Functional Changes of the Genitourinary and Gastrointestinal Systems before and after the Treatment of Endometrial Cancer—A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
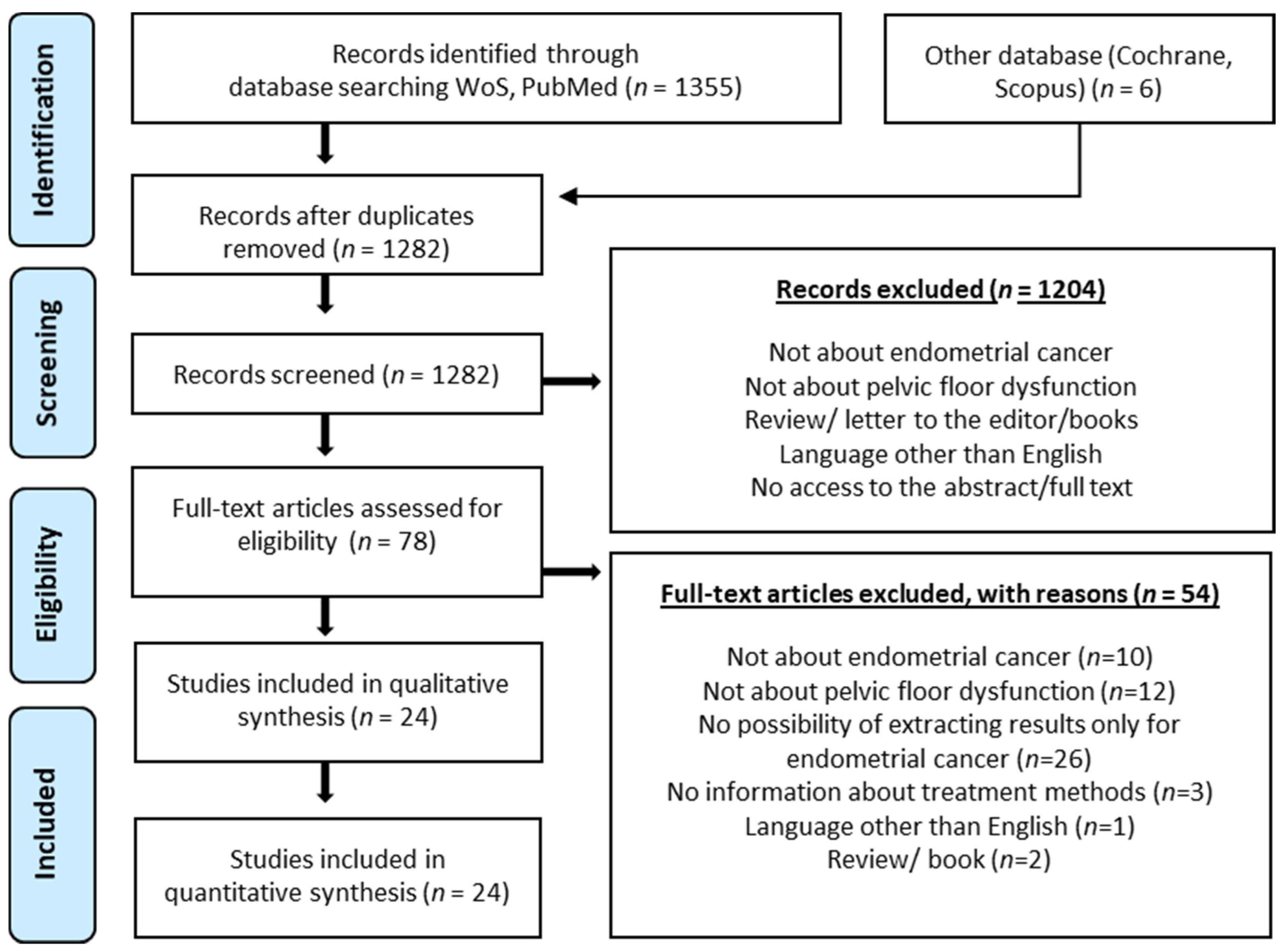
2. Materials and Methods
3. Results
3.1. Evaluation of Pelvic Floor Disorders in Women before Oncological Treatment
3.2. Evaluation of Pelvic Floor Disorders in Women after Surgical Treatment of Endometrial Cancer
3.3. Evaluation of Pelvic Floor Disorders in Women after Radiotherapy in Endometrial Cancer Treatment
3.4. Evaluation of Pelvic Floor Dysfunction in Women after Chemoradiotherapy in the Treatment of Endometrial Cancer
3.5. Management of Urogynecologic Disorders during Cancer Treatment in Women
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- The Global Cancer Observatory. Available online: https://gco.iarc.fr (accessed on 15 January 2021).
- Krajowy Rejestr Nowotworów. Available online: http://onkologia.org.pl/nowotwory-trzonu-macicy-kobiet-c54 (accessed on 15 January 2021).
