Longitudinal Analyses of the Reciprocity of Depression and Anxiety after Traumatic Brain Injury and Its Clinical Implications
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Ethical Approval
2.3. Measures
2.4. Statistical Analysis
2.5. Role of the Funding Source
3. Results
3.1. Descriptive Statistics
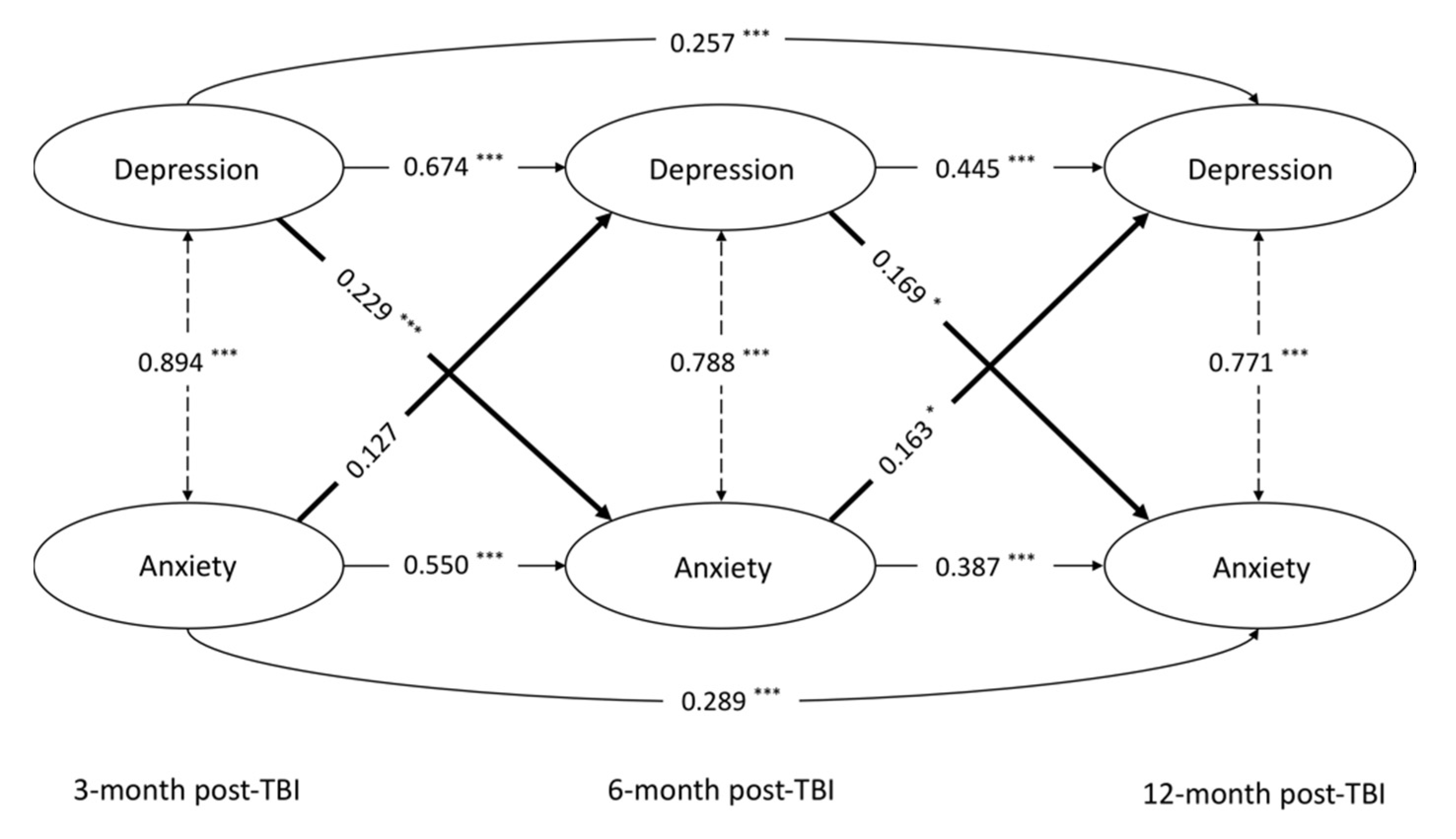
3.2. Longitudinal Cross-Lagged Model
3.3. Factors Associated with Higher Levels of MD and GAD after TBI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallya, S.; Sutherland, J.; Pongracic, S.; Mainland, B.; Ornstein, T.J. The manifestation of anxiety disorders after traumatic brain injury: A review. J. Neurotrauma 2015, 32, 411–421. [Google Scholar] [CrossRef]
- Osborn, A.; Mathias, J.; Fairweather-Schmidt, A.; Anstey, K. Anxiety and comorbid depression following traumatic brain injury in a community-based sample of young, middle-aged and older adults. J. Affect. Disord. 2017, 213, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.F.; Mohanty, S.; Sharma, V.; Tiwari, Y.; Choudhary, A.; Singh, V.P. Double extradural hematoma: An analysis of 46 cases. Neurol. India 2004, 52, 450–452. [Google Scholar]
- Hoffman, A.N.; Taylor, A.N. Stress reactivity after traumatic brain injury: Implications for comorbid post-traumatic stress disorder. Behav. Pharmacol. 2019, 30, 115–121. [Google Scholar] [CrossRef]
- Chen, J.-K.; Johnston, K.M.; Petrides, M.; Ptito, A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch. Gen. Psychiatry 2008, 65, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, R.; Robinson, R.G. Mood disorders following traumatic brain injury. Int. Rev. Psychiatry 2003, 15, 317–327. [Google Scholar] [CrossRef]
- Osborn, A.; Mathias, J.; Fairweather-Schmidt, A. Depression following adult, non-penetrating traumatic brain injury: A meta-analysis examining methodological variables and sample characteristics. Neurosci. Biobehav. Rev. 2014, 47, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Osborn, A.J.; Mathias, J.L.; Fairweather-Schmidt, A.K. Prevalence of anxiety following adult traumatic brain injury: A meta-analysis comparing measures, samples and postinjury intervals. Neuropsychology 2016, 30, 247–261. [Google Scholar] [CrossRef]
- Gilbody, S.; Richards, D.; Brealey, S.; Hewitt, C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): A diagnostic meta-analysis. J. Gen. Intern. Med. 2007, 22, 1596–1602. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, J.D.; Hammond, F.M. Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 2013, 94, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Robinson, R.G.; Starkstein, S.E.; Arndt, S. Influence of major depression on 1-year outcome in patients with traumatic brain injury. J. Neurosurg. 1994, 81, 726–733. [Google Scholar] [CrossRef]
