Predictors of the Development of Protracted Bacterial Bronchitis following Presentation to Healthcare for an Acute Respiratory Illness with Cough: Analysis of Three Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
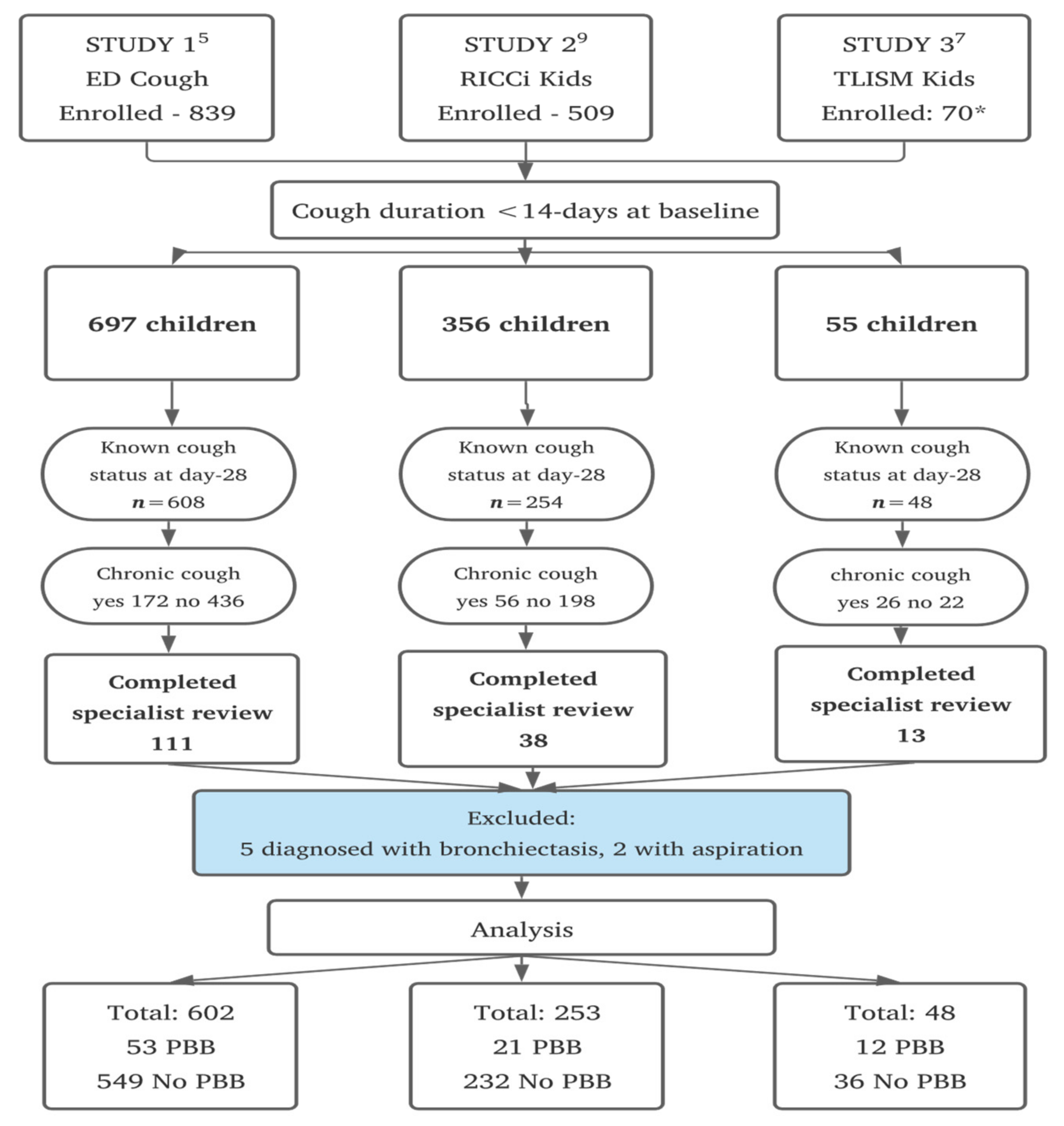
2.3. Participants
2.4. Data Collection
2.5. Primary Outcome
2.6. Laboratory Methods
2.7. Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchant, J.M.; Masters, I.B.; Taylor, S.M.; Cox, N.C.; Seymour, G.J.; Chang, A.B. Evaluation and outcome of young children with chronic cough. Chest 2006, 129, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kantar, A.; Chang, A.B.; Shields, M.D.; Marchant, J.M.; Grimwood, K.; Grigg, J.; Priftis, K.N.; Cutrera, R.; Midulla, F.; Brand, P.L.P.; et al. ERS statement on protracted bacterial bronchitis in children. Eur. Respir. J. 2017, 50, 1602139. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Redding, G.J.; Everard, M.L. Chronic wet cough: Protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr. Pulmonol. 2008, 43, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Bush, A.; Grimwood, K. Bronchiectasis in children: Diagnosis and treatment. Lancet 2018, 392, 866–879. [Google Scholar] [CrossRef]
- Drescher, B.J.; Chang, A.B.; Phillips, N.; Acworth, J.; Marchant, J.; Sloots, T.P.; David, M.; O’Grady, K.A. The development of chronic cough in children following presentation to a tertiary paediatric emergency department with acute respiratory illness: Study protocol for a prospective cohort study. BMC Pediatr. 2013, 13, 125. [Google Scholar] [CrossRef]
- O’Grady, K.F.; Drescher, B.J.; Goyal, V.; Phillips, N.; Acworth, J.; Marchant, J.M.; Chang, A.B. Chronic cough postacute respiratory illness in children: A cohort study. Arch. Dis. Child. 2017, 102, 1044–1048. [Google Scholar] [CrossRef]
- Hall, K.K.; Chang, A.B.; Sloots, T.P.; Anderson, J.; Kemp, A.; Hammill, J.; Otim, M.; O’Grady, K.A. The respiratory health of urban indigenous children aged less than 5 years: Study protocol for a prospective cohort study. BMC Pediatr. 2015, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.K.; Chang, A.B.; Anderson, J.; Arnold, D.; Goyal, V.; Dunbar, M.; Otim, M.; O’Grady, K.F. The Incidence and Short-term Outcomes of Acute Respiratory Illness with Cough in Children from a Socioeconomically Disadvantaged Urban Community in Australia: A Community-Based Prospective Cohort Study. Front. Pediatr. 2017, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, K.F.; Grimwood, K.; Toombs, M.; Sloots, T.P.; Otim, M.; Whiley, D.; Anderson, J.; Rablin, S.; Torzillo, P.J.; Buntain, H.; et al. Effectiveness of a cough management algorithm at the transitional phase from acute to chronic cough in Australian children aged <15 years: Protocol for a randomised controlled trial. BMJ Open 2017, 7, e013796. