholar] [CrossRef]
- Wang, G.; Deng, J.; Li, J.; Wu, C.; Dong, H.; Wu, S.; Zhong, Y. The Role of High-Density Lipoprotein in COVID-19. Front. Pharmacol. 2021, 12, 720283. [Google Scholar] [CrossRef] [PubMed]
- Vicenzi, M.; Ruscica, M.; Iodice, S.; Rota, I.; Ratti, A.; Di Cosola, R.; Corsini, A.; Bollati, V.; Aliberti, S.; Blasi, F. The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients. J. Clin. Med. 2020, 9, 2943. [Google Scholar] [CrossRef]
- Aliberti, S.; Amati, F.; Pappalettera, M.; Di Pasquale, M.; D’Adda, A.; Mantero, M.; Gramegna, A.; Simonetta, E.; Oneta, A.M.; Privitera, E.; et al. COVID-19 multidisciplinary high dependency unit: The Milan model. Respir. Res 2020, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Iodice, S.; Favero, C.; Angelici, L.; Albetti, B.; Cacace, R.; Cantone, L.; Carugno, M.; Cavalleri, T.; De Giorgio, B.; et al. Susceptibility to particle health effects, miRNA and exosomes: Rationale and study protocol of the SPHERE study. BMC Public Health 2014, 14, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asztalos, B.F.; de la Llera-Moya, M.; Dallal, G.E.; Horvath, K.V.; Schaefer, E.J.; Rothblat, G.H. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 2005, 46, 2246–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, C.J.; Xie, Y.; Brewster, P.S.; Ghosh, S.; Dube, P.; Sarsour, T.; Kleinhenz, A.L.; Crawford, E.L.; Malhotra, D.; James, R.W.; et al. Circulating Lactonase Activity but Not Protein Level of PON-1 Predicts Adverse Outcomes in Subjects with Chronic Kidney Disease. J. Clin. Med. 2019, 8, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Rodriguez, M.; Medina-Cuadros, M.; Martinez-Gallego, G.; Sillero-Arenas, M. Total cholesterol, HDL-cholesterol, and risk of nosocomial infection: A prospective study in surgical patients. Infect. Control Hosp. Epidemiol. 1997, 18, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Jacobs, D.R., Jr.; Sidney, S.; Claxton, A.J.; Feingold, K.R. Cohort study of serum total cholesterol and in-hospital incidence of infectious diseases. Epidemiol. Infect. 1998, 121, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Ye, X.; Raimann, J.G.; Wang, Y.; Topping, A.; Usvyat, L.A.; Stuard, S.; Canaud, B.; van der Sande, F.M.; Kooman, J.P.; et al. Lipid levels are inversely associated with infectious and all-cause mortality: International MONDO study results. J. Lipid Res. 2018, 59, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorby, C.D.; Gherardi, E.; Dovey, L.; Godliman, C.; Bowyer, D.E. Transforming growth factor-beta 1 and interleukin-1 beta stimulate LDL receptor activity in Hep G2 cells. Atherosclerosis 1992, 97, 21–28. [Google Scholar] [CrossRef]
- Liao, W.; Floren, C.H. Tumor necrosis factor up-regulates expression of low-density lipoprotein receptors on HepG2 cells. Hepatology 1993, 17, 898–907. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [Green Version]
- Vu, C.N.; Ruiz-Esponda, R.; Yang, E.; Chang, E.; Gillard, B.; Pownall, H.J.; Hoogeveen, R.C.; Coraza, I.; Balasubramanyam, A. Altered relationship of plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: Further evidence for a unique form of metabolic syndrome in HIV patients. Metabolism 2013, 62, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jerico, C.; Guerrero, C.; Miret, M.; Naf, S.; Pardo, A.; et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef]