- Steadman-Pare, D.; Colantonio, A.; Ratcliff, G.; Chase, S.; Vernich, L. Factors associated with perceived quality of life many years after traumatic brain injury. J. Head Trauma Rehabil. 2001, 16, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Rauen, K.; Reichelt, L.; Probst, P.; Schäpers, B.; Müller, F.; Jahn, K.; Plesnila, N. Quality of life up to 10 years after traumatic brain injury: A cross-sectional analysis. Health Qual. Life Outcomes 2020, 18, 166. [Google Scholar] [CrossRef]
- Rockhill, C.M.; Jaffe, K.; Zhou, C.; Fan, M.-Y.; Katon, W.; Fann, J.R. Health care costs associated with traumatic brain injury and psychiatric illness in adults. J. Neurotrauma 2012, 29, 1038–1046. [Google Scholar] [CrossRef]
- Hanel, G.; Henningsen, P.; Herzog, W.; Sauer, N.; Schaefert, R.; Szecsenyi, J.; Löwe, B. Depression, anxiety, and somatoform disorders: Vague or distinct categories in primary care? Results from a large cross-sectional study. J. Psychosom. Res. 2009, 67, 189–197. [Google Scholar] [CrossRef]
- Van der Horn, H.J.; Spikman, J.M.; Jacobs, B.; van der Naalt, J. Postconcussive complaints, anxiety, and depression related to vocational outcome in minor to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2013, 94, 867–874. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. The comorbidity of major depression and anxiety disorders: Recognition and management in primary care. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 244. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Robinson, R.G.; Moser, D.; Tateno, A.; Crespo-Facorro, B.; Arndt, S. Major depression following traumatic brain injury. Arch. Gen. Psychiatry 2004, 61, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.; Löwe, B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. Gen. Hosp. Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef]
- Whelan-Goodinson, R.; Ponsford, J.L.; Schönberger, M.; Johnston, L. Predictors of psychiatric disorders following traumatic brain injury. J. Head Trauma Rehabil. 2010, 25, 320–329. [Google Scholar] [CrossRef]
