Sedation versus General Anesthesia for Cardiac Catheterization in Infants: A Retrospective, Monocentric, Cohort Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Procedural Characteristics
2.3. Anesthetic Technique
2.4. Early Outcome
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Adverse Events
3.3. Predictors of High-Severity Adverse Events
3.4. Predictors of Requirement for Additional Hemodynamic Support
4. Discussion
4.1. Adverse Events
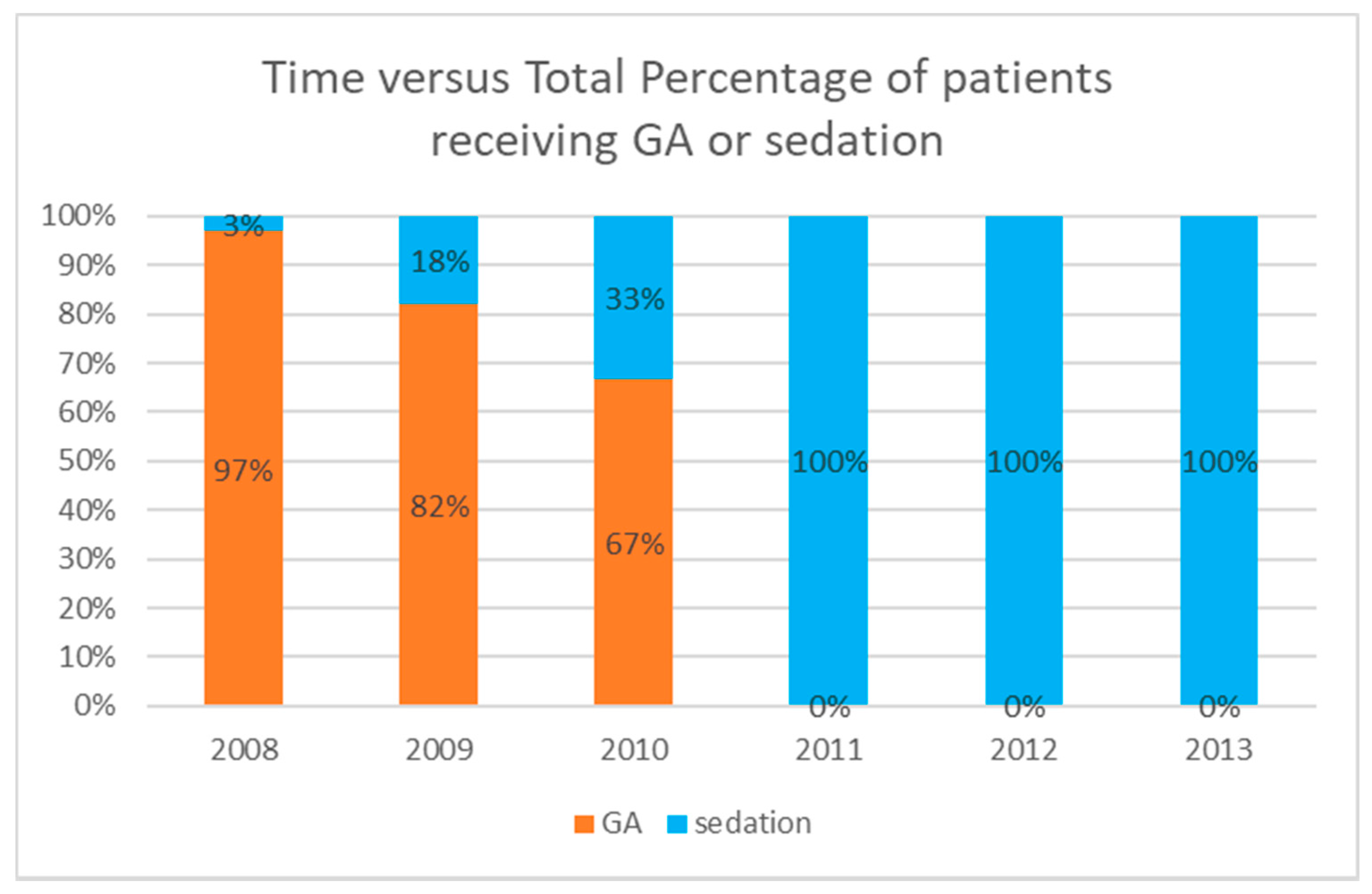
4.2. Sedation
4.3. Weight
4.4. Age Cluster
4.5. Age
4.6. Pulmonary Hypertension
4.7. Two-Ventricle
4.8. Hemodynamics
4.9. Experience
4.10. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollinger, I.; Mittnacht, A. Cardiac Catheterisation and Other Radiographic Examinations. In Pediatric Cardiac Anaesthesia, 4th ed.; Lake, C.L., Booker, P.D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 7, pp. 112–115. [Google Scholar]
- Lin, C.H.; Desai, S.; Nicolas, R.; Gauvreau, K.; Foerster, S.; Sharma, A.; Armsby, L.; Marshall, A.C.; Odegard, K.; DiNardo, J.; et al. Sedation and Anaesthesia in Pediatric and Congenital Cardiac Catheterization: A Prospective Multicenter Experience. Pediatr. Cardiol. 2015, 36, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.; Gauvreau, K.; Marshall, A.; Kreutzer, J.; Beekman, R.; Hirsch, R.; Foerster, S.; Balzer, D.; Vincent, J.; Hellenbrand, W.; et al. Procedure-Type Risk Categories for Pediatric and Congenital Cardiac Catheterization. Circ. Cardiovasc. Interv. 2011, 4, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cote, C.J.; Wilson, S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: An update. Pediatrics 2019, 143, e20191000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; pp. 119–138. [Google Scholar]
- Wood, S. Thin plate regression splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, R.N.; Moore, J.; Beekman, R.H.; Benson, L.; Bergersen, L.; Holzer, R.; Jayaram, N.; Jenkins, K.; Ringel, R.; Rome, J.; et al. Procedural characteristics and adverse events in diagnostic and interventional catheterizations in paediatric and adult CHD: Initial report from the IMPACT Registry. Cardiol. Young 2016, 26, 70–78. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, M.L.; Millenson, M.E.; Steven, J.M.; Gillespie, M.J.; Dori, Y.; Glatz, A.C.; Rome, J.J. Operator-Directed Procedural Sedation in the Congenital Cardiac Catheterization Laboratory. JACC Cardiovasc. Interv. 