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Zaino, R.J. FIGO staging of endometrial adenocarcinoma: A critical review and proposal. Int. J. Gynecol. Pathol. 2009, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer 2016, 26, 16–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Neron, M.; Bastide, S.; de Tayrac, R.; Masia, F.; Ferrer, C.; Labaki, M.; Boileau, L.; Letouzey, V.; Huberlant, S. Impact of gynecologic cancer on pelvic floor disorder symptoms and quality of life: An observational study. Sci. Rep. 2019, 9, 2250. [Google Scholar] [CrossRef] [Green Version]
- Rechberger, T.; Miotła, P.; Futyma, K.; Bartuzi, A.; Basta, A.; Opławski, M.; Stangel-Wójcikiewicz, K.; Baranowski, W.; Doniec, J.; Rogowski, A.; et al. Czynniki ryzyka defektów dna miednicy u kobiet zakwalifikowanych do operacji rekonstrukcyjnych: Polskie badanie wieloośrodkowe. Ginekol. Pol. 2010, 81, 821–827. [Google Scholar]
- Chase, D.M.; Watanabe, T.; Monk, B.J. Assessment and significance of quality of life in women with gynecologic cancer. Future Oncol. 2010, 6, 1279–1287. [Google Scholar] [CrossRef]
- Nout, R.A.; van de Poll-Franse, L.V.; Lybeert, M.L.; Wárlám-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, B.; Van Putten, W.L.; Creutzberg, C.L. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the postoperative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J. Clin. Oncol. 2011, 29, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Nout, R.A.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; Oerlemans, S.; et al. Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: Results from the randomized PORTEC-2 trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretschneider, C.E.; Sheyn, D.; Mahajan, S.T.; Ferrando, C.A. Adverse events after concurrent procedures for gynecologic malignancies and pelvic floor disorders. Obstet. Gynecol. 2018, 132, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Soisson, S.; Ganz, P.A.; Gaffney, D.; Rowe, K.; Snyder, J.; Wan, Y.; Herget, K.; Deshmukh, V.; Newman, M.; Fraser, A.; et al. Long-term, adverse genitourinary outcomes among endometrial cancer survivors in a large, population-based cohort study. Gynecol. Oncol. 2018, 148, 499–506. [Google Scholar] [CrossRef]
- Thomas, S.G.; Sato, H.R.; Glantz, J.C.; Doyle, P.J.; Buchsbaum, G.M. Prevalence of symptomatic pelvic floor disorders among gynecologic oncology patients. Obstet. Gynecol. 2013, 122, 976–980. [Google Scholar] [CrossRef]
- Lipetskaia, L.; Sharma, S.; Johnson, M.S.; Ostergard, D.R.; Francis, S. Urinary incontinence and quality of life in endometrial cancer patients after robotic-assisted laparoscopic hysterectomy with lymph node dissection. J. Obstet. Gynaecol. 2019, 39, 986–990. [Google Scholar] [CrossRef]
- Bretschneider, C.E.; Doll, K.M.; Bensen, J.T.; Gehrig, P.A.; Wu, J.M.; Geller, E.J. Prevalence of pelvic floor disorders in women with suspected gynecological malignancy: A survey-based study. Int. Urogynecol. J. 2016, 27, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Higgs, P.; Janda, M.; Asher, R.; Gebski, V.; Forder, P.; Obermair, A. Pelvic floor functional outcomes after total abdominal vs total laparoscopic hysterectomy for endometrial cancer. Am. J. Obstet. Gynecol. 2018, 218, 419.e1–419.e14. [Google Scholar] [CrossRef] [Green Version]
- Nosti, P.A.; McDermott, C.D.; Schilder, J.M.; Stehman, F.B.; Woodman, P.J. Symptoms of pelvic floor disorders and quality of life measures in postoperative patients with endometrial cancer. Clin. Ovarian Other Gynecol. Cancer 2012, 5, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Segal, S.; John, G.; Sammel, M.; Andy, U.U.; Chu, C.; Arya, L.A.; Brown, J.; Schmitz, K. Urinary incontinence and other pelvic floor disorders after radiation therapy in endometrial cancer survivors. Maturitas 2017, 105, 83–88. [Google Scholar] [CrossRef]
- Opławski, M.; Kojs, Z. An assessment of the urinary function and the comfort of life in patients after endometrial cancer combined treatment. Curr. Gynecol. Oncol. 2015, 13, 78–84. [Google Scholar] [CrossRef]
- Bahng, A.Y.; Dagan, A.; Bruner, D.W.; Lin, L.L. Determination of prognostic factors for vaginal mucosal toxicity associated with intravaginal high-dose rate brachytherapy in patients with endometrial cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 667–673. [Google Scholar] [CrossRef]