- Teymoori, A.; Gorbunova, A.; Haghish, F.E.; Real, R.; Zeldovich, M.; Wu, Y.J.; Polinder, S.; Asendorf, T.; Menon, D.; Steinbuchel, N. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J. Clin. Med. 2020, 9, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, A.; Wong, J.L.; Bagge, C.L.; Freedenthal, S.; Gutierrez, P.M.; Lozano, G. The Depression Anxiety Stress Scales-21 (DASS-21): Further examination of dimensions, scale reliability, and correlates. J. Clin. Psychol. 2012, 68, 1322–1338. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. International Statistical Classification of Diseases and Related Health Problems, 11th ed.; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Barker-Collo, S.; Theadom, A.; Jones, K.; Starkey, N.; Kahan, M.; Feigin, V.L. Depression and anxiety across the first 4 years after mild traumatic brain injury: Findings from a community-based study. Brain Inj. 2018, 32, 1651–1658. [Google Scholar] [CrossRef]
- Ren, D.; Fan, J.; Puccio, A.M.; Okonkwo, D.O.; Beers, S.R.; Conley, Y. Group-based trajectory analysis of emotional symptoms among survivors after severe traumatic brain injury. J. Head Trauma Rehabil. 2017, 32, E29–E37. [Google Scholar] [CrossRef] [PubMed]
- Zahniser, E.; Nelson, L.D.; Dikmen, S.S.; Machamer, J.E.; Stein, M.B.; Yuh, E.L.; Manley, G.T.; Temkin, N.R.; Adeoye, O.; Badjatia, N.; et al. The temporal relationship of mental health problems and functional limitations following mTBI: A TRACK-TBI and TED study. J. Neurotrauma 2019, 36, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Stevens, L.F.; Sutter, M.; Lequerica, A.H.; Krch, D.; Kolakowsky-Hayner, S.A.; Arango-Lasprilla, J.C. Reciprocal causation between functional independence and mental health 1 and 2 years after traumatic brain injury: A cross-lagged panel structural equation model. Am. J. Phys. Med. Rehabil. Jun 2017, 96, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.R.; Ponsford, J.L.; Johnston, L.; Schönberger, M. The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: A prospective study. Psychol. Med. 2011, 41, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.C.; Haagsma, J.A.; Cnossen, M.C.; Olff, M.; Van Beeck, E.F.; Polinder, S. Prevalence of and risk factors for anxiety and depressive disorders after traumatic brain injury: A systematic review. J. Neurotrauma 2016, 33, 1969–1994. [Google Scholar] [CrossRef] [PubMed]
- Demakis, G.J.; Hammond, F.M.; Knotts, A. Prediction of depression and anxiety 1 year after moderate-severe traumatic brain injury. Appl. Neuropsychol. 2010, 17, 183–189. [Google Scholar] [CrossRef]