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, K.F.; Grimwood, K.; Torzillo, P.J.; Rablin, S.; Lovie-Toon, Y.; Kaus, M.; Arnold, D.; Roberts, J.; Buntain, H.; Adsett, D.; et al. Effectiveness of a chronic cough management algorithm at the transitional stage from acute to chronic cough in children: A multicenter, nested, single-blind, randomised controlled trial. Lancet Child Adolesc. Health 2019, 3, 889–898. [Google Scholar] [CrossRef]
- Gibson, P.G.; Chang, A.B.; Glasgow, N.J.; Holmes, P.W.; Katelaris, P.; Kemp, A.S.; Landau, L.I.; Mazzone, S.; Newcombe, P.; Van Asperen, P.; et al. CICADA: Cough in Children and Adults: Diagnosis and Assessment. Australian cough guidelines summary statement. Med. J. Aust. 2010, 192, 265–271. [Google Scholar] [CrossRef]
- Chang, A.B.; Oppenheimer, J.J.; Weinberger, M.; Grant, C.C.; Rubin, B.K.; Irwin, R.S.; Panel, C.E.C. Etiologies of Chronic Cough in Pediatric Cohorts: CHEST Guideline and Expert Panel Report. Chest 2017, 152, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Robertson, C.F.; van Asperen, P.P.; Glasgow, N.J.; Masters, I.B.; Teoh, L.; Mellis, C.M.; Landau, L.I.; Marchant, J.M.; Morris, P.S. A cough algorithm for chronic cough in children: A multicenter, randomized controlled study. Pediatrics 2013, 131, e1576–e1583. [Google Scholar] [CrossRef]
- Asilsoy, S.; Bayram, E.; Agin, H.; Apa, H.; Can, D.; Gulle, S.; Altinoz, S. Evaluation of chronic cough in children. Chest 2008, 134, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.T.Y.; Laird, P.; Stevenson, P.G.; Schultz, A. Frequency of protracted bacterial bronchitis and management pre-respiratory referral. J. Paediatr. Child Health 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gedik, A.H.; Cakir, E.; Torun, E.; Demir, A.D.; Kucukkoc, M.; Erenberk, U.; Uzuner, S.; Nursoy, M.; Ozkaya, E.; Aksoy, F.; et al. Evaluation of 563 children with chronic cough accompanied by a new clinical algorithm. Ital. J. Pediatr. 2015, 41, 73. [Google Scholar] [CrossRef][Green Version]
- Chang, A.B.; Robertson, C.F.; Van, A.P.P.; Glasgow, N.; Mellis, C.M.; Masters, I.B.; Teoh, L.; Tjhung, I.; Morris, P.S.; Petsky, H.L.; et al. A multi-centre study on chronic cough in children: Burden and etiologies based on a standardized management pathway. Chest 2012, 142, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Craven, V.; Everard, M.L. Protracted bacterial bronchitis: Reinventing an old disease. Arch. Dis. Child. 2012, 98, 72–76. [Google Scholar] [CrossRef]
- Au-Yeung, Y.T.; Chang, A.B.; Grimwood, K.; Lovie-Toon, Y.; Kaus, M.; Rablin, S.; Arnold, D.; Roberts, J.; Parfitt, S.; Anderson, J.; et al. Risk Factors for Chronic Cough in Young Children: A Cohort Study. Front. Pediatr. 2020, 8, 444. [Google Scholar] [CrossRef]