- Kauffmann, G.; Wu, T.; Al-Hallaq, H.; Hasan, Y. Triple-tandem high-dose-rate brachytherapy for early-stage medically inoperable endometrial cancer: Initial report on acute toxicity and dosimetric comparison to stereotactic body radiation therapy. Brachytherapy 2017, 16, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Emirdar, V.; Nayki, U.; Ertas, I.E.; Nayki, C.; Kulhan, M.; Yildirim, Y. Urodynamic assessment of short-term effects of pelvic radiotherapy on bladder function in patients with gynecologic cancers. Pol. J. Obstet. Gynecol. 2016, 87, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Kuku, S.; Fragkos, C.; McCormack, M.; Forbes, A. Radiation-induced bowel injury: The impact of radiotherapy on survivorship after treatment for gynaecological cancers. Br. J. Cancer 2013, 109, 1504–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecasteele, K.; Tummers, P.; Makar, A.; Van Eijkeren, M.; Delrue, L.; Denys, H.; Fonteyne, V.; Lambert, B.; Beerens, A.S.; Van den Broecke, R.; et al. Postoperative intensity-modulated arc therapy for cervical and endometrial cancer: A prospective report on toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Barillot, I.; Tavernier, E.; Peignaux, K.; Williaume, D.; Nickers, P.; Leblanc-Onfroy, M.; Lerouge, D. Impact of postoperative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: Results of the phase II RTCMIENDOMETRE French multicentre trial. Radiother. Oncol. 2014, 111, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Roszak, A.; Wareńczak-Florczak, Ż.; Bratos, K.; Milecki, P. Incidence of radiation toxicity in cervical cancer and endometrial cancer patients treated with radiotherapy alone versus adjuvant radiotherapy. Rep. Pract. Oncol. Radiother. 2012, 17, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samper-Ternent, R.; Zhang, D.; Kuo, Y.F.; Hatch, S.; Freeman, J. Late GI and bladder toxicities after radiation for uterine cancer. Gynecol. Oncol. 2011, 120, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Tropé, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Fyles, A.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Robison, K.; Lokich, E.; Raman, S.; Luis, C.; Raker, C.; Clark, M.A.; Wohlrab, K. Cancer of the uterus and treatment of stress incontinence: A pilot study. Am. J. Obstet. Gynecol. 2016, 214, 760. [Google Scholar] [CrossRef] [Green Version]
- Bochenska, K.; Mueller, M.; Geynisman-Tan, J.; Leader-Cramer, A.; Davé, B.; Lewicky-Gaupp, C.; Kenton, K. Concomitant repair of pelvic floor disorders in women undergoing surgery for gynecologic malignancies. Female Pelvic. Med. Reconstr. Surg. 2019, 25, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Brown, H.W.; Brubaker, L.; Cornu, J.N.; Daly, J.O.; Cartwright, R. Urinary incontinence in women. Nat. Rev. Dis. Primers 2017, 20, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erekson, E.A.; Sung, V.W.; DiSilvestro, P.A.; Myers, D.L. Urinary symptoms and impact on quality of life in women after treatment for endometrial cancer. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2009, 20, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skręt, A.; Kotarski, J.; Baranowski, W.; Basta, A.; Malinowski, A.; Naróg, M.; Markwitz-Nowak, R.; Rechberger, T.; Skręt-Magierło, J.; Tarkowski, R. Recommendations of the Polish Society of Obstetrics and Gynaecology Regarding Prevention and Treatment of Pelvic Organ Prolapse and Urinary Incontinence in Patients Qualified to Hysterectomy. Ginekol. Pol. 2009, 80, 459–465. [Google Scholar] [PubMed]
- Casarin, J.; Multinu, F.; Ubl, D.S.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Butler, K.A.; Ghezzi, F.; Habermann, E.B.; Mariani, A. Adoption of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet. Gynecol. 2018, 131, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Scaletta, G.; Dinoi, G.; Capozzi, V.; Cianci, S.; Pelligra, S.; Ergasti, R.; Fagotti, A.; Scambia, G.; Fanfani, F. Comparison of minimally invasive surgery with laparotomic approach in the treatment of high risk endometrial cancer: A systematic review. Eur. J. Surg. Oncol. 2020, 46, 782–788. [Google Scholar] [CrossRef]
- Paek, J.; Kang, E.; Lim, P.C. Comparative analysis of genitourinary function after type C1 robotic nerve-sparing radical hysterectomy versus type C2 robotic radical hysterectomy. Surg. Oncol. 2019, 30, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.S.; Naik, R. Pelvic floor dysfunction and radical hysterectomy. Int. J. Gynecol. Cancer 2006, 16, 354–363. [Google Scholar] [CrossRef]
- Aytan, H.; Ertunç, D.; Tok, E.C.; Yaşa, O.; Nazik, H. Prevalence of pelvic organ prolapse and related factors in a general female population. Turk. J. Obstet. Gynecol. 2014, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.E.; Tate, S.B.; Nicholas, J. Correlation of symptoms with degree of pelvic organ support in a general population of women: What is pelvic organ prolapse? Am. J. Obstet. Gynecol. 2003, 189, 372–377. [Google Scholar] [CrossRef]