- Rao, V.; Bertrand, M.; Rosenberg, P.; Makley, M.; Schretlen, D.J.; Brandt, J.; Mielke, M. Predictors of new-onset depression after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.S.; McCauley, S.R.; Josic, C.P.; Boake, C.; Brown, S.A.; Goodman, H.S.; Merritt, S.G.; Brundage, S.I. Predicting depression following mild traumatic brain injury. Arch. Gen. Psychiatry 2005, 62, 523–528. [Google Scholar]
- Deb, S.; Burns, J. Neuropsychiatric consequences of traumatic brain injury: A comparison between two age groups. Brain Inj. 2007, 21, 301–307. [Google Scholar] [CrossRef]
- Carroll, E.L.; Manktelow, A.E.; Outtrim, J.G.; Chatfield, D.; Forsyth, F.; Hutchinson, P.J.A.; Tenovuo, O.; Posti, J.P.; Wilson, L.; Sahakian, B.J.; et al. Influence of concomitant extracranial injury on functional and cognitive recovery from mild versus moderate to severe traumatic brain injury. J. Head Trauma Rehabil. 2020, 35, E513–E523. [Google Scholar] [CrossRef]
- Maas, A.I.; Menon, D.K.; Steyerberg, E.W.; Citerio, G.; Lecky, F.; Manley, G.T.; Hill, S.; Legrand, V.; Sorgner, A.; CENTER-TBI Participants and Investigators. Collaborative european neurotrauma effectiveness research in traumatic brain injury (center-tbi) a prospective longitudinal observational study. Neurosurgery 2015, 76, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: A European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019, 18, 923–934. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The glasgow coma scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Gennarelli, T.A.; Wodzin, E. AIS 2005: A contemporary injury scale. Injury 2006, 37, 1083–1091. [Google Scholar] [CrossRef]
- Sewalt, C.A.; Venema, E.; Wiegers, E.J.A.; Lecky, F.E.; Schuit, S.C.E.; Hartog, D.D.; Steyerberg, E.W.; Lingsma, H.F. Trauma models to identify major trauma and mortality in the prehospital setting. Br. J. Surg. 2020, 107, 373. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, N.; Lingsma, H.F.; Perel, P.; Lecky, F.; Roozenbeek, B.; Lu, J.; Shakur, H.; Weir, J.; Steyerberg, E.W.; Maas, A.I. Prognostic value of major extracranial injury in traumatic brain injury: An individual patient data meta-analysis in 39,274 patients. Neurosurgery 2012, 70, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.L.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollen, K.A. Structural equation models. Encycl. Biostat. 2005, 7. [Google Scholar]
- Curran, P.J.; Bollen, K.A. The Best of Both Worlds: Combining Autoregressive and Latent Curve Models; American Psychological Association: Washington, DC, USA, 2001; pp. 107–135. [Google Scholar]