- Wurzel, D.F.; Marchant, J.M.; Yerkovich, S.T.; Upham, J.W.; Mackay, I.M.; Masters, I.B.; Chang, A.B. Prospective characterization of protracted bacterial bronchitis in children. Chest 2014, 145, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Upham, J.W.; Masters, I.B.; Redding, G.R.; Gibson, P.G.; Marchant, J.M.; Grimwood, K. Protracted bacterial bronchitis: The last decade and the road ahead. Pediatr. Pulmonol. 2016, 51, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Simons, E.; Foty, R.G.; Subbarao, P.; To, T.; Dell, S.D. Misdiagnosis of asthma in schoolchildren. Pediatr. Pulmonol. 2017, 52, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Naaktgeboren, C.A.; Bont, L.J.; van der Ent, C.K.; de Hoog, M.L.A. Defining asthma in children: How well do parents, doctors and spirometry agree? ERJ Open Res. 2020, 6, 00348-2019. [Google Scholar] [CrossRef]
- Saglani, S.; Menzie-Gow, A.N. Approaches to Asthma Diagnosis in Children and Adults. Front. Pediatr. 2019, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Glomb, W.B. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 260S–283S. [Google Scholar] [CrossRef]
- McFadden, E.R., Jr. Exertional dyspnea and cough as preludes to acute attacks of bronchial asthma. N. Engl. J. Med. 1975, 292, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Oppenheimer, J.J.; Irwin, R.S.; Panel, C.E.C. Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Petsky, H.L.; Acworth, J.P.; Clark, R.; Thearle, D.M.; Masters, I.B.; Chang, A.B. Asthma and protracted bronchitis: Who fares better during an acute respiratory infection? J. Paediatr. Child Health 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Upham, J.W.; Yerkovich, S.T.; Chang, A.B.; Marchant, J.M.; Carroll, M.; Simpson, J.L.; Gibson, P.G. Mediators of neutrophil function in children with protracted bacterial bronchitis. Chest 2014, 146, 1013–1020. [Google Scholar] [CrossRef]
- Hodge, S.; Upham, J.W.; Pizzutto, S.; Petsky, H.L.; Yerkovich, S.; Baines, K.J.; Gibson, P.; Simpson, J.L.; Buntain, H.; Chen, A.C.H.; et al. Is Alveolar Macrophage Phagocytic Dysfunction in Children With Protracted Bacterial Bronchitis a Forerunner to Bronchiectasis? Chest 2016, 149, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; O’Brien, K.L.; Nair, H.; Liu, L.; Theodoratou, E.; Qazi, S.; Luksic, I.; Fischer Walker, C.L.; Black, R.E.; Campbell, H.; et al. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health 2013, 3, 010401. [Google Scholar] [PubMed]