- Dai, W.; Lund, H.; Chen, Y.; Zhang, J.; Osinski, K.; Jones, S.Z.; Kreuziger, L.B.; Lopez, J.A.; Benjamin, I.J.; Silverstein, R.L.; et al. Hypertriglyceridemia during hospitalization independently associates with mortality in patients with COVID-19. J. Clin. Lipidol. 2021, 15, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Alipour, A.; van Oostrom, A.J.; Izraeljan, A.; Verseyden, C.; Collins, J.M.; Frayn, K.N.; Plokker, T.W.; Elte, J.W.; Castro Cabezas, M. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 792–797. [Google Scholar] [CrossRef]
- Tucker, B.; Sawant, S.; McDonald, H.; Rye, K.A.; Patel, S.; Ong, K.L.; Cochran, B.J. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: Results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis 2021, 319, 1–9. [Google Scholar] [CrossRef]
- Aung, N.; Khanji, M.Y.; Munroe, P.B.; Petersen, S.E. Causal Inference for Genetic Obesity, Cardiometabolic Profile and COVID-19 Susceptibility: A Mendelian Randomization Study. Front. Genet. 2020, 11, 586308. [Google Scholar] [CrossRef] [PubMed]
- Scalsky, R.J.; Chen, Y.J.; Desai, K.; O’Connell, J.R.; Perry, J.A.; Hong, C.C. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK Biobank. PLoS ONE 2021, 16, e0248602. [Google Scholar] [CrossRef]
- Howie, D.; Ten Bokum, A.; Necula, A.S.; Cobbold, S.P.; Waldmann, H. The Role of Lipid Metabolism in T Lymphocyte Differentiation and Survival. Front. Immunol. 2017, 8, 1949. [Google Scholar] [CrossRef]
- Reilly, N.A.; Lutgens, E.; Kuiper, J.; Heijmans, B.T.; Wouter Jukema, J. Effects of fatty acids on T cell function: Role in atherosclerosis. Nat. Rev. Cardiol. 2021, 18, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.; Alexandraki, I.; Bertholf, R.L.; Mooradian, A.D. Transient dyslipidemia mimicking the plasma lipid profile of Tangier disease in a diabetic patient with gram negative sepsis. Ann. Clin. Lab. Sci. 2011, 41, 150–153. [Google Scholar]
- Madsen, C.M.; Varbo, A.; Tybjaerg-Hansen, A.; Frikke-Schmidt, R.; Nordestgaard, B.G. U-shaped relationship of HDL and risk of infectious disease: Two prospective population-based cohort studies. Eur. Heart J. 2018, 39, 1181–1190. [Google Scholar] [CrossRef]
- Trinder, M.; Walley, K.R.; Boyd, J.H.; Brunham, L.R. Causal Inference for Genetically Determined Levels of High-Density Lipoprotein Cholesterol and Risk of Infectious Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Does HDL (High-Density Lipoprotein) Play a Causal Role in Host Defenses Against Infection? Arterioscler. Thromb. Vasc. Biol. 2020, 40, 5–6. [Google Scholar] [CrossRef]
- Hilser, J.R.; Han, Y.; Biswas, S.; Gukasyan, J.; Cai, Z.; Zhu, R.; Tang, W.H.W.; Deb, A.; Lusis, A.J.; Hartiala, J.A.; et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J. Lipid Res. 2021, 62, 100061. [Google Scholar] [CrossRef]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of Blood Lipid Concentrations Over the Adult Life Course and Risk of Cardiovascular Disease and All-Cause Mortality: Observations From the Framingham Study Over 35 Years. J. Am. Heart Assoc. 2019, 8, e011433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R.; Grunfeld, C. Effect of inflammation on HDL structure and function. Curr. Opin. Lipidol. 2016, 27, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Wang, X.; Noffsinger, V.P.; Jennings, D.; de Beer, M.C.; de Beer, F.C.; Tannock, L.R.; Webb, N.R. Serum amyloid A is not incorporated into HDL during HDL biogenesis. J. Lipid Res. 2020, 61, 328–337. [Google Scholar] [CrossRef]