2019, 12, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Cua, C.; Kreutzer, J.; Armsby, L.; El-Said, H.; Moore, J.W.; Gauvreau, K.; Bergersen, L.; Holzer, R.J. Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter. Cardiovasc. Interv. 2013, 82, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hegde, S.; Marshall, A.C.; Porras, D.; Gauvreau, K.; Balzer, D.T.; Beekman, R.H., 3rd; Torres, A.; Vincent, J.A.; Moore, J.W.; et al. Risk and Management of Life threatening adverse events during cardiac catheterization for congenital heart disease. Pediatr. Cardiol. 2014, 35, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.J.; Derrick, G.; McEwan, A.; Haworth, S.G.; Sury, M.R.J. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br. J. Anaesth. 2007, 98, 657–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Griend, B.F.; Lister, N.A.; McKenzie, I.M.; Martin, N.; Ragg, P.G.; Sheppard, S.J.; Davidson, A.J. Postoperative Mortality in Children After 101,885 Anesthetics at a Tertiary Pediatric Hospital. Anesth. Analg. 2011, 112, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Carmosino, M.J.; Friesen, R.H.; Doran, A.; Ivy, D.D. Perioperative Complications in Children with Pulmonary Hypertension Undergoing Noncardiac Surgery or Cardiac Catheterization. Anesth. Analg. 2007, 104, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Twite, M.D.; Friesen, R.H. The Anesthetic Management of Children with Pulmonary Hypertension in the Cardiac Catheterization Laboratory. Anesthesiol. Clin. 2014, 32, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, N.; Beekman, R.H., 3rd; Benson, L.; Holzer, R.; Jenkins, K.; Kennedy, K.F.; Martin, G.R.; Moore, J.W.; Ringel, R.; Rome, J.; et al. Adjusting for Risk Associated with Pediatric and Congenital Cardiac Catheterization: A Report from the NCDR IMPACT Registry. Circulation 2015, 132, 1863–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bähner, T.; Heinze, I.; Dewald, O.; Mueller, M.; Schindler, E.; Schirmer, U.; Hoeft, A.; Baumgartner, E.; Ellerkmann, R.K. Anästhesiologische Versorgung an deutschen Zentren für Kinderherzchirurgie. Aktueller Stand der personellen und strukturellen Organisation. Anästh. Intensivmed. 2016, 57, 716–728. [Google Scholar]
