Pre-Procedural Right Ventricular Longitudinal Strain and Post-Procedural Tricuspid Regurgitation Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI)
Abstract
:1. Introduction
2. Methods
3. Statistics
4. Results
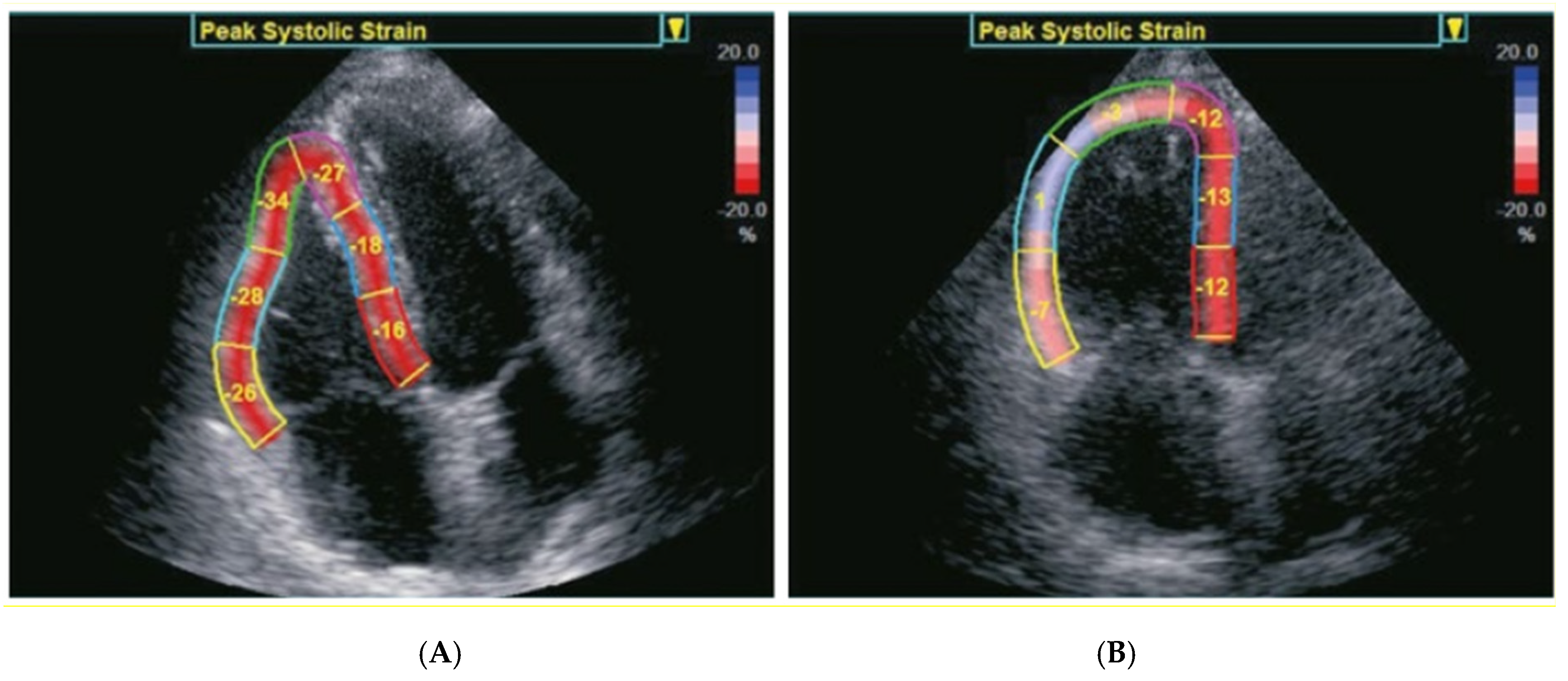
4.1. Echocardiographic Assessment
4.2. Echocardiographic Predictors of Long-Term Mortality
5. Discussion
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, X.; Yu, L.; Sun, Y.; Jaiswal, S.; Zhu, Q.; Chen, H.; He, Y.; Wang, L.; Ren, K.; et al. Impact of tricuspid regurgitation and right ventricular dysfunction on outcomes after transcatheter aortic valve replacement: A systematic review and meta-analysis. Clin. Cardiol. 2019, 42, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, L.F.; Obuchowski, N.A.; Rodriguez, L.; Popovic, Z.; Kwon, D.; Marwick, T.H. Accuracy and Interobserver Concordance of Echocardiographic Assessment of Right Ventricular Size and Systolic Function: A Quality Control Exercise. J. Am. Soc. Echocardiogr. 2012, 25, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ternacle, J.; Berry, M.; Cognet, T.; Kloeckner, M.; Damy, T.; Monin, J.-L.; Couetil, J.-P.; Dubois-Rande, J.-L.; Gueret, P.; Lim, P. Prognostic Value of Right Ventricular Two-Dimensional Global Strain in Patients Referred for Cardiac Surgery. J. Am. Soc. Echocardiogr. 2013, 26, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, A.; Albanese, M.; Salerno, N.; Aquila, I.; Sabatino, J.; Sorrentino, S.; Leo, I.; Cacia, M.; Signorile, V.; Mongiardo, A.; et al. Predictors of outcomes in patients with mitral regurgitation undergoing percutaneous valve repair. Sci. Rep. 2020, 10, 17144. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Pislaru, S.V.; Soo, W.M.; Huang, R.; Greason, K.L.; Mathew, V.; Sandhu, G.S.; Eleid, M.F.; Suri, R.M.; Oh, J.K.; et al. Impact of right ventricular size and function on survival following transcatheter aortic valve replacement. Int. J. Cardiol. 2016, 221, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Asami, M.; Stortecky, S.; Praz, F.; Lanz, J.; Räber, L.; Franzone, A.; Piccolo, R.; Siontis, G.C.M.; Heg, D.; Valgimigli, M.; et al. Prognostic Value of Right Ventricular Dysfunction on Clinical Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Koschutnik, M.; Dannenberg, V.; Nitsche, C.; Donà, C.; Siller-Matula, J.M.; Winter, M.P.; Andreas, M.; Zafar, A.; Bartko, P.E.; Beitzke, D.; et al. Right ventricular function and outcome in patients undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Koifman, E.; Didier, R.; Patel, N.; Jerusalem, Z.; Kiramijyan, S.; Ben-Dor, I.; Negi, S.I.; Wang, Z.; Goldstein, S.A.; Lipinski, M.J.; et