- Douros, K.; Everard, M.L. Time to Say Goodbye to Bronchiolitis, Viral Wheeze, Reactive Airways Disease, Wheeze Bronchitis and All That. Front. Pediatr. 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Escribano Montaner, A.; Garcia de Lomas, J.; Villa Asensi, J.R.; Asensio de la Cruz, O.; de la Serna Blazquez, O.; Santiago Burruchaga, M.; Mondejar Lopez, P.; Torrent Vernetta, A.; Feng, Y.; Van Dyke, M.K.; et al. Bacteria from bronchoalveolar lavage fluid from children with suspected chronic lower respiratory tract infection: Results from a multi-center, cross-sectional study in Spain. Eur. J. Pediatr. 2018, 177, 181–192. [Google Scholar] [CrossRef]
- Hare, K.M.; Chang, A.B.; Smith-Vaughan, H.C.; Bauert, P.A.; Spain, B.; Beissbarth, J.; Grimwood, K. Do combined upper airway cultures identify lower airway infections in children with chronic cough? Pediatr. Pulmonol. 2019, 54, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.F.; Parameswaran, G.I. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 2009, 49, 124–131. [Google Scholar] [CrossRef]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar]
- Murphy, T.F.; Brauer, A.L.; Grant, B.J.; Sethi, S. Moraxella catarrhalis in chronic obstructive pulmonary disease: Burden of disease and immune response. Am. J. Respir. Crit. Care Med. 2005, 172, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Welp, A.L.; Bomberger, J.M. Bacterial Community Interactions During Chronic Respiratory Disease. Front. Cell Infect. Microbiol. 2020, 10, 213. [Google Scholar] [CrossRef]
- Gallucci, M.; Pedretti, M.; Giannetti, A.; di Palmo, E.; Bertelli, L.; Pession, A.; Ricci, G. When the Cough Does Not. Improve: A Review on Protracted Bacterial Bronchitis in Children. Front. Pediatr. 2020, 8, 433. [Google Scholar]
- O’Grady, K.F.; Grimwood, K.; Sloots, T.P.; Whiley, D.M.; Acworth, J.P.; Phillips, N.; Marchant, J.; Goyal, V.; Chang, A.B. Upper airway viruses and bacteria and clinical outcomes in children with cough. Pediatr. Pulmonol. 2017, 52, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Latzin, P.; Fuchs, O. Asthma diagnosis in children: More evidence needed. Lancet Child Adolesc. Health 2017, 1, 83–85. [Google Scholar] [CrossRef]
- Marchant, J.M.; Newcombe, P.A.; Juniper, E.F.; Sheffield, J.K.; Stathis, S.L.; Chang, A.B. What is the burden of chronic cough for families? Chest 2008, 134, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lovie-Toon, Y.G.; Chang, A.B.; Newcombe, P.A.; Vagenas, D.; Anderson-James, S.; Drescher, B.J.; Otim, M.E.; O’Grady, K.F. Longitudinal study of quality of life among children with acute respiratory infection and cough. Qual. Life Res. 2018, 27, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Prime, S.J.; Carter, H.E.; McPhail, S.M.; Petsky, H.L.; Chang, A.B.; Graves, N.; Marchant, J.M. Chronic wet cough in Australian children: Societal costs and quality of life. Pediatr. Pulmonol. 2021, 56, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Ruffles, T.J.C.; Marchant, J.M.; Masters, I.B.; Yerkovich, S.T.; Wurzel, D.F.; Gibson, P.G.; Busch, G.; Baines, K.J.; Simpson, J.L.; Smith-Vaughan, H.C.; et al. Outcomes of protracted bacterial bronchitis in children: A 5-year prospective cohort study. Respirology 2021, 26, 241–248. [Google Scholar] [CrossRef]