- Baehner, T.; Kiefer, N.; Ghamari, S.; Graeff, I.; Huett, C.; Pflugradt, S.; Sendzik, B.; Heinze, I.; Mueller, M.; Schindler, E.; et al. A National Survey: Current Clinical Practice in Pediatric Anesthesia for Congenital Heart Surgery. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.; Koster, A.; Becke, K. Personelle, räumliche, apparative und organisatorische Voraussetzungen sowie Anforderungen bei der Erbringung von Anästhesieleistungen für herzchirurgische und kardiologische Eingriffe bei Kindern und Jugendlichen mit angeborenen Herzfehlern. Anästh. Intensivmed. 2017, 85, 518–524. [Google Scholar]





| Severity Level | Definition | |
|---|---|---|
| Low | 1-None (very mild) | No harm, no change in condition, may have required monitoring to assess for potential change in condition with no intervention indicated. |
| 2-Minor | Transient change in condition, not life threatening, condition returns to baseline, required monitoring, required minor intervention such as holding a medication, or obtaining lab test. | |
| High | 3-Moderate | Transient change in condition may be life threatening if not treated, condition returns to baseline, required monitoring, required intervention such as reversal agent, additional medication, transfer to the intensive care unit for monitoring, or moderate trans-catheter intervention to correct condition. |
| 4-Major | Change in condition, life-threatening if not treated, change in condition may be permanent, may have required an intensive care unit admission or emergency readmission to hospital, may have required invasive monitoring, required interventions such as electrical cardioversion or unanticipated intubation or required major invasive procedures or trans-catheter interventions to correct condition. | |
| 5-Catastrophic | Any death and emergency surgery or heart lung bypass support (ECMO) to prevent death with failure to wean from bypass support. | |
| GA Procedures | Sedation Procedures | p Value | |||
|---|---|---|---|---|---|
| (n = 368) | (n = 435) | ||||
| Mean age (m) | 6.5 ± 6.0 | 7.3 ± 6.2 | 0.07 | ||
| Mean weight (kg) | 5.9 ± 2.5 | 6.3 ± 2.5 | 0.01 | ||
| ASA physical status | 0.00 | ||||
| 1 | 0 | (0%) | 0 | (0%) | |
| 2 | 0 | (0%) | 0 | (0%) | |
| 3 | 38 | (10%) | 80 | (18%) | |
| 4 | 330 | (90%) | 355 | (82%) | |
