Current and Future Directions of Breast MRI
Abstract
:1. Introduction
2. High-Risk Screening
3. MRI Evaluation of Newly Diagnosed Breast Cancer
4. Response to Neoadjuvant Chemotherapy
5. Imaging Biomarkers
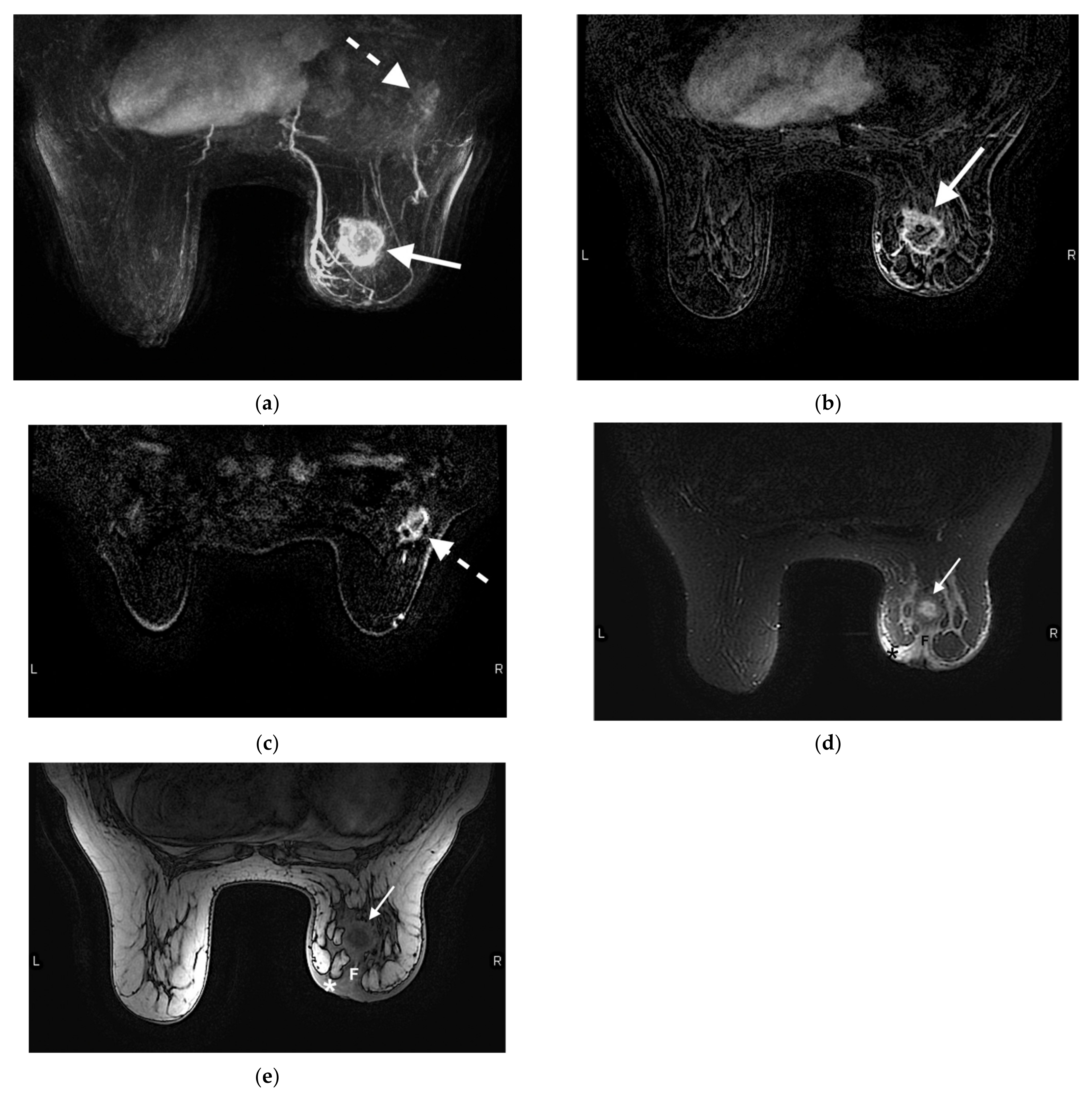
6. Abbreviated Breast MRI
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Duric, N.; Littrup, P. Breast ultrasound tomography. In Breast Imaging; Kuziak, C.M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- American Society of Breast Surgeons. Position Statement on Screening Mammography. Available online: https://www.breastsurgeons.org/docs/statements/Position-Statement-on-Screening-Mammography.pdf (accessed on 30 September 2021).