| Variable | PBB Diagnosis | Total | p-Value ŧ | |
|---|---|---|---|---|
| No n = 817 (90.5%) | Yes n = 86 (9.5%) | n = 903 (100%) | ||
| Study | ||||
| ED Cough | 549 (67.2) | 53 (61.6) | 602 (66.7) | 0.001 |
| RICCi Kids | 232 (28.4) | 21 (24.4) | 253 (28.0) | |
| TLSIMM Kids | 36 (4.4) | 12 (14.0) | 48 (5.3) | |
| Age group (years) | ||||
| <1 | 149 (18.2) | 32 (37.2) | 181 (20.0) | <0.001 |
| 1-<2 | 208 (25.5) | 31 (36.1) | 239 (26.5) | |
| 2-<5 | 248 (30.3) | 20 (23.3) | 268 (29.7) | |
| ≥5 | 212 (26.0) | 3 (3.5) | 215 (23.8) | |
| Sex | ||||
| Male | 482 (59.0) | 52 (60.5) | 534 (59.1) | 0.792 |
| Female | 335 (41.0) | 34 (39.5) | 369 (40.9) | |
| Indigenous status of the child | ||||
| Other | 689 (84.3) | 68 (79.1) | 757 (83.8) | 0.207 |
| First Nations Australian | 128 (15.7) | 18 (20.9) | 146 (16.1) | |
| Birthweight | ||||
| <2500 g | 72 (8.8) | 6 (7.0) | 78 (8.6) | 0.564 |
| ≥2500 g | 745 (91.2) | 80 (93.0) | 825(91.4) | |
| Gestational age | ||||
| <37-weeks | 82 (10.0) | 9 (10.5) | 91 (10.1) | 0.900 |
| ≥37-weeks | 735 (90.0) | 77 (89.5) | 812 (89.9) | |
| Season of enrolment | ||||
| Summer | 155 (19.0) | 15 (17.4) | 170 (18.8) | 0.236 |
| Autumn | 271 (33.2) | 22 (25.6) | 293 (32.4) | |
| Winter | 273 (33.4) | 38 (44.2) | 311 (34.4) | |
| Spring | 118 (14.4) | 11 (12.8) | 129 (14.3) | |
| Attends childcare | ||||
| Yes | 368 (45.0) | 53 (61.6) | 421 (46.6) | 0.004 |
| No | 370 (45.3) | 31 (36.1) | 401 (44.4) | |
| Not applicable (≥6-years of age) | 79 (9.7) | 2 (2.3) | 81 (9.0) | |
| Exposed to indoor tobacco smoke | ||||
| Yes | 114 (13.9) | 11 (12.8) | 125 (13.8) | 0.766 |
| No | 703 (86.1) | 75 (87.2) | 778 (86.2) | |
| Number of other children in the house | ||||
| 0 | 230 (28.1) | 36 (41.8) | 266 (29.5) | 0.027 |
| 1 | 347 (42.5) | 28 (32.6) | 375 (41.5) | |
| ≥2 | 240 (29.4) | 22 (25.6) | 262 (29.0) | |
| Pets at home | ||||
| Yes | 429 (52.5) | 52 (60.5) | 481 (53.2) | 0.160 |
| No | 388 (47.5) | 34 (39.5) | 422 (46.7) | |
| Previous history of cough lasting >4-weeks | ||||
| Yes | 160 (19.6) | 29 (33.7) | 189 (20.9) | 0.002 |
| No | 657 (80.4) | 57 (66.3) | 714 (79.1) | |
| History of wheeze (parent-reported) | ||||
| Yes | 447 (54.7) | 49 (57.0) | 496 (54.9) | 0.688 |
| No | 370 (45.3) | 37 (43.0) | 407 (45.1) | |
| History of eczema | ||||
| Yes | 207 (25.3) | 13 (15.1) | 220 (24.4) | 0.036 |
| No | 610 (74.7) | 73 (84.9) | 683 (75.6) | |