| Status | 0.96 | ||||
| Native | 172 | 47% | 202 | 46% | |
| Palliated | 132 | 36% | 154 | 35% | |
| Repaired | 64 | 17% | 79 | 18% | |
| Single ventricle physiology | 155 (42%) | 178 (41%) | 0.77 | ||
| Cyanosis | 207 (56%) | 227 (52%) | 0.25 | ||
| Extracardiac anomalies | 33 (9%) | 52 (12%) | 0.21 | ||
| Pulmonary hypertension | 16 (4%) | 22 (5%) | 0.74 | ||
| Indication | 0.70 | ||||
| Diagnostic | 161 (44%) | 196 (45%) | |||
| Interventional | 207 (56%) | 239 (55%) | |||
| Procedure type risk categories | <0.01 | ||||
| 1 | 23 | 6% | 30 | 7% | |
| 2 | 193 | 52% | 285 | 66% | |
| 3 | 126 | 34% | 99 | 23% | |
| 4 | 26 | 7% | 21 | 5% | |
| GA Procedures | Sedation Procedures | p Value | Missing Values | |||
|---|---|---|---|---|---|---|
| (n = 368) | (n = 435) | |||||
| Procedural | ||||||
| Base excess | −2.6 ± 3.1 | −3.7 ± 3 | <0.01 | 7% | ||
| Puffer requirement | 64 (17%) | 32 (7%) | <0.01 | 0% | ||
| Lactate | 1 ± 0.7 | 0.8 ± 1.1 | 0.01 | 17% | ||
| Blood saturation | 85 ± 12 | 88 ± 10 | <0.01 | 6% | ||
| Central venous saturation | 57 ± 12 | 62 ± 11 | <0.01 | 24% | ||
| Left atrial pressure | 10 ± 4 | 9 ± 5 | <0.01 | 45% | ||
| Lowest mean arterial pressure | 47 ± 10 | 56 ± 11 | <0.01 | 20% | ||
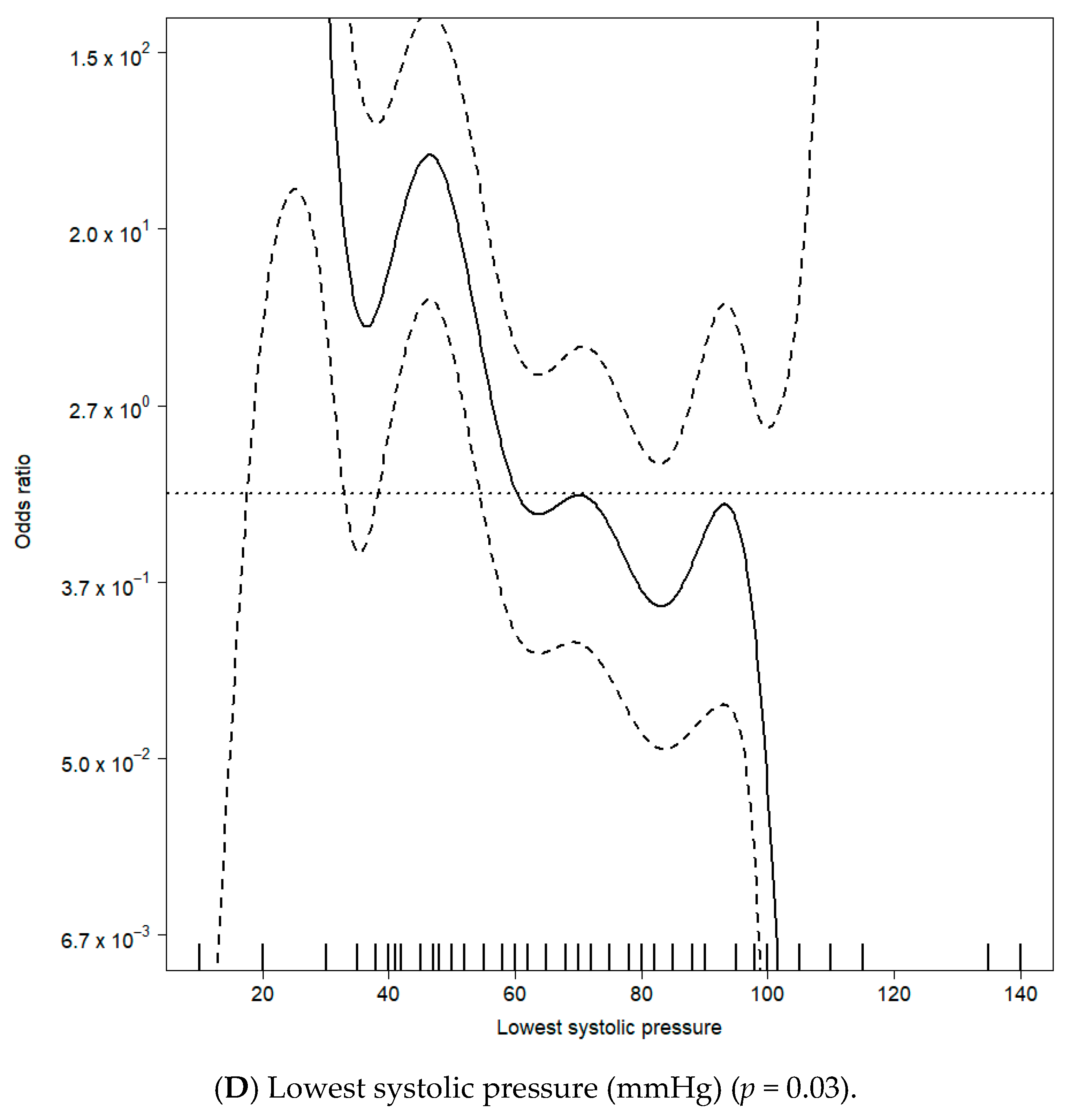
| Lowest systemic arterial pressure | 60 ± 13 | 76 ± 15 | <0.01 | 1% | ||
| Early post-procedural | ||||||
| 24 h mortality | 0 | 0% | 2 | 0.5% | 0.99 | |
| Intensive care admission | 48 | 13% | 27 | 6% | <0.01 | |
| Transfusion | 75 | 20% | 55 | 13% | <0.01 | |
| Hospital length of stay * | 4.9 ± 4.0 | 4.1 ± 2.5 | 0.01 | |||
| Severity Level Adverse Event | ||||||
| 1 | 199 | 54% | 369 | 85% | <0.01 | |
| 2 | 93 | 25% | 26 | 6% | ||
| 3 | 65 | 18% | 29 | 7% | ||
| 4 | 9 | 2% | 8 | 2% | ||
| 5 | 1 | 0% | 3 | 1% | ||
| Low severity (level 1–2) | 292 | 79% | 395 | 91% | <0.01 | |
| High severity (level 3–5) | 75 | 20% | 40 | 9% | ||
| Requirement for additional hemodynamic support | 127 (34%) | 35 (8%) | <0.01 | 0% | ||
| Cause | n = 115 (100%) |
|---|---|
| Requirement for ICU monitoring * | 39 (34%) |
| Hypotension | 31 (27%) |
| Respiratory failure | 26 (23%) |
| Rhythm or conduction disturbance | 19 (17%) |
| Resuscitation, independently of cause | 16 (14%) |
| n | High-Severity Adverse Event, n (% [95%CI]) | |
|---|---|---|