- Monticciolo, D.L.; Newell, M.S.; Hendrick, R.E.; Helvie, M.A.; Moy, L.; Monsees, B.; Kopans, D.B.; Eby, P.R.; Sickles, E.A. Breast Cancer Screening for Average-Risk Women: Recommendations From the ACR Commission on Breast Imaging. J. Am. Coll. Radiol. 2017, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Siu, A.L. U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, M.; Pisano, E.P.; Schnall, M.; Sener, S.; et al. ACS Guidelines for Breast Screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, J.G.; Armstrong, K.; Lehman, C.D.; Fletcher, S.W. Screening for breast cancer. JAMA 2005, 293, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Salam, B.; Khalid, Q.S.B.; Alam, S.; Sayani, R. Diagnostic Accuracy of Digital Mammography in the Detection of Breast Cancer. Cureus 2018, 10, e2448. [Google Scholar] [CrossRef] [Green Version]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef]
- Kuhl, C.K. MR imaging for surveillance of women at high familial risk for breast cancer. Magn. Reson. Imaging Clin. 2006, 14, 391–402. [Google Scholar] [CrossRef]
- Berg, W.A. Current Status of Supplemental Screening in Dense Breasts. J. Clin. Oncol. 2016, 34, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Brem, R.F.; Lenihan, M.J.; Lieberman, J.; Torrente, J. Screening breast ultrasound: Past, present, and future. AJR Am. J. Roentgenol. 2015, 204, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Brekelmans, C.T.M.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.A.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obdeijn, I.-M.; Winter-Warnars, G.A.O.; Mann, R.; Hooning, M.J.; Hunink, M.G.M.; Tilanus-Linthorst, M.M.A. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast Cancer Res. Treat 2014, 144, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.; Scaranelo, A.; Aboras, H.; Ghai, S.; Kulkarni, S.; Fleming, R.; Bukhanov, K.; Crystal, P. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology 2017, 285, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sippo, D.A.; Burk, K.S.; Mercaldo, S.F.; Rutledge, G.M.; Edmonds, C.; Guan, Z.; Hughes, K.S.; Lehman, C.D. Performance of Screening Breast MRI across Women with Different Elevated Breast Cancer Risk Indications. Radiology 2019, 292, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am. Coll. Radiol. 2018, 15 Pt A, 408–414. [Google Scholar] [CrossRef]
- Schacht, D.V.; Yamaguchi, K.; Lai, J.; Kulkarni, K.; Sennett, C.A.; Abe, H. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer: Results from screening breast MRI. AJR Am. J. Roentgenol. 2014, 202, 289–292. [Google Scholar] [CrossRef]
- Weinstock, C.; Campassi, C.; Goloubeva, O.; Wooten, K.; Kesmodel, S.; Bellevance, E.; Feigenberg, S.; Ioffe, O.; Tkaczuk, K.H.R. Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. Springerplus 2015, 4, 459. [Google Scholar] [CrossRef] [Green Version]
- Lehman, C.D.; Lee, J.M.; DeMartini, W.B.; Hippe, D.S.; Rendi, M.H.; Kalish, G.; Porter, P.; Gralow, J.; Partridge, S.C. Screening MRI in Women With a Personal History of Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djv349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gweon, H.M.; Cho, N.; Han, W.; Yi, A.; Moon, H.-G.; Noh, D.-Y.; Moon, W.K. Breast MR imaging screening in women with a history of breast conservation therapy. Radiology 2014, 272, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Giess, C.S.; Poole, P.S.; Chikarmane, S.A.; Sippo, D.A.; Birdwell, R.L. Screening Breast MRI in Patients Previously Treated for Breast Cancer: Diagnostic Yield for Cancer and Abnormal Interpretation Rate. Acad. Radiol. 2015, 22, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Port, E.R.; Park, A.; Borgen, P.I.; Morris, E.; Montgomery, L.L. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann. Surg. Oncol. 2007, 14, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Degnim, A.C.; Santen, R.J.; Dupont, W.D.; Ghosh, K. Atypical hyperplasia of the breast--risk assessment and management options. N. Engl. J. Med. 2015, 372, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, B.L.; Gangnon, R.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Natl. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghjyan, L.; Colditz, G.A.; Collins, L.C.; Schnitt, S.J.; Rosner, B.; Vachon, C.; Tamimi, R.M. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J. Natl. Cancer Inst. 2011, 103, 1179–1189. [Google Scholar] [CrossRef] [Green Version]
- Bakker, M.F.; De Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.; Lobbes, M.B.; et al. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Senate Bill No. 595. Penn. 2020. Available online: https://legiscan.com/PA/text/SB595/2019 (accessed on 21 September 2021).