| Asthma diagnosis in past 12-months | ||||
| Yes | 160 (19.6) | 4 (4.6) | 164 (18.2) | 0.001 |
| No | 657 (80.4) | 82 (95.4) | 739 (81.8) | |
| Cough type at presentation | ||||
| Dry | 286 (35.0) | 19 (22.1) | 305 (33.8) | 0.009 |
| Wet | 313 (38.3) | 32 (37.2) | 345 (38.2) | |
| Variable | 218 (26.7) | 35 (40.7) | 253 (28.0) | |
| Steroids (inhaled or oral) for current illness § | ||||
| Yes | 277 (33.9) | 17 (19.8) | 294 (32.6) | 0.008 |
| No | 540 (66.1) | 69 (80.2) | 609 (67.4) | |
| Antibiotics for current illness §§ | ||||
| Yes | 225 (27.5) | 22 (25.6) | 247 (27.3) | 0.698 |
| No | 592 (72.5) | 64 (74.4) | 656 (72.7) | |
| Diagnosis at discharge | ||||
| Upper respiratory tract infection £ | 242 (29.6) | 37(43.0) | 279 (30.9) | Not done |
| Asthma/reactive airways disease | 223 (27.3) | 8 (9.3) | 231 (25.6) | |
| Croup | 81 (9.9) | 9 (10.5) | 90 (10.0) | |
| Bronchiolitis | 72 (8.8) | 3 (3.5) | 75 (8.3) | |
| Pneumonia/lower respiratory tract infection | 52 (6.3) | 7 (8.3) | 59 (6.5) | |
| Bronchitis | 4 (0.5) | 1 (1.2) | 5 (0.5) | |
| Other ꝉ | 88 (10.8) | 8 (9.3) | 96 (10.6) | |
| Not documented | 55 (6.7) | 13 (15.1) | 68 (7.5) | |
| Hospitalized for current illness | ||||
| Yes | 278 (34.0) | 20 (23.3) | 298 (33.0) | 0.043 |
| No | 539 (66.0) | 66 (76.7) | 605 (67.0) | |
| Variable | PBB/n | Crude RR | 95% CI | Adjusted RR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Female sex | 34/369 | 1.03 | 0.68–1.56 | 0.93 | 0.54–1.58 | 0.778 |
| Australian First Nations child | 18/146 | 1.42 | 0.87–2.31 | 1.05 | 0.82–1.35 | 0.689 |
| Age group (months) | ||||||
| <12-months | 32/181 | 4.30 | 2.41–7.66 | 4.31 | 1.42–13.1 | 0.010 |
| 12-<24-months | 31/239 | 3.10 | 1.75–5.50 | 2.00 | 1.35–2.96 | 0.001 |
| >24-months | 23/483 | Ref | Ref | |||
| Childcare attendance | 53/421 | 1.86 | 1.22–2.83 | 2.32 | 1.48–3.63 | <0.001 |
| Prior history of cough lasting >4-weeks | 29/189 | 1.79 | 1.17–2.76 | 2.63 | 1.72–4.01 | <0.001 |
| Parent reported wheeze past 12-months | 49/496 | 1.32 | 0.80–2.20 | 1.73 | 1.17–2.58 | 0.007 |
| Diagnosis of asthma in past 12-months | 4/164 | 0.25 | 0.10–0.61 | 0.55 | 0.27–1.13 | 0.105 |
| Diagnosis of asthma and parent reported wheeze past 12-months * | 2/126 | 0.15 | 0.04–0.59 | 0.35 | 0.16–0.75 | 0.007 |
| History of eczema | 13/220 | 0.55 | 0.31–0.98 | 0.73 | 0.48–1.11 | 0.144 |
| Clinical diagnosis on the day of enrolment | ||||||
| Upper respiratory tract infection £ | 37/279 | Ref | ||||
| Asthma/RAD | 8/231 | 0.23 | 0.11–0.51 | 0.32 | 0.15–0.67 | 0.002 |
| Bronchiolitis | 3/75 | 0.27 | 0.08–0.91 | 0.14 | 0.06–0.31 | <0.001 |
| Bronchitis | 1/5 | 1.64 | 0.18–15.0 | 3.24 | 0.14–73.77 | 0.461 |
| Croup | 9/90 | 0.73 | 0.34–1.57 | 0.73 | 0.38–1.39 | 0.343 |