- Rortveit, G.; Subak, L.L.; Thom, D.H.; Creasman, J.M.; Vittinghoff, E.; Van Den Eeden, S.K.; Brown, J.S. Urinary incontinence, fecal incontinence and pelvic organ prolapse in a population-based, racially diverse cohort: Prevalence and risk factors. Female Pelvic. Med. Reconstr. Surg. 2010, 16, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Nygaard, I.; Barber, M.D.; Burgio, K.L.; Kenton, K.; Meikle, S.; Schaffer, J.; Spino, C.; Whitehead, W.E.; Wu, J.; Brody, D.J. Prevalence of symptomatic pelvic floor disorders in US women. J. Am. Med. Assoc. 2008, 300, 1311–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bestvina, C.M.; Fleming, G.F. Chemotherapy for Endometrial Cancer in Adjuvant and Advanced Disease Settings. Oncologist 2016, 21, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Donovan, K.A.; Boyington, A.R.; Judson, P.L.; Wyman, J.F. Bladder and bowel symptoms in cervical and endometrial cancer survivors. Psychooncology 2014, 23, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Trimbos, J.B.; Maas, C.P.; Peters, A.A.W.; Kenter, G.G.; Maas, C.P.; Deruiter, M.C. A nerve-sparing radical hysterectomy: Guidelines and feasibility in Western patients. Int. J. Gynecol. Cancer 2001, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Enzlin, P.; Verhaeghe, J.; Poppe, W.; Vergote, I.; Amant, F. Sexual functioning in women after surgical treatment for endometrial cancer: A prospective controlled study. J. Sex. Med. 2015, 12, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Shisler, R.; Sinnott, J.A.; Wang, V.; Hebert, C.; Salani, R.; Felix, A.S. Life after endometrial cancer: A systematic review of patient-reported outcomes. Gynecol. Oncol. 2018, 148, 403–413. [Google Scholar] [CrossRef]
- Angioli, R.; Plotti, F.; Cafa, E.V.; Dugo, N.; Capriglione, S.; Terranova, C.; Montera, R.; Guzzo, F.; Panici, P.B. Quality of life in patients with endometrial cancer treated with or without systematic lymphadenectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Brennen, R.; Lin, K.Y.; Denehy, L.; Frawley, H.C. The effect of pelvic floor muscle interventions on pelvic floor dysfunction after gynecological cancer treatment: A systematic review. Physical. Ther. 2020, 100, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Kołomańska-Bogucka, D.; Nowakowski, C.; Tim, S. Urinary Incontinence in Women: Modern Methods of Physiotherapy as a Support for Surgical Treatment or Independent Therapy. J. Clin. Med. 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
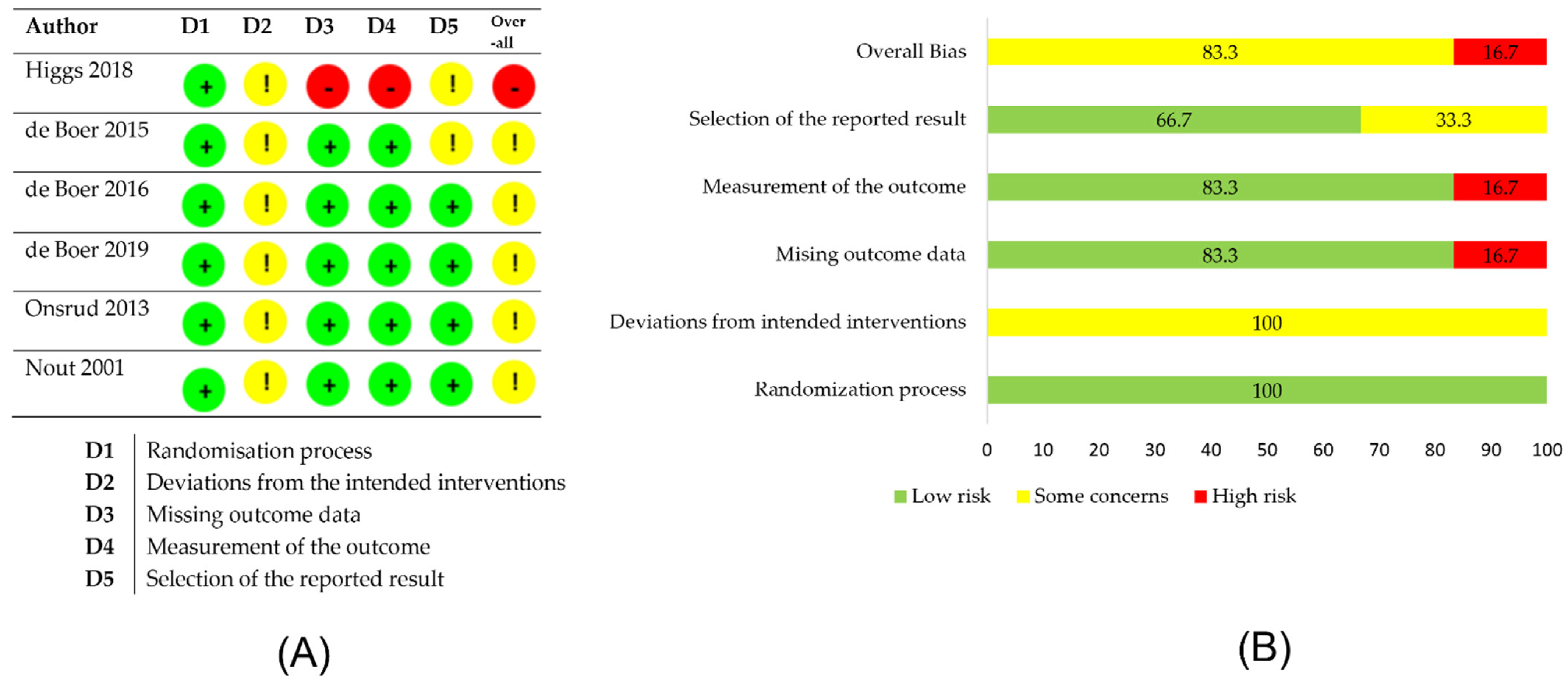
| Author | Type of Study | Bias Due to Confounding | Bias in Selection of Participants | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Result | Overall |
|---|---|---|---|---|---|---|---|
| Emirdar, 2016 | Prospective cohort | Serious | Low | Low | Moderate | Low | Moderate |
| Bretschneider, 2018 | Retrospective cohort | Serious | Serious | Moderate | Moderate | Serious | Serious |
| Bahng, 2012 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Bochenska, 2018 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Bretschneider, 2016 | Cross-sectional | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Kaufmann, 2016 | Comparative study | Serious | Serious | Low | Moderate | Serious | Serious |
| Kuku, 2013 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Lipetskaia, 2019 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Samper-Ternert, 2011 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Nosti, 2012 | Cross-sectional | Moderate | Moderate | Low | Moderate | Moderate | Moderate |
| Opławski, 2015 | Cross-sectional | Serious | Serious | Moderate | Moderate | Serious | Serious |
| Robison, 2016 | Prospective cohort | Serious | Serious | Moderate | No info | No info | Critical |
| Roszak, 2012 | Prospective | Serious | Low | Moderate | Moderate | Moderate | Moderate |
| Segal, 2018 | Retrospective | Serious | Moderate | Moderate | Moderate | Serious | Serious |
| Soisson, 2017 | Prospective cohort | Moderate | Moderate | Moderate | Serious | Serious | Serious |
| Thomas, 2013 | Cross-sectional | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Vandecasteele, 2011 | Prospective cohort | Moderate | Moderate | Moderate | Moderate | Serious | Serious |
| Author | Surgery | RTH | ChTH | UI | FI | POP | RTH Toxicity |
|---|---|---|---|---|---|---|---|
| Bretschneider CE et al., 2016 [19] | + | + | |||||
| Thomas SG et al. 2012 [17] | + | + | + | ||||
| Lipetskaia et al., 2019 [18] | + | + | |||||
| Higgs et al., 2017 [20] | + | + | + | ||||
| Nosti et al., 2012 [21] | + | + | + | ||||
| Segal, S. et al., 2017 [22] | + | + | + | + | + | ||
| de Boer et al., 2015 [14] | + | + | + | + | |||
| Opławski M et al., 2015 [23] | + | + | + | ||||
| Bahng AY et al., 2012 [24] | + | + | + | ||||
| Nout et al., 2011 [13] | + | + | + | ||||
| Kauffmann G et al., 2017 [25] | + | + | |||||
| Soisson, S et al., 2018 [16] | + | + | + | ||||
| Emirdar et al., 2016 [26] | + | + | + | ||||
| Kuku et al., 2013 [27] | + | + | + | ||||
| Vandecasteele, K et al., 2012 [28] | + | + | + | ||||