- Brennan, M.E.; Houssami, N.; Lord, S.J.; Macaskill, P.; Irwig, L.; Dixon, J.M.; Warren, R.M.; Ciatto, S. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: Systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J. Clin. Oncol. 2009, 27, 5640–5649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plana, M.N.; Carreira, C.; Muriel, A.; Chiva, M.; Abraira, V.; Emparanza, J.I.; Bonfill, X.; Zamora, J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: Systematic review of diagnostic accuracy and meta-analysis. Eur. Radiol. 2012, 22, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, A.; Ortiz-Perez, T.; Ebuoma, L.; Sepulveda, K.; Severs, F.; Roark, A.; Wang, T.; Sedgwick, E. Is breast magnetic resonance imaging (MRI) useful for diagnosis of additional sites of disease in patients recently diagnosed with pure ductal carcinoma in situ (DCIS)? Eur. J. Radiol. 2017, 96, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Wardelmann, E.; Kuhn, W.; Maass, N.; Schrading, S. Impact of Preoperative Breast MR Imaging and MR-guided Surgery on Diagnosis and Surgical Outcome of Women with Invasive Breast Cancer with and without DCIS Component. Radiology 2017, 284, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Wilke, L.G.; Czechura, T.; Wang, C.; Lapin, B.; Liederbach, E.; Winchester, D.P.; Yao, K. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: A report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014, 149, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- McCahill, L.E.; Single, R.M.; Bowles, E.J.A.; Feigelson, H.S.; James, T.A.; Barney, T.; Engel, J.M.; Onitilo, A.A. Variability in reexcision following breast conservation surgery. JAMA 2012, 307, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobbes, M.B.; Vriens, I.J.; Van Bommel, A.C.; Nieuwenhuijzen, G.A.; Smidt, M.L.; Boersma, L.J.; Van Dalen, T.; Smorenburg, C.; Struikmans, H.; Siesling, S.; et al. Breast MRI increases the number of mastectomies for ductal cancers, but decreases them for lobular cancers. Breast Cancer Res. Treat. 2017, 162, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-Y.; Long, J.B.; Killelea, B.K.; Evans, S.B.; Roberts, K.B.; Silber, A.L.; Davidoff, A.J.; Sedghi, T.; Gross, C.P. Associations of preoperative breast magnetic resonance imaging with subsequent mastectomy and breast cancer mortality. Breast Cancer Res. Treat. 2018, 172, 453–461. [Google Scholar] [CrossRef]
- Sardanelli, F.; Trimboli, R.M.; Houssami, N.; Gilbert, F.J.; Helbich, T.H.; Benito, M.A.; Balleyguier, C.; Bazzocchi, M.; Bult, P.; Calabrese, M.; et al. Solving the preoperative breast MRI conundrum: Design and protocol of the MIPA study. Eur. Radiol. 2020, 30, 5427–5436. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.M.; Morrow, M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res. Treat. 2017, 165, 273–283. [Google Scholar] [CrossRef]
- Kupstas, A.; Ibrar, W.; Hayward, R.D.; Ockner, D.; Wesen, C.; Falk, J. A novel modality for intraoperative margin assessment and its impact on re-excision rates in breast conserving surgery. Am. J. Surg. 2018, 215, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.-S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Croshaw, R.; Shapiro-Wright, H.; Svensson, E.; Erb, K.; Julian, T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann. Surg. Oncol. 2011, 18, 3160–3163. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Trahan, T.J.; Xiao, J.; Taghipour, M.; Mena, E.; Connolly, R.M.; Subramaniam, R.M. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist 2016, 21, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, R.A.; Yau, C.; Rosen, M.; Tandon, V.J.; Hylton, N.; Esserman, L.J. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann. Surg. Oncol. 2013, 20, 3823–3830. [Google Scholar] [CrossRef] [Green Version]