| Pneumonia, LRTI | 7/59 | 0.88 | 0.37–2.08 | 1.05 | 0.74–1.68 | 0.879 |
| Other ꝉ | 8/96 | 0.59 | 0.27–1.32 | 0.84 | 0.71–0.99 | 0.036 |
| Not documented § | 13/68 | 1.54 | 0.77–3.10 | 1.67 | 0.93–3.01 | 0.083 |
| PBB | ||||
|---|---|---|---|---|
| No n = 619 (%) | Yes n = 68 (%) | Total n = 687 (%) | p-Value ŧ | |
| Organism detected | 0.146 | |||
| None | 84 (13.6) | 8 (11.8) | 92 (13.4) | |
| Bacteria only | 166 (26.8) | 20 (29.4) | 206 (28.0) | |
| Virus only | 72 (11.6) | 2 (2.9) | 74 (10.8) | |
| Both virus and bacteria | 297 (48.0) | 38 (55.9) | 335 (48.8) | |
| Total bacteria detected | 0.141 | |||
| 0 | 156 (25.2) | 10 (14.7) | 166 (24.2) | |
| 1 | 189 (30.5) | 20 (29.4) | 209 (30.4) | |
| 2 | 169 (27.3) | 25 (36.8) | 194 (28.2) | |
| 3 | 94 (15.2) | 10 (14.7) | 104 (15.1) | |
| 4 | 10 (1.6) | 3 (4.4) | 13 (1.9) | |
| 5 | 1 (0.2) | 0 | 1 (0.1) | |
| Total viruses detected | 0.169 | |||
| 0 | 151 (24.4) | 12 (17.6) | 163 (23.7) | |
| 1 | 241 (38.9) | 23 (33.8) | 264 (38.4) | |
| 2 | 181 (29.2) | 24 (35.3) | 205 (29.8) | |
| 3 | 40 (6.5) | 7 (10.3) | 47 (6.8) | |
| 4 | 6 (1.0) | 2 (2.9) | 8 (1.2) | |
| Total viruses and/or bacteria detected | 0.015 | |||
| 0 | 81 (13.1) | 8 (11.8) | 89 (12.9) | |
| 1 | 123 (19.9) | 6 (8.8) | 129 (18.8) | |
| 2 | 106 (17.1) | 9 (13.2) | 115 (16.7) | |
| 3 | 104 (16.8) | 12 (17.6) | 116 (16.9) | |
| 4 | 105 (17.0) | 23 (33.8) | 128 (18.6) | |
| 5+ | 100 (16.2) | 10 (14.7) | 110 (16.0) | |
| Specific bacteria detected | ||||
| Streptococcus pneumoniae | 307 (49.6) | 32 (47.1) | 339 (49.3) | 0.691 |
| Haemophilus influenzae | 207 (33.4) | 28 (41.2) | 235 (34.2) | 0.198 |
| Moraxella catarrhalis | 311 (50.2) | 49 (72.1) | 360 (52.4) | <0.001 |
| Bordetella pertussis | 5 (0.8) | 0 | 5 (0.7) | - |
| Mycoplasma pneumoniae | 8 (1.3) | 0 | 8 (1.2) | - |
| Chlamydia pneumoniae | 3 (0.5) | 0 | 3 (0.4) | - |
| Specific viruses detected | ||||
| Rhinovirus | 172 (27.8) | 20 (29.4) | 192 (27.9) | 0.735 |
| Adenovirus | 29 (4.7) | 3 (4.4) | 32 (4.6) | 0.934 |
| Respiratory syncytial virus (A & B) | 104 (16.8) | 7 (10.3) | 111 (16.1) | 0.087 |
| Parainfluenza (1, 2 & 3) | 35 (5.6) | 6 (8.8) | 41 (6.0) | 0.283 |
| Influenza (A & B) | 22 (3.5) | 2 (2.9) | 24 (3.5) | 1.000 |
| Enterovirus | 24 (3.9) | 1 (1.5) | 25 (3.6) | 0.499 |
| Human bocavirus-1 | 16 (2.3) | 2 (2.9) | 18 (2.6) | 0.693 |
| Human metapneumovirus | 14 (2.0) | 2 (2.9) | 16 (2.3) | 0.664 |
| Human coronavirus HUK1, OC43, NL63 & 229E | 18 (2.9) | 3 (3.7) | 21 (3.1) | 0.454 |
| Human polyomavirus: KI & WU | 6 (1.0) | 4 (5.9) | 10 (1.5) | 0.012 |
| Baseline | Specialist Review | Both Timepoints in the Same Children | |
|---|---|---|---|
| Organism detected | |||
| None | 6 (10.0) | 8 (13.3) | 2 (3.3) |