| Pulmonary arteries (dilatation or stent) intervention | 104 | 16 (15% [9–24%]) |
| Aortic arch (dilatation or stent) intervention | 91 | 6 (7% [2–14%]) |
| Patent ductus arteriosus closure | 63 | 3 (5% [1–13%]) |
| Aortopulmonary collateral closure | 53 | 6 (11% [4–23%]) |
| Balloon valvotomy | 40 | 10 (25% [13–41%]) |
| Rashkind procedure | 21 | 10 (48% [26–70%]) |
| Shunt (Blalock or Sano) intervention | 20 | 5 (25% [9–49%]) |
| Patent ductus arteriosus stenting | 15 | 3 (20% [4–48%]) |
| Right ventricle outflow tract procedure | 9 | 3 (33% [7–70%]) |
| Pulmonary artery banding dilatation | 7 | 3 (43% [10–82%]) |
| Systemic veins (dilatation or stent) intervention | 6 | 1 (17% [0–64%]) |
| Pulmonary veins (dilatation or stent) intervention | 5 | 1 (20% [0–72%]) |
| Biopsy | 4 | 2 (50% [7–93%]) |
| Ventricle septal defect closure | 3 | 0 (0% [NA]) |
| Other (fenestration occlusion, paraprosthesis leak closure) | 2 | 1 (50% [1–99%]) |
| Number | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| High-Severity Adverse Events (%) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | p Value | ||
| Use of sedation | 435 | 40 (9.2%) | 1.2 (0.8–1.8) | 1.2 (0.7–2.2) | 0.46 |
| Status | |||||
| Native | 374 | 67 (17.9%) | 1.0 | 1.0 | |
| Palliated | 286 | 35 (12.2%) | 3 (2.1–4.4) | 3.2 (1.2–8.9) | 0.02 |
| Corrected | 143 | 13 (9.1%) | 0.5 (0.2–1) | 0.5 (0.2–1.4) | 0.20 |
| Physiology | |||||
| Single-ventricle | 333 | 44 (13.2%) | 1.0 | 1.0 | |
| Two-ventricle | 470 | 71 (15.1%) | 0.3 (0.2–0.5) | 7.3 (2.7–20.2) | <0.01 |
| Cyanosis | 434 | 77 (17.7%) | 5.5 (3.5–8.4) | 4.6 (2.2–9.8) | <0.01 |
| Extracardiac anomalies | 85 | 13 (15.3%) | 0.7 (0.4–1.6) | 0.7 (0.3–1.8) | 0.44 |
| Pulmonary hypertension | 38 | 8 (21.1%) | 1.4 (0.5–3.5) | 5.6 (2.0–15.5) | <0.01 |
| Interventional catheterization | 446 | 71 (15.9%) | 3.4 (2.2–5.2) | 1.8 (1.1–3.2) | 0.02 |
| Procedure-type risk category | |||||
| 1 | 53 | 1 (1.9%) | 1.0 | 1.0 | |
| 2 | 478 | 52 (10.9%) | 17.1 (1.2–239.7) | 10.6 (0.8–142.5) | 0.08 |
| 3 | 225 | 48 (21.3%) | 11.9 (0.8–170.7) | 4.7 (0.3–67.0) | 0.25 |
| 4 | 47 | 14 (29.8%) | 33 (2.1–510.4) | 28.9 (1.8–455.1) | 0.02 |
| Number | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Requirement for Additional Hemodynamic Support (%) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | p Value | ||