- Masten, A.S.; Cicchetti, D. Developmental cascades. Dev. Psychopathol. 2010, 22, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selig, J.P.; Little, T.D. Autoregressive and Cross-Lagged Panel Analysis for Longitudinal Data; American Psychological Association: Washington, DC, USA, 2012. [Google Scholar]
- Kearney, M.W. Cross lagged panel analysis. In The SAGE Encyclopedia of Communication Research Methods; Sage Publications: Thousand Oaks, CA, USA, 2017; pp. 312–314. [Google Scholar]
- Muthén, L.; Muthén, B. Mplus Version 7 User’s Guide. 1998–2015. In Statistical Analysis with Latent Variables; Muthén & Muthén: Los Angeles, CA, USA, 2012. [Google Scholar]
- Beauducel, A.; Herzberg, P.Y. On the performance of maximum likelihood versus means and variance adjusted weighted least squares estimation in CFA. Struct. Equ. Modeling 2006, 13, 186–203. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bombardier, C.H.; Hoekstra, T.; Dikmen, S.; Fann, J.R. Depression trajectories during the first year after traumatic brain injury. J. Neurotrauma 2016, 33, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Alway, Y.; Gould, K.R.; Johnston, L.; McKenzie, D.; Ponsford, J. A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychol. Med. 2016, 46, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Godwin, E.E.; Lukow, H.R.; Lichiello, S. Promoting resilience following traumatic brain injury: Application of an interdisciplinary, evidence-based model for intervention. Fam. Relat. 2015, 64, 347–362. [Google Scholar] [CrossRef]
- Kreutzer, J.S.; Marwitz, J.H.; Sima, A.; Bergquist, T.F.; Johnson-Greene, D.; Felix, E.R.; Whiteneck, G.G.; Dreer, L.E. Resilience following traumatic brain injury: A traumatic brain injury model systems study. Arch. Phys. Med. Rehabil. 2015, 97, 708–713. [Google Scholar] [CrossRef]
- Wardlaw, C.; Hicks, A.J.; Sherer, M.; Ponsford, J.L. Psychological resilience is associated with participation outcomes following mild to severe traumatic brain injury. original research. Front. Neurol. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.; A Spielman, L.; Hibbard, M.R.; Silver, J.M.; Chandna, T.; A Gordon, W. Psychiatric challenges in the first 6 years after traumatic brain injury: Cross-sequential analyses of Axis I disorders. Arch. Phys. Med. Rehabil. 2004, 85, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Whelan-Goodinson, R.; Ponsford, J.; Schönberger, M. Association between psychiatric state and outcome following traumatic brain injury. J. Rehabil. Med. 2008, 40, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seel, R.T.; Kreutzer, J.S.; Rosenthal, M.; Hammond, F.M.; Corrigan, J.D.; Black, K. Depression after traumatic brain injury: A National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Arch. Phys. Med. Rehabil. 2003, 84, 177–184. [Google Scholar] [CrossRef]