| Bacteria only | 16 (26.7) | 25 (41.7) | 7 (11.7) |
| Virus only | 2 (3.3) | 3 (5.0) | 0 |
| Both virus and bacteria | 36 (60.0) | 24 (40.0) | 14 (23.3) |
| Specific bacteria detected | |||
| Streptococcus pneumoniae | 28 (46.7) | 38 (63.3) | 22 (36.7) |
| Haemophilus influenzae | 24 (40.0) | 24 (40.0) | 14 (23.3) |
| Moraxella catarrhalis | 44 (73.0) | 43 (71.7) | 33 (55.0) |
| Bordetella pertussis | 0 | 0 | 0 |
| Mycoplasma pneumoniae | 0 | 0 | 0 |
| Chlamydia pneumoniae | 0 | 0 | 0 |
| Specific viruses detected | |||
| Rhinovirus | 19 (31.7) | 12 (20.0) | 2 (3.3) |
| Adenovirus | 3 (5.0) | 6 (10.0) | 0 |
| Respiratory syncytial virus (A & B) | 7 (11.7) | 1 (1.7) | 0 |
| Parainfluenza (1, 2 & 3) | 6 (10.0) | 2 (3.3) | 1 (1.7) |
| Influenza (A & B) | 2 (3.3) | 1 (1.7) | 0 |
| Enterovirus | 1 (1.7) | 5 (8.3) | 0 |
| Human bocavirus-1 | 2 (3.3) | 2 (3.3) | 0 |
| Human metapneumovirus | 0 | 0 | 0 |
| Human coronavirus HKU1, OC43, NL63 & 229E | 2 (3.3) | 5 (8.3) | 0 |
| Human polyomavirus—KI & WU | 3 (5.0) | 5 (8.3) | 1 (1.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Grady, K.-A.F.; Mahon, J.; Arnold, D.; Grimwood, K.; Hall, K.K.; Goyal, V.; Marchant, J.M.; Phillips, N.; Acworth, J.; King, A.; et al. Predictors of the Development of Protracted Bacterial Bronchitis following Presentation to Healthcare for an Acute Respiratory Illness with Cough: Analysis of Three Cohort Studies. J. Clin. Med. 2021, 10, 5735. https://doi.org/10.3390/jcm10245735
O’Grady K-AF, Mahon J, Arnold D, Grimwood K, Hall KK, Goyal V, Marchant JM, Phillips N, Acworth J, King A, et al. Predictors of the Development of Protracted Bacterial Bronchitis following Presentation to Healthcare for an Acute Respiratory Illness with Cough: Analysis of Three Cohort Studies. Journal of Clinical Medicine. 2021; 10(24):5735. https://doi.org/10.3390/jcm10245735
Chicago/Turabian StyleO’Grady, Kerry-Ann F., Juliana Mahon, Daniel Arnold, Keith Grimwood, Kerry K. Hall, Vikas Goyal, Julie M. Marchant, Natalie Phillips, Jason Acworth, Alex King, and et al. 2021. "Predictors of the Development of Protracted Bacterial Bronchitis following Presentation to Healthcare for an Acute Respiratory Illness with Cough: Analysis of Three Cohort Studies" Journal of Clinical Medicine 10, no. 24: 5735. https://doi.org/10.3390/jcm10245735
APA StyleO’Grady, K.-A. F., Mahon, J., Arnold, D., Grimwood, K., Hall, K. K., Goyal, V., Marchant, J. M., Phillips, N., Acworth, J., King, A., Scott, M., & Chang, A. B. (2021). Predictors of the Development of Protracted Bacterial Bronchitis following Presentation to Healthcare for an Acute Respiratory Illness with Cough: Analysis of Three Cohort Studies. Journal of Clinical Medicine, 10(24), 5735. https://doi.org/10.3390/jcm10245735