| Use of sedation | 435 | 35 (8.0%) | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) | <0.01 |
| Status | |||||
| Native | 374 | 67 (17.9%) | 1.0 | 1.0 | |
| Palliated | 286 | 70 (24.5%) | 1.9 (1.3–2.7) | 2.4 (1.0–5.7) | 0.05 |
| Corrected | 143 | 25 (17.5%) | 0.4 (0.2–0.8) | 1.5 (0.7–3.0) | 0.28 |
| Physiology | |||||
| Single-ventricle | 333 | 83 (24.9%) | 1.0 | 1.0 | |
| Two-ventricle | 470 | 79 (16.8%) | 0.5 (0.3–0.7) | 1.4 (0.6–3.2) | 0.48 |
| Cyanosis | 434 | 112 (25.8%) | 3.1 (2–4.8) | 1.7 (0.9–3.4) | 0.11 |
| Extracardiac anomalies | 85 | 15 (16.6%) | 1.1 (0.6–1.9) | 1.7 (0.8–3.6) | 0.15 |
| Pulmonary hypertension | 38 | 8 (21.1%) | 3 (1.4–6.3) | 7.1 (3.0–16.9) | <0.01 |
| Interventional catheterization | 446 | 85 (19.1%) | 0.7 (0.5–1.1) | 0.9 (0.5–1.4) | 0.53 |
| Procedure type risk category | |||||
| 1 | 53 | 7 (13.2%) | 1.0 | 1.0 | |
| 2 | 478 | 72 (15.2%) | 23.3 (1.6–346.9) | 1.6 (0.5–5.5) | 0.48 |
| 3 | 225 | 66 (29.3%) | 16.1 (1.1–244.6) | 3.4 (0.9–12.9) | 0.07 |
| 4 | 47 | 17 (36.2%) | 46.5 (2.9–752.6) | 4.5 (1.0–21.1) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikus, M.; Welchowski, T.; Schindler, E.; Schneider, M.; Mini, N.; Vergnat, M. Sedation versus General Anesthesia for Cardiac Catheterization in Infants: A Retrospective, Monocentric, Cohort Evaluation. J. Clin. Med. 2021, 10, 5648. https://doi.org/10.3390/jcm10235648
Mikus M, Welchowski T, Schindler E, Schneider M, Mini N, Vergnat M. Sedation versus General Anesthesia for Cardiac Catheterization in Infants: A Retrospective, Monocentric, Cohort Evaluation. Journal of Clinical Medicine. 2021; 10(23):5648. https://doi.org/10.3390/jcm10235648
Chicago/Turabian StyleMikus, Marian, Thomas Welchowski, Ehrenfried Schindler, Martin Schneider, Nathalie Mini, and Mathieu Vergnat. 2021. "Sedation versus General Anesthesia for Cardiac Catheterization in Infants: A Retrospective, Monocentric, Cohort Evaluation" Journal of Clinical Medicine 10, no. 23: 5648. https://doi.org/10.3390/jcm10235648
APA StyleMikus, M., Welchowski, T., Schindler, E., Schneider, M., Mini, N., & Vergnat, M. (2021). Sedation versus General Anesthesia for Cardiac Catheterization in Infants: A Retrospective, Monocentric, Cohort Evaluation. Journal of Clinical Medicine, 10(23), 5648. https://doi.org/10.3390/jcm10235648