- Alway, Y.; Gould, K.R.; McKay, A.; Johnston, L.; Ponsford, J. Factors associated with posttraumatic stress disorder following moderate to severe traumatic brain injury: A prospective study. Depress. Anxiety 2016, 33, 19–26. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Levin, H.S.; Contant, C.F.; Song, J.X. Postconcussional disorder following mild to moderate traumatic brain injury: Anxiety, depression, and social support as risk factors and comorbidities. J. Clin. Exp. Neuropsychol. 2001, 23, 792–808. [Google Scholar] [CrossRef]
- Shultz, S.R.; Sun, M.; Wright, D.K.; Brady, R.D.; Liu, S.; Beynon, S.; Schmidt, S.F.; Kaye, A.H.; Hamilton, J.A.; O’brien, T.J. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 2015, 35, 1339–1347. [Google Scholar] [CrossRef] [Green Version]
- Spitz, G.; Alway, Y.; Gould, K.R.; Ponsford, J.L. Disrupted white matter microstructure and mood disorders after traumatic brain injury. J. Neurotrauma 2017, 34, 807–815. [Google Scholar] [CrossRef]
- Moreno-López, L.; Sahakian, B.; Manktelow, A.; Menon, D.K.; Stamatakis, E.A. Depression following traumatic brain injury: A functional connectivity perspective. Brain Inj. 2016, 30, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- First, M.B. Structured clinical interview for the DSM (SCID). In The Encyclopedia of Clinical Psychology; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–6. [Google Scholar]
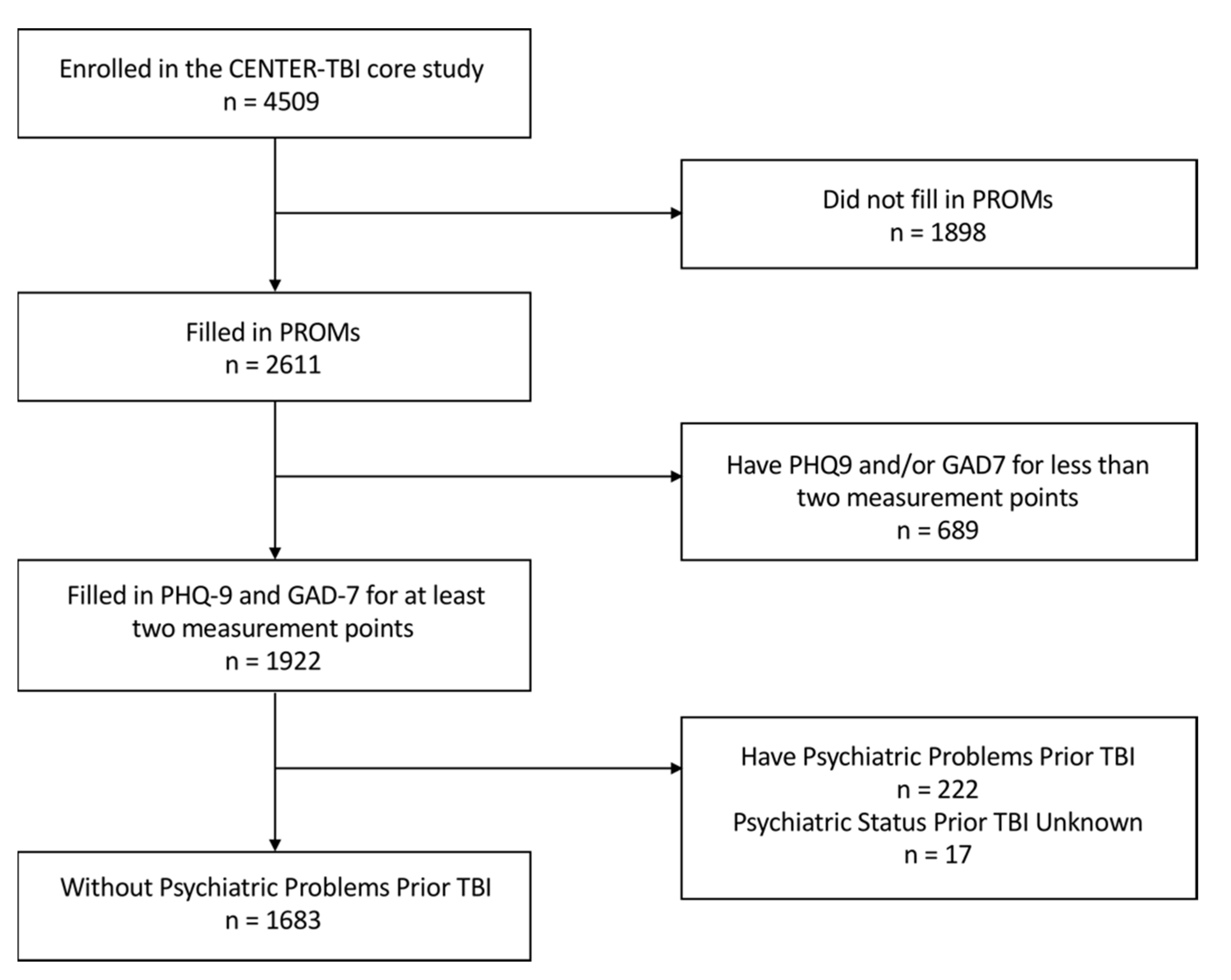
| Number/Mean Score | Percentage/Standard Deviation | |
|---|---|---|
| Female | 563 | 33.5% |
| Age 1 | 49.25 | 19.55 |
| Age group | ||
| Adolescent (16–24) | 263 | 15.6% |
| Young adult (25–34) | 198 | 11.8% |
| Adult (35–44) | 220 | 13.1% |
| Middle age (45–54) | 280 | 16.6% |
| Upper middle age (55–64) | 291 | 17.3% |
| Senior (≥65) | 431 | 25.6% |
| Years of education 1 | 13.95 | 4.15 |
| Education level | ||
| None | 13 | 0.9% |
| Currently studying | 45 | 2.9% |
| Primary school | 187 | 12.2% |
| Secondary/high school | 529 | 34.6% |
| Post high school training | 309 | 20.2% |
| College/university | 446 | 29.2% |
| Employment status | ||
| Employed, full-time | 739 | 46.2% |
| Employed, part-time | 132 | 11.4% |
| Sick leave | 6 | 0.4% |
| Unemployed | 91 | 5.7% |
| Retired | 399 | 24.9% |
| Student | 166 | 10.4% |
| Homemaker | 17 | 1.1% |
| Relationship status | ||
| Never been married | 481 | 29.7% |