| Barillot I et al., 2014 [29] | + | + | + | ||||
| Roszak A et al., 2012 [30] | + | + | + | ||||
| Samper-Ternent, R et al., 2011[31] | + | + | + | ||||
| Onsrud M et al., 2013 [32] | + | + | + | ||||
| de Boer et al., 2016 [33] | + | + | + | + | |||
| de Boer et al., 2019 [9] | + | + | + | + | |||
| Robison et al., 2016 [34] | + | + | |||||
| Bretschneider C et al., 2018 [15] | + | + | + | ||||
| Bochenska et al., 2018 [35] | + | + |
| Author | Objective | Participants | Assessment | Pelvic Floor Dysfunction in EC, n (%) | Summary | ||||
|---|---|---|---|---|---|---|---|---|---|
| UI | SUI | UUI | FI | POP | |||||
| Bretschneider et al., 2016 [19] | To assess prevalence of PFD in women with suspected GM. | 152 women with GM aged 58.1 ± 13.3 including 94 women with EC. BMI: 33.6 ± 8.8 | RSC, ICIQ-FLUTS | 35 (37) | 27 (29) | 23 (24) | 3 (3) | N/I | Pelvic floor disorders are common in women with suspected gynecological malignancies. |
| Thomas et al., 2012 [17] | To assess prevalence of PFD in women with suspected GM. | 549 women aged 59.6±0.9: 347 (63.2%) women with GM, including 189 (49.9%) women with EC. 202 (36.8%) with benign gynecological condition BMI: 32.2 ± 0.5 | One page questionnaire on pelvic floor dysfunction based on PFDI | 102 (55) | 67 (36) | 27 (14.5) | N/I | 13 (7) | The prevalence of UI at baseline was similar among patients with all types of gynecologic cancer. No significant difference was observed in prevalence or severity of UI and POP between women with benign vs. malignant gynecology disease. |
| Author | Objective | Participants | Assessment | Intervention | PFD Preop. | PFD Postop. | Outcomes |
|---|---|---|---|---|---|---|---|
| Lipetskaia et al., 2019 [18] | To assess the long term effect of lymph node dissection on LUTS in patients treated for EC. | 74 women with EC—FIGO stage I Control group: 37 women aged 58 ± 11 Study group: 37 women aged 60 ± 11 | UDI-6, IIQ-7 after 13 ± 8 months | Robotic-assisted TLH and BSO; Control group: w/o additional intervention Study group: with lymph node dissection | Total UI: 28% | Total UI: 74.3% Control group: IIQ-7 14.9+/−23 UDI-6 30.0+/−25.3 Study group: IIQ-7 10.5+/−22.9(p = 0.419) UDI-6 20.7+/−22.9(p = 0.104) The odds ratio for developing new-onset UI—2.4 with 95% CI 0.62–9.5 (p 1⁄4 0.18). | There was a significant increase in the incidence of urinary incontinence after robotic-assisted TLH and BSO. No statistically significant increase in the incidence of UI in the study group. No statistically significant differences in IIQ-7 and UDI-6 score depending on lymph node dissection. |
| Higgs et al., 2017 [20] | To assess PFD after treatment for early stage EC in patients who underwent TAH or TLH. | 381 women with EC FIGO stage IA 195 women aged 62.6 ± 10.9 underwent TAH 186 women aged 63.0 ±9.5 underwent TLH | PFDI preop. and 6, 18, 30, 42, 54 months postop. | Patients were randomly allocated to TAH or TLH | Moderate to severe symptoms TAH: US: 6% POP: 5% CRAS: 4% TLH: US: 11% POP: 8% CRAS: 7% | Moderate to severe symptoms after 6 months TAH: US: 5% POP: 2% CRAS: 5% TLH: US: 6% POP: 3% CRAS: 4% | No statistically significant increase in the incidence of PFD in terms of urinary, bowel and prolapse symptoms in both groups after 6, 18, 30, 42 and 54 months postop. After 6 months all patients showed improvement from baseline in the POP and urinary stress domain. |
| Nosti et al., 2012 [21] | To assess prevalence of PFD in postoperative patients with EC and the impact of these issues on QoL. | 25 women aged 62 (±12) with EC FIGO stage IB. BMI 32(19–46) | PFDI-20 and PFIQ-7 after 19 (6–42) months | TAH with BSO ± lymph node sampling | N/I | Any symptoms: US: 76% POP: 44% CRAS: 68% Moderate to severe symptoms: US: 24% POP: 12% CRAS: 12% | Pelvic symptoms were reported by 84% patients (mild: 76%; severe: 8%) Quality of life issues associated with PFS: 44% patients. Pelvic symptoms were reported at a much higher rate than seen in the general public. |
| Author | Objective | Participants | Assessment | Intervention | Follow-Up Time | Outcomes |
|---|---|---|---|---|---|---|
| Segal, S. et al., 2017 [22] | To assess the prevalence of UI, FI, POP and sexual dysfunction in patients who underwent RTH for EC. | 159 women with EC FIGO stage I (radiation data available for 149 patients). Control group: 87 women Study group: 62 women | ISI, QUID, FISI, PFDI-20 question No 3, PISQ-12 | Surgery: hysterectomy via laparotomy, (85) minimally invasive techniques (60), or no surgery (4) Control group: no RTH Study group: 28 VBT, 34 EBRT | After 8–10 years from diagnosis | Symptoms no RTH vs. RTH UI 57.5% vs., 48.5% p = 0.47 FI 48.3% vs. 45.2% p = 0.66 POP 3.4% vs. 6.5% p = 0.33 Sexual function score (median) 32 vs. 21 p = 0.03 |