al. Impact of right ventricular function on outcome of severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Am. Heart J. 2017, 184, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Granot, Y.; Merdler, I.; Finkelstein, A.; Arbel, Y.; Banai, S.; Topilsky, Y.; Scwartz, L.A.; Segev, A.; Barbash, I.; Fefer, P.; et al. Prognostic implication of right ventricular dysfunction and tricuspid regurgitation following transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2021, 98, E758–E767. [Google Scholar] [CrossRef] [PubMed]
- Schueler, R.; Öztürk, C.; Laser, J.V.; Wirth, F.; Werner, N.; Welz, A.; Nickenig, G.; Sinning, J.M.; Hammerstingl, C. Right ventricular assessment in patients undergoing transcatheter or surgical aortic valve replacement. Catheter. Cardiovasc. Interv. 2020, 96, E711–E722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedofsky, D.; Koifman, E.; Jarrett, H.; Miyoshi, T.; Rogers, T.; Ben-Dor, I.; Satler, L.F.; Torguson, R.; Waksman, R.; Asch, F.M. Association of Right Ventricular Longitudinal Strain with Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2020, 33, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.A.; Rozenbaum, Z.; Ghantous, E.; Kramarz, J.; Biner, S.; Ghermezi, M.; Shimiaie, J.; Finkelstein, A.; Banai, S.; Aviram, G.; et al. Impact of Right Ventricular Dysfunction and Tricuspid Regurgitation on Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2017, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, A.A.; Marzluf, B.A.; Graf, A.; Bachmann, A.; Kocher, A.; Bonderman, D.; Mascherbauer, J. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J. Am. Coll. Cardiol. 2014, 64, 2633–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age (years) | 83.8 ± 5 |
| Male (n, %) | 87 (38) |
| Weight (kg) | 72 ± 14.4 |
| Height (cm) | 166 ± 8.5 |
| COPD (n, %) | 36 (15.7) |
| Diabetes (n, %) | 48 (21) |
| NYHA Class II (n, %) | 36 (15.7) |
| NYHA Class III (n, %) | 177 (77.3) |
| NYHA Class IV (n, %) | 16 (7) |
| Hypertension (n, %) | 180 (78.6) |
| Coronary artery disease (n, %) | 124 (54.1%) |
| Chronic kidney disease (n, %) | 82 (35.8) |
| Atrial Fibrillation (n, %) | 80 (34.9) |
| Previous Surgery (n, %) | 41 (17.9) |
| Logistic Euroscore II | 5.7 ± 5.0 |
| STS score | 5.6 ± 3.6 |
| Parameter | Pre−TAVI | Post−TAVI | p-Value |
|---|---|---|---|
| RV basal wall LS (%) | −21.5 ± 7.9 | −20.8 ± 8.3 | 0.25 |
| RV basal time to peak strain (ms) | 376.4 ± 76 | 379.4 ± 94 | 0.71 |
| RV middle wall LS (%) | −21.5 ± 8.2 | −21.1 ± 8.3 | 0.52 |