| Married | 762 | 47.1% |
| Living together | 152 | 9.4% |
| Divorced | 115 | 7.1% |
| Separated | 29 | 1.8% |
| Widowed | 78 | 4.8% |
| Clinical pathway | ||
| ADM | 679 | 40.3% |
| ER | 319 | 19.0% |
| ICU | 685 | 40.7% |
| GCS score 1 | 12.85 | 3.74 |
| GCS category | ||
| Mild | 1252 | 76.6% |
| Moderate | 123 | 7.5% |
| Severe | 259 | 15.9% |
| MEI | 510 | 30.3% |
| Major trauma | 838 | 50.1% |
| GOSE score 1,2 | 6.77 | 1.42 |
| GOSE category | ||
| Good recovery | 1114 | 66.2% |
| Moderate disability | 415 | 24.7% |
| Severe disability | 154 | 9.2% |
| Prevalence * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cronbach’s α | Mean | SD | Min–Max [Q1, Q2, Q3] | Mild | Moderate | Moderately Severe | Severe | |
| (1) PHQ-9 at 3 m | 1519 | 0.86 | 4.71 | 4.89 | 0–25 [1, 3, 7] | 24.9% | 9.7% | 4.5% | 1.30% |
| (2) PHQ-9 at 6 m | 1600 | 0.87 | 4.41 | 4.85 | 0–27 [1, 3, 6] | 22.9% | 8.4% | 3.9% | 1.80% |
| (3) PHQ-9 at 12 m | 1156 | 0.87 | 4.46 | 4.98 | 0–27 [1, 3, 7] | 20.4% | 10.5% | 3.2% | 1.80% |
| (4) GAD-7 at 3 m | 1514 | 0.90 | 3.29 | 4.20 | 0–21 [0, 2, 5] | 18.0% | 6.4% | - | 3.10% |
| (5) GAD-7 at 6 m | 1601 | 0.90 | 3.14 | 4.10 | 0–21 [0, 2, 5] | 18.2% | 5.2% | - | 2.70% |
| (6) GAD-7 at 12 m | 1160 | 0.90 | 3.11 | 4.03 | 0–21 [0, 2, 5] | 17.8% | 5.5% | - | 2.50% |
| Major Depression Assessed with the PHQ-9 | Generalized Anxiety Disorder Assessed with the GAD-7 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | χ2 | df | p-Value | CFI | TLI | RMSEA [90%CI] | χ2 | df | p-Value | CFI | TLI | RMSEA [90%CI] |
| Baseline model | 980.700 | 294 | <0.001 | 0.982 | 0.979 | 0.037 [0.035–0.040] | 635.441 | 165 | <0.001 | 0.990 | 0.987 | 0.041 [0.038–0.045] |
| Loading invariance | 998.087 | 310 | <0.001 | 0.982 | 0.980 | 0.036 [0.034–0.039] | 665.040 | 177 | <0.001 | 0.989 | 0.987 | 0.040 [0.037–0.044] |
| Threshold invariance | 1004.896 | 344 | <0.001 | 0.983 | 0.983 | 0.034 [0.031–0.036] | 675.662 | 203 | <0.001 | 0.990 | 0.989 | 0.037 [0.034–0.040] |
| Unique factor invariance | 910.185 | 362 | <0.001 | 0.986 | 0.986 | 0.030 [0.028–0.032] | 621.769 | 217 | <0.001 | 0.991 | 0.991 | 0.033 [0.030–0.036] |
| Model | χ2 | df | p-Value | CFI | TLI | RMSEA [90%CI] | |
|---|---|---|---|---|---|---|---|
| 1 | autoregressive paths between adjacent time points | 2522.981 | 1025 | <0.001 | 0.982 | 0.980 | 0.029 [0.028–0.031] |
| 2 | add cross-lagged paths between adjacent time points | 2523.358 | 1021 | <0.001 | 0.982 | 0.980 | 0.030 [0.028–0.031] |