- Denis, F.; Desbiez-Bourcier, A.; Chapiron, C.; Arbion, F.; Body, G.; Brunereau, L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur. J. Surg. Oncol. 2004, 30, 1069–1076. [Google Scholar] [CrossRef]
- Schrading, S.; Kuhl, C.K. Breast Cancer: Influence of Taxanes on Response Assessment with Dynamic Contrast-enhanced MR Imaging. Radiology 2015, 277, 687–696. [Google Scholar] [CrossRef]
- Chen, J.-H.; Bahri, S.; Mehta, R.S.; Carpenter, P.M.; McLaren, C.E.; Chen, W.-P.; Fwu, P.T.; Hsiang, D.J.B.; Lane, K.T.; Butler, J.A.; et al. Impact of factors affecting the residual tumor size diagnosed by MRI following neoadjuvant chemotherapy in comparison to pathology. J. Surg. Oncol. 2014, 109, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig, B.; Lewin, A.A.; Du, L.; Heacock, L.; Toth, H.K.; Heller, S.L.; Gao, Y.; Moy, L. Breast MRI for Evaluation of Response to Neoadjuvant Therapy. Radiographics 2021, 41, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Boughey, J.C.; Jones, K.N.; Shah, S.S.; Glazebrook, K.N. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann. Surg. Oncol. 2013, 20, 3199–3204. [Google Scholar] [CrossRef]
- Duric, N.; Littrup, P.; Sak, M.; Li, C.; Chen, D.; Roy, O.; Bey-Knight, L.; Brem, R. A Novel Marker, Based on Ultrasound Tomography, for Monitoring Early Response to Neoadjuvant Chemotherapy. J. Breast Imaging 2020, 2, 569–576. [Google Scholar] [CrossRef]
- Giess, C.S.; Yeh, E.D.; Raza, S.; Birdwell, R.L. Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 2014, 34, 234–247. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- DeMartini, W.B.; Liu, F.; Peacock, S.; Eby, P.; Gutierrez, R.L.; Lehman, C.D. Background parenchymal enhancement on breast MRI: Impact on diagnostic performance. AJR Am. J. Roentgenol. 2012, 198, W373–W380. [Google Scholar] [CrossRef]
- Ellis, R.L. Optimal timing of breast MRI examinations for premenopausal women who do not have a normal menstrual cycle. AJR Am. J. Roentgenol. 2009, 193, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Sippo, D.A.; Rutledge, G.M.; Burk, K.S.; Mercaldo, S.F.; Dontchos, B.N.; Edmonds, C.E.; Lehman, C.D. Effect of Background Parenchymal Enhancement on Cancer Risk Across Different High-Risk Patient Populations Undergoing Screening Breast MRI. AJR Am. J. Roentgenol. 2019, 212, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Arasu, V.A.; Miglioretti, D.L.; Sprague, B.L.; Alsheik, N.H.; Buist, D.S.; Henderson, L.M.; Herschorn, S.D.; Lee, J.M.; Onega, T.; Rauscher, G.H.; et al. Population-based assessment of the association between magnetic resonance imaging background parenchymal enhancement and future primary breast cancer risk. J. Clin. Oncol. 2019, 37, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- King, V.; Kaplan, J.; Pike, M.C.; Liberman, L.; Dershaw, D.D.; Lee, C.; Brooks, J.D.; Morris, E. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J. 2012, 18, 527–534. [Google Scholar] [CrossRef]