| de Boer et al., 2015 [14] | To assess the long-term outcome, HRQL, urinary and bowel symptoms and sexual functioning of patients with stage I high-intermediate risk EC treated with EBRT or VBT | 427 women with FIGO stage I high-intermediate risk EC | EORTC-QLQ-C30 | 214 women aged 69.3(51–89) received EBRT 213 women aged 69.8(46–85) received VBT | After 7 and 10 years | Symptoms (severe to moderate): UU EBRT 39.3%, VBT 25.5% p = 0.05 UI EBRT 11.9% VBT 8.7% p = 0.89 FL EBRT 10.6%, VBT 1.8% p = 0.0 Symptoms (mild, moderate or severe): UU EBRT 67,9% VBT 61,3% UI EBRT 42,9% VBT 45,2% FL EBRT 24,7% VBT 15% |
| Opławski M et al., 2015 [23] | To evaluate urinary tract function and QoL in EC patients after combined treatment. | 46 women: 23 EC stage IA patients (G1-G2) 23 non-oncological patients | Medical history, gynecologic and urodynamic evaluation, STAI, BDI | Study group: Radical hysterectomy and VBT Control Group: Non-oncological hysterectomy with uterine appendage removal | 6–12 months after surgical treatment | Significant difference between the two groups in terms of urogynecological outcomes (p = 0.0193). Study group (%): SUI = 26 MUI = 39 OAB = 13 Control group (%): SUI = 4 MUI = 22 OAB = 9 |
| Bahng AY et al., 2012 [24] | To evaluate prognostic factors and occurrence rates of radiation-induced vaginal mucosal toxicity in patients who have received BRT for EC. | 100 EC patients aged 41–84 | CTCAE v. 4.02. | Total hysterectomy + BSO with or without lymph node dissection and adjuvant VBT Study group: vaginal dilator use Control group: no dilator | 24 months (4 months to 14 years) | The incidence of Grade 1 or asymptomatic vaginal toxicity was 33% and Grade 2–3 or symptomatic vaginal toxicity was 14% Use of vaginal dilator at least 1×/week was associated with decreased vaginal mucosal toxicity |
| Nout et al., 2011 [13] | To assess the long-term outcome and HRQL of patients with EC treated with or without pelvic RTH. | 714 patients with stage I EC 113 women after EBRT returned questionnaire 133 women with NAT returned questionnaire | EORTC, SF-36 | TLH with BSO Study group: EBRT Control group: NAT | After 13.3 years (2.8–18.5) | Women treated with EBRT reported lower scores on all scales of the SF-36 SF-36 scores: UU EBRT 46, NAT 32 p = 0.001 UI EBRT 30, NAT 16 p < 0.001 Need to remain close to toilet EBRT 26 NAT 10 p < 0.001 Fecal urgency EBRT 44 NAT 25 p < 0.001 FI EBRT 19 NAT 8 p = 0.002 Diarrhoea EBRT 25 NAT 10 p < 0.001 |
| Kauffmann G at al., 2017 [25] | To evaluate acute and late toxicity after triple-tandem high—dose VBT for medically inoperable EC. | 6 women with medically inoperable EC stage I aged 57 (53–70). BMI: 49.8 (44.8–76.5) | History, physical examination, CTCAE v. 4 | Triple-tandem high - dose VBT with (3) or without (3) preceding EBRT | Median follow-up of 6.5 months | EBRT + VBT: Acute GI toxicity 3/3 patients (grade 1–2) Acute GU toxicity 3/3 patients (grade 1–2) HDR VBT: Acute GI toxicity 2/6 patients (grade 1–2) Acute GU toxicity 2/6 patients (grade 1) |
| Soisson, S et al., 2018 [16] | To evaluate the urinary system and genital system disorders among EC survivors. | 2648 EC survivors and 10,503 individuals from the general population | ICD-9 diagnosis codes (genitourinary/urinary system, genital organs), ambulatory inpatient and surgery records | Study groups: S/S + ChTH/S + RTH/S + ChRTH Control group: general population | After 1–5 and 5–10 years | Urinary system disorders—HR: Surgery Surgery+ RTH (1–5 years): 1.46 Surgery+ RTH (5–10 years): 1.24 Genital system disorders: Surgery Surgery+ RTH (1–5 years): 1.26 Surgery+ RTH (5–10 years): 1.09 |
| Emirdar et al., 2016 [26] | To assess the short-term effects of adjuvant or primary curative RTH on the urinary system in women with gynecologic cancer. | 55 women: Group 1: 10 women with early stage cervical cancer aged 46.6 ± 8.6 Group 2: 36 women with EC aged 59.5 ± 5.2 Group 3: 9 women with IIB or advanced cervical cancer aged 46.6 ± 10.2 | Urodynamic examination | Group 2: TAH + BSO, pelvic + para-aortic lymph node dissection and omentectomy + adjuvant RTH | Before and 6 months after treatment | Group 2 (EC): Incontinence: preT 27.8%, postT 38.9%, p = 0.046 Positive UM: preT 33.3% postT 22.2% p = 0.157 FUUV: preT 195 ± 80.1 postT 186.9 ± 89.1 p = 0.649 NUUV: PreT 351.2 ± 119.0 PostT 301.8 ± 101.7 p = 0.037 SUUV PreT 485.3 ± 145.3 PostT 393.8 ± 122.8 p = 0.000 Bladder capacity (ml) PreT 600.2 ± 124.8 PostT 490.0 ± 92.6 p = 0.000 Residual urine (ml) preT 4.0 ± 1.3 postT 4.1 ± 1.0 p = 0.914 MVP preT 129.3 ± 40.1 postT 130.2 ± 56.9 p = 0.793 MDP preT 69.4 ± 23.8 postT 77.7 ± 41.6 p = 0.338 |