| 3 | add autoregressive paths between distant time points | 2338.889 | 1019 | <0.001 | 0.984 | 0.983 | 0.028 [0.026–0.029] |
| 4 | add cross-lagged paths between distant time points | 2347.931 | 1017 | <0.001 | 0.984 | 0.983 | 0.028 [0.026–0.029] |
| Depression | Anxiety | ||||
|---|---|---|---|---|---|
| Covariate 1 | Reference | β3 | p-Value 4 | β3 | p-Value 4 |
| Sex | Male | 0.211 | <0.001 | 0.207 | <0.001 |
| Age | - | −0.068 | 0.020 | −0.088 | 0.0030 |
| Years of education | - | −0.057 | 0.086 | −0.046 | 0.158 |
| Employment status | Employed | −0.047 | 0.44 | −0.102 | 0.098 |
| Relationship status | Stable relationship | 0.172 | 0.0042 | 0.106 | 0.080 |
| Clinical pathway | |||||
| ER | Adm | 0.051 | 0.047 | 0.006 | 0.85 |
| ICU | Adm | 0.333 | <0.001 | 0.222 | <0.001 |
| GCS score | - | −0.110 | <0.001 | −0.043 | 0.15 |
| MEI | No | 0.244 | <0.001 | 0.231 | <0.001 |
| Major trauma | No | 0.219 | <0.001 | 0.154 | 0.0093 |
| GOSE score at 6m after TBI 2 | - | −0.443 | <0.0001 | −0.337 | <0.001 |
| - | −0.216 | <0.001 | −0.142 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zeldovich, M.; Rauen, K.; Wu, Y.-J.; Covic, A.; Muller, I.; Haagsma, J.A.; Polinder, S.; Menon, D.; Asendorf, T.; et al. Longitudinal Analyses of the Reciprocity of Depression and Anxiety after Traumatic Brain Injury and Its Clinical Implications. J. Clin. Med. 2021, 10, 5597. https://doi.org/10.3390/jcm10235597
Wang B, Zeldovich M, Rauen K, Wu Y-J, Covic A, Muller I, Haagsma JA, Polinder S, Menon D, Asendorf T, et al. Longitudinal Analyses of the Reciprocity of Depression and Anxiety after Traumatic Brain Injury and Its Clinical Implications. Journal of Clinical Medicine. 2021; 10(23):5597. https://doi.org/10.3390/jcm10235597
Chicago/Turabian StyleWang, Biyao, Marina Zeldovich, Katrin Rauen, Yi-Jhen Wu, Amra Covic, Isabelle Muller, Juanita A. Haagsma, Suzanne Polinder, David Menon, Thomas Asendorf, and et al. 2021. "Longitudinal Analyses of the Reciprocity of Depression and Anxiety after Traumatic Brain Injury and Its Clinical Implications" Journal of Clinical Medicine 10, no. 23: 5597. https://doi.org/10.3390/jcm10235597
APA StyleWang, B., Zeldovich, M., Rauen, K., Wu, Y.-J., Covic, A., Muller, I., Haagsma, J. A., Polinder, S., Menon, D., Asendorf, T., Andelic, N., von Steinbuechel, N., & CENTER-TBI Participants and Investigators. (2021). Longitudinal Analyses of the Reciprocity of Depression and Anxiety after Traumatic Brain Injury and Its Clinical Implications. Journal of Clinical Medicine, 10(23), 5597. https://doi.org/10.3390/jcm10235597