- Brem, R.F.; Ruda, R.C.; Yang, J.L.; Coffey, C.M.; Rapelyea, J.A. Breast-Specific γ-Imaging for the Detection of Mammographically Occult Breast Cancer in Women at Increased Risk. J. Nucl. Med. 2016, 57, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.; Sorenson, L.; Bennetts, L.; Winter, K.; Bryn, S.; Johnson, W.; Glissmeyer, M.; Garreau, J.; Blanchard, D. Breast-specific gamma imaging is a cost effective and efficacious imaging modality when compared with MRI. Am. J. Surg. 2014, 207, 698–701. [Google Scholar] [CrossRef]
- Hruska, C.B.; Geske, J.R.; Conners, A.L.; Whaley, D.H.; Rhodes, D.J.; O’Connor, M.K.; Carter, R.E.; Scott, C.G.; Vachon, C.M. Background Parenchymal Uptake on Molecular Breast Imaging and Breast Cancer Risk: A Cohort Study. AJR Am. J. Roentgenol. 2021, 216, 1193–1204. [Google Scholar] [CrossRef]
- McLaughlin, S.A. Current controversies surrounding breast cancer screening. J. Hematol Oncol. 2015, 11, 26–30. [Google Scholar]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.-D.; Bieling, H.B. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Osei, K.V.; Mehta, A.K.; Thigpen, D.M.; Rapelyea, J.; Friedman, S.; Brem, R.F. Abbreviated breast MRI for screening high-risk women: Comparison with the full clinical protocol. J. Breast Imaging 2021, 3, 196–200. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Korhonen, K.; Cirelli, C.; Schnall, M.D.; McDonald, E.S.; Pantel, A.R.; Zuckerman, S.; Borthakur, A.; Conant, E.F. Abbreviated Breast Magnetic Resonance Imaging for Supplemental Screening of Women With Dense Breasts and Average Risk. J. Clin Oncol. 2020, 38, 3874–3882. [Google Scholar] [CrossRef]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Partovi, S.; Sin, D.; Lu, Z.; Sieck, L.; Marshall, H.; Pham, R.; Plecha, D. Fast MRI breast cancer screening—Ready for prime time. Clin. Imaging 2020, 60, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Mango, V.L.; Harvey, J.A.; Plecha, D.M.; Conant, E.F. Implementation of Abbreviated Breast MRI for Screening: AJR Expert Panel Narrative Review [published online ahead of print, 11 August 2021]. AJR Am. J. Roentgenol. 2021. [Google Scholar] [CrossRef]
- Chikarmane, S.A.; Tai, R.; Meyer, J.E.; Giess, C.S. Prevalence and Predictive Value of BI-RADS 3, 4, and 5 Lesions Detected on Breast MRI: Correlation with Study Indication. Acad. Radiol. 2017, 24, 435–441. [Google Scholar] [CrossRef]
- Kuhl, C.K. Abbreviated Magnetic Resonance Imaging (MRI) for Breast Cancer Screening: Rationale, Concept, and Transfer to Clinical Practice. Annu. Rev. Med. 2019, 70, 501–519. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houser, M.; Barreto, D.; Mehta, A.; Brem, R.F. Current and Future Directions of Breast MRI. J. Clin. Med. 2021, 10, 5668. https://doi.org/10.3390/jcm10235668
Houser M, Barreto D, Mehta A, Brem RF. Current and Future Directions of Breast MRI. Journal of Clinical Medicine. 2021; 10(23):5668. https://doi.org/10.3390/jcm10235668
Chicago/Turabian StyleHouser, Margaret, David Barreto, Anita Mehta, and Rachel F. Brem. 2021. "Current and Future Directions of Breast MRI" Journal of Clinical Medicine 10, no. 23: 5668. https://doi.org/10.3390/jcm10235668
APA StyleHouser, M., Barreto, D., Mehta, A., & Brem, R. F. (2021). Current and Future Directions of Breast MRI. Journal of Clinical Medicine, 10(23), 5668. https://doi.org/10.3390/jcm10235668