| RV middle wall time to peak strain (ms) | 368.6 ± 72 | 371.8 ± 89 | 0.65 |
| RV apical wall LS (%) | −15.7 ± 8.4 | −17.1 ± 7.9 | 0.04 |
| RV apical wall time to peak strain (ms) | 407.9 ± 119.7 | 405 ± 112.6 | 0.8 |
| Average RV free−wall LS (%) | −20.0 ± 7.6 | −19.8 ± 7.8 | 0.7 |
| RA volume (mL) | 43.8 ± 30.3 | 41.6 ± 25.5 | 0.08 |
| RV end−diastolic basal diameter (mm) | 36.3 ± 6.6 | 36.7 ± 6.5 | 0.32 |
| RV EDA (mm2) | 14.6 ± 3.9 | 14.7 ± 4.1 | 0.6 |
| RV ESA (mm2) | 8.8 ± 3.2 | 8.9 ± 3.4 | 0.7 |
| FAC (%) | 40 ± 12.2 | 40.2 ± 12.4 | 0.8 |
| TAPSE (mm) | 16.6 ± 4.1 | 16.4 ± 4.2 | 0.28 |
| Long-Term All-Cause Mortality | ||||
|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Average baseline RV free-wall LS | 1.05 (1.01–1.10) | 0.044 | 1.05 (1.01–1.10) | 0.049 |
| Pre-TAVI TR2 (>mild) | 2.95 (1.46–5.97) | 0.003 | 1.31 (0.55–3.15) | 0.53 |
| Post-TAVI TR (>mild) | 4.39 (2.22–8.70) | <0.0001 | 3.77 (1.62–8.75) | 0.002 |
| TAPSE | 1.0 (0.98–1.18) | 0.89 | ||
| FAC | 0.98 (0.96–1.01) | 0.12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, H.; Polimeni, A.; Brandt, V.; Rudolph, V.; Rudolph, T.K.; Bleiziffer, S.; Friedrichs, K.P.; Faber, L.; Dimitriadis, Z. Pre-Procedural Right Ventricular Longitudinal Strain and Post-Procedural Tricuspid Regurgitation Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI). J. Clin. Med. 2021, 10, 5877. https://doi.org/10.3390/jcm10245877
Omran H, Polimeni A, Brandt V, Rudolph V, Rudolph TK, Bleiziffer S, Friedrichs KP, Faber L, Dimitriadis Z. Pre-Procedural Right Ventricular Longitudinal Strain and Post-Procedural Tricuspid Regurgitation Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI). Journal of Clinical Medicine. 2021; 10(24):5877. https://doi.org/10.3390/jcm10245877
Chicago/Turabian StyleOmran, Hazem, Alberto Polimeni, Verena Brandt, Volker Rudolph, Tanja K. Rudolph, Sabine Bleiziffer, Kai P. Friedrichs, Lothar Faber, and Zisis Dimitriadis. 2021. "Pre-Procedural Right Ventricular Longitudinal Strain and Post-Procedural Tricuspid Regurgitation Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI)" Journal of Clinical Medicine 10, no. 24: 5877. https://doi.org/10.3390/jcm10245877
APA StyleOmran, H., Polimeni, A., Brandt, V., Rudolph, V., Rudolph, T. K., Bleiziffer, S., Friedrichs, K. P., Faber, L., & Dimitriadis, Z. (2021). Pre-Procedural Right Ventricular Longitudinal Strain and Post-Procedural Tricuspid Regurgitation Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI). Journal of Clinical Medicine, 10(24), 5877. https://doi.org/10.3390/jcm10245877