| Author | Study Objective | Participants | Follow Up | Assessment | Intervention (EC Patients) | Acute GI Toxicity | Acute Bladder Toxicity | Late GI Toxicity | Late Bladder Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Kuku et al., 2013 [27] | To describe the symptoms of radiotherapy-induced bowel injury. | 541 women: 219 women with CC aged 52 (27–81) 322 women with EC aged 63 (40–80). Selected for analysis: 77 CC patients, 73 EC patients | 3 months up to 10 years | Clinical examination, routine screening for bowel and bladder toxicity | S: TAH/TLH +BSO with peritoneal washings + ChTH (36% of patients) RTH: EBRT (100% of patients) Dose: 45 Gy in 25 fractions + 12 Gy in 2 fractions to the vaginal vault | N/I | N/I | 73 EC patients (23%) reported bowel toxicity: defecation urgency 8.7%, frequency < 4/day 65.3%, diarrhea 48%, pain 45.3%, bloating 30.7%, FI 21.3% | N/I |
| Vandecasteele, K et al., 2012 [28] | To evaluate acute and late toxicity after postoperative IMAT for EC. | 65 women: 41 women with EC aged 67 (50 to 83) 24 women with CC aged 49 (35–71) | Weekly during IMAT + after IMAT: 1,3 months, then every 3–6 months (years 1–5) | RTOG scoring system, the scale of GI urgency and incontinence in-house developed scales for rectal blood loss and UI, Radiation-Induced Lower Intestine scoring scale | S: TAH + BSO with or w/o pelvic lymphadenectomy OR resection of the local recurrence RTH: IMAT followed by VBT or an external boost if VBT was not feasible Para-aortic irradiation if PALN were affected Dose: 46 Gy in 23 fractions + 11–21 Gy | 93% Most of the toxicity was grade 1 Frequency 85%, abdominal cramps 56%, urgency 34%, nausea 29%, incontinence 7% | 63% Most of the toxicity was grade 1. Nycturia 46%, urge 41%, pollakiuria 34%, dysuria 32%, Incontinence 14% | 36% Most of the toxicity was grade 1, no grade 3 toxicity Frequency 20%, cramps 8%, urgency 4%, Mucus loss 4%, Abdominal discomfort 4% | 36% All grade 1–2 Incontinence 20%, pollakiuria 12%, urge 8%, dysuria 4%, nycturia 4% |
| Barillot I et al., 2014 [29] | To evaluate acute toxicity (grade 2 or higher) after IMRT for EC. | 46 women with EC (stage I-II) aged 65.5 (57–75) | During IMRT + within 90 days | CTCAE-3.0 | S: TAH/TLH +BSO. Pelvic lymphadenectomy in 96% of patients RTH: IMRT, 36 patients received additional HDR VBT Dose: 45.5 Gy (median) | 85%. Grade 2: <30%. Grade 3: 0% | 39.5%. Grade 2: <20% Grade 3: 0% | N/I | N/I |
| Roszak A et al., 2012 [30] | To evaluate acute and late toxicity after radiotherapy for gynecological cancer. | 263 patients with CC (n = 128) and EC (n = 135) treated with definitive (CC) or adjuvant RTH (CC and EC) | Weekly during treatment + 2 years | EORTC/RTOG toxicity scale | S: not specified RTH: EBRT + HDR VBT. Dose: 43.4 Gy + 18 Gy in 63 fractions | 26.5% Most was grade 0 (73.3%) and grade 1–2 (17.8%) | 18.5% Most was grade 0 (81,5%) and grade 1–2 (17.0%) | 7.4% Most of the toxicity was grade 0 (92.6%). There were no patients with toxicity of grade 3 or above | 1.5% Most of the toxicity was grade 0 (98.5%). There were no patients with toxicity of grade 3 or above |
| Samper-Ternent, R et al., 2011 [31] | To evaluate acute and late toxicity after radiotherapy for EC. | 8797 women with EC | 60 months | Database search for any gastrointestinal or bladder diagnosis based on ICD-9-CM codes | S: 87% had surgery, type not specified Study group: EBRT or radioactive implants or BOTH Control group: w/o radiation. Dose: not specified | Radiation 21.9% No radiation 17.5% (p < 0.0001). Any grade Inflammation 12.5%, hemorrhage 4.9%, Obstrucion 3.9% | Radiation 14.3% No radiation 15.5% (p = 0.1398) Any grade hemorrhage 4.7%, incontinence 4.6% | Radiation 60.8% No Radiation 53.1% (p < 0.0001) Any grade inflammation 42.2%, hemorrhage 31.5%, obstruction 12.4% | Radiation 35.8% No Radiation 31.9% (p = 0.0004) Any grade incontinence 17.3%, hemorrhage 17.3%, cystitis 10% |
| Onsrud M et al., 2013 [32] | To evaluate long-term effects of EBRT treatment for early-stage EC. | 568 EC patients, 280 with adjuvant VBT, 288 with adjuvant VBT + EBRT | 20+ years | French-Italian Glossary (toxicity) | S: TAH + BSO Study group: VBT + EBRT Control group: VBT Dose: 60 Gy (VBT), 40 Gy (EBRT) in 20 fractions | Study group: 27.4% grade 2 toxicity or higher, 2.9% grade 3–4 toxicity. Control group: 4.5% grade 2 toxicity, no reports of grade 3 or 4 toxicity Median survival time: study group: 20.48 years, control group 20.5 years (p = 0.186) | |||
| Author | Objective | Participants | Assessment | Intervention | Outcomes |
|---|---|---|---|---|---|