- Wilson, P.G.; Thompson, J.C.; Shridas, P.; McNamara, P.J.; de Beer, M.C.; de Beer, F.C.; Webb, N.R.; Tannock, L.R. Serum Amyloid A Is an Exchangeable Apolipoprotein. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1890–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Velia, E.; Mavingui, P.; et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef]
- Pieri, M.; Ciotti, M.; Nuccetelli, M.; Perrone, M.A.; Calio, M.T.; Lia, M.S.; Minieri, M.; Bernardini, S. Serum Amyloid A Protein as a useful biomarker to predict COVID-19 patients severity and prognosis. Int. Immunopharmacol. 2021, 95, 107512. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, W.J.; Lan, M.Q.; Gan, F.R.; Liu, Y.Y.; Sun, L.; Chen, J.L.; Sun, Y.F.; Tao, C.M. Association between serum amyloid A levels and predicting disase severity in COVID-19 patients: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Souza Junior, D.R.; Silva, A.R.M.; Rosa-Fernandes, L.; Reis, L.R.; Alexandria, G.; Bhosale, S.D.; Ghilardi, F.R.; Dalcoquio, T.F.; Bertolin, A.J.; Nicolau, J.C.; et al. HDL proteome remodeling associates with COVID-19 severity. J. Clin. Lipidol. 2021, 15, 796–804. [Google Scholar] [CrossRef]
- Fumagalli, C.; Rozzini, R.; Vannini, M.; Coccia, F.; Cesaroni, G.; Mazzeo, F.; Cola, M.; Bartoloni, A.; Fontanari, P.; Lavorini, F.; et al. Clinical risk score to predict in-hospital mortality in COVID-19 patients: A retrospective cohort study. BMJ Open 2020, 10, e040729. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Tomas, E.; Iftimie, S.; Castane, H.; Baiges-Gaya, G.; Hernandez-Aguilera, A.; Gonzalez-Vinas, M.; Castro, A.; Camps, J.; Joven, J. Clinical Performance of Paraoxonase-1-Related Variables and Novel Markers of Inflammation in Coronavirus Disease-19. A Machine Learning Approach. Antioxidants 2021, 10, 991. [Google Scholar] [CrossRef]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of paraoxonase 1 (PON1) as an antioxidant and antiatherogenic enzyme in the cardiovascular complications of type 2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.W.; Ding, F.H.; Shen, Y.; Liu, Z.H.; Wang, F.; Zhang, R.Y.; Shen, W.F.; Lu, L.; Wang, X.Q. Relationship of High-Density Lipoprotein-Associated Arylesterase Activity to Systolic Heart Failure in Patients with and without Type 2 Diabetes. Sci. Rep. 2019, 9, 5979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potocnjak, I.; Degoricija, V.; Trbusic, M.; Teresak, S.D.; Radulovic, B.; Pregartner, G.; Berghold, A.; Tiran, B.; Marsche, G.; Frank, S. Metrics of High-Density Lipoprotein Function and Hospital Mortality in Acute Heart Failure Patients. PLoS ONE 2016, 11, e0157507. [Google Scholar] [CrossRef] [Green Version]
- Li, X.M.; Tang, W.H.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; Didonato, J.A.; Fisher, E.A.; et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| SARS-CoV2 Infection | ||
|---|---|---|
| Negative (n = 65) | Positive (n = 108) | |
| Age, years | 58 ± 12.9 | 60 ± 13.1 |
| Gender | ||
| Male | 32 (49.2%) | 75 (69.4%) |
| Female | 33 (50.8%) | 33 (30.6%) |
| BMI, kg/m2 | 28.2 ± 4.9 | 27.9 ± 4.3 |
| Smoke | ||
| Yes | 5 (7.7%) | 3 (2.8%) |
| No | 60 (92.3%) | 90 (83.3%) |
| Missing | - | 15 (13.9%) |
| TC, mg/dL | 207.3 ± 35.9 | 138.1 ± 46.5 |
| LDL-c, mg/dL | 123.8 ± 6.6 | 78.0 ± 24.6 |
| HDL-c, mg/dL | 60.4 ± 14.3 | 27.1 ± 9.7 |
| non-HDL-c, mg/dL | 147.0 ± 36.8 | 110.9 ± 44.5 |
| Triglyceride, mg/dL | 116.7 ± 72.5 | 165.9 ± 62.5 |
| Antihypertensive medications | ||
| Yes | 11 (16.9%) | 16 (14.8%) |
| No | 54 (83.1%) | 92 (85.2%) |
| Outcome | SARS-CoV2 Infection | Univariate Model | Adjusted Model | ||
|---|---|---|---|---|---|
| Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | ||
| SAA (ng/mL) | Negative | 32.7 (27.3; 50.2) | <0.0001 | 35.9 (27.3; 47.2) | <0.0001 |
| Positive | 57.8 (50.2; 66.5) | 60.5 (45.4; 80.5) | |||
| PON-1 activity (µU/mL) | Negative | 702.5 (612.8; 805.3) | <0.0001 | 618.3 (502.1; 761.4) | <0.0001 |
| Positive | 1579.3 (1420.5; 1755.8) | 1394.0 (1123.8; 1729.2) | |||
| Outcome | Age | SARS-CoV2 Infection | Mean (95% CI) | p-Value | p-Value for Interaction |
|---|---|---|---|---|---|
| SAA (ng/mL) | mean − SD: 46 years | Negative | 47.1 (34.3; 64.7) | 0.1780 | 0.0087 |
| Positive | 58.3 (42.4; 80.4) | ||||
| mean: 59 years | Negative | 34.4 (26.2; 45.0) | <0.0001 | ||
| Positive | 59.3 (44.8; 78.5) | ||||
| mean + SD: 72 years | Negative | 25.1 (17.7; 35.5) | <0.0001 | ||
| Positive | 60.3 (44.3; 82.3) |
| Outcome | Survival Status | Univariate Model | Adjusted Model | ||
|---|---|---|---|---|---|
| Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | ||
| SAA (ng/mL) | Death | 60.1 (44.2; 81.7) | 0.8616 | 64.3 (37.8; 109.3) | 0.9530 |
| Alive | 58.3 (49.8; 68.3) | 63.4 (40.2; 99.9) | |||
| PON-1 activity (µU/mL) | Death | 1512.1 (1237.5; 1847.6) | 0.6523 | 1687.4 (1340.2; 2124.4) | 0.5480 |
| Alive | 1592.5 (1436.4; 1765.5) | 1558.0 (1398.5; 1735.6) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papotti, B.; Macchi, C.; Favero, C.; Iodice, S.; Adorni, M.P.; Zimetti, F.; Corsini, A.; Aliberti, S.; Blasi, F.; Carugo, S.; et al. HDL in COVID-19 Patients: Evidence from an Italian Cross-Sectional Study. J. Clin. Med. 2021, 10, 5955. https://doi.org/10.3390/jcm10245955
Papotti B, Macchi C, Favero C, Iodice S, Adorni MP, Zimetti F, Corsini A, Aliberti S, Blasi F, Carugo S, et al. HDL in COVID-19 Patients: Evidence from an Italian Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(24):5955. https://doi.org/10.3390/jcm10245955
Chicago/Turabian StylePapotti, Bianca, Chiara Macchi, Chiara Favero, Simona Iodice, Maria Pia Adorni, Francesca Zimetti, Alberto Corsini, Stefano Aliberti, Francesco Blasi, Stefano Carugo, and et al. 2021. "HDL in COVID-19 Patients: Evidence from an Italian Cross-Sectional Study" Journal of Clinical Medicine 10, no. 24: 5955. https://doi.org/10.3390/jcm10245955
APA StylePapotti, B., Macchi, C., Favero, C., Iodice, S., Adorni, M. P., Zimetti, F., Corsini, A., Aliberti, S., Blasi, F., Carugo, S., Bollati, V., Vicenzi, M., & Ruscica, M. (2021). HDL in COVID-19 Patients: Evidence from an Italian Cross-Sectional Study. Journal of Clinical Medicine, 10(24), 5955. https://doi.org/10.3390/jcm10245955