| de Boer et al., 2016 [33] | To assess the benefit of adjuvant ChRTH compared with RTH alone for women with high-risk EC. To assess 2-year toxicity and QoL. | 686 women with EC underwent TAH/TLH with BSO and next were randomly allocated (1:1) to receive either CHRTH or RTH alone. RTH group: 333 women aged 61.9 (55.9–68.1) CHRTH group: 327 women aged 62.5 (56.5–68.0) | Common Terminology Criteria for Adverse Events version 3.0 and EORTC QLQ-C30, CX24, OV28 after RTH and at 6, 12, 24, 36 and 60 months after randomization | RTH group underwent pelvic RTH 48.6 Gy in 1.8 Gy fractions, five times a week for 5.5 weeks. CHRTH group additionally received 2 cycles of cisplatin 50 mg/m2 in the first and fourth week of RTH, followed by 4 cycles of carboplatin area under the curve (AUC) 5 and paclitaxel 175 mg/m2 at 21-day intervals (and a 28-day interval between the second concurrent and first adjuvant cycle). | CHRTH for high-risk EC caused significantly higher incidence of severe adverse gastrointestinal events and reduced health-related quality of life during treatment compared with RTH alone, but with rapid recovery. After 6 and 12 months, there was no significant difference between groups in bowel and urinary symptoms. |
| de Boer et al., 2019 [20] | To compare five-year survival, recurrence and adverse events in women with high-risk EC treated with ChRTH compared with RTH alone. | 686 women with EC underwent TAH/TLH with BSO and next were randomly allocated (1:1) to receive either CHRTH or RTH alone. RTH group: 330 women aged 62.0 (55.8–68.2) CHRTH group: 330 women aged 62.4 (56.5–67.9) | Common Terminology Criteria for Adverse Events version 3.0 5 years after randomization | RTH group underwent pelvic RTH 48.6 Gy in 1.8 Gy fractions, five times a week. CHRTH group additionally received 2 cycles of cisplatin 50 mg/m2 in the first and fourth week of RTH, followed by 4 cycles of carboplatin area under the curve (AUC) 5 and paclitaxel 175 mg/m2 at 21-day intervals (and a 28-day interval between the second concurrent and first adjuvant cycle). | CHRTH for high-risk endometrial cancer did not improve five-year overall survival, but increase failure-free survival. No significant differences between treatment groups in genitourinary and gastrointestinal adverse events at 60 months. |
| Soisson at al., 2018 [16] | To assess the urinary system and genital system disorders among EC survivors. | 2648 EC survivors and 10,503 individuals from the general population. | ICD-9 diagnosis codes (genitourinary/urinary system, genital organs), ambulatory inpatient and surgery records | Surgery / surgery + ChTH/surgery + RTH/surgery + ChTH + RTH | EC survivors have higher incidence of urinary system and genital system disorders than general population. Patients treated with surgery in combination with RTH and/or ChTH were at higher risk for both urinary system and genital organ disorders compared to those treated with surgery alone. Stage at diagnosis was not associated with risk for genital organ disorders. Higher EC grade was associated with higher risk for urinary system disorders. Higher BMI was not strongly associated with urinary system or genital organ disorders |
| Author | Objective | Participants | Assessment | Intervention | Objective | Outcomes |
|---|---|---|---|---|---|---|
| Bochenska et al., 2018 [35] | To evaluate the rate of POPUI procedures in women undergoing surgery for a gynecologic malignancy. | 23,501 women with EC (n = 14,711), ovarian cancer (n = 5961), cervical cancer (n = 1922) | Primary surgery with or w/o concurrent procedure for PFD (SUI, POP, both) | ACS-NSQIP database analysis | n/i | 434 (2.9%) EC patients underwent concomitant POPUI procedures. In the whole group 76.9% of surgeries were performed for repair of POP, 23.1% were performed for SUI. No statistically significant difference in serious adverse events 30 days post operatively between groups. |
| Bretschneider C et al., 2018 [15] | To evaluate the outcomes after concurrent surgeries for gynecology cancer and PFD—retrospective study. | 25,138 cases of gynecological cancer | Primary surgery with or w/o concurrent procedure for PFD (SUI, POP, both) | ACS NSQIP database analysis ICD-9 codes | 30 days | 2.3% (589) patients underwent concurrent procedure for PFD, most commonly it was POP procedure. There was no statistically significant difference in adverse effects incidence between groups. The group that might benefit the most from concurrent PFD surgery is EC patients rather than CC or OC patients. |
| Robison at al., 2016 [34] | To assess whether EC patients could be screened for SUI before treatment and then referred for concurrent treatment of EC and SUI. | 59 women aged 62.1 (37–85) with early stage (IA-IIIC) EC | anti-incontinence concurrent surgery OR non-surgical SUI treatment OR SUI observation | author’s questionnaire, urogynecological examination | 32 days (14–60) | Baseline incidence of SUI: 39% (20) 80% (16) of patients screened positive for SUI wanted urogynecological consult before the surgery. 8 (53%) patients with EC and SUI wanted to undergo anti-incontinence concurrent surgery. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oplawski, M.; Średnicka, A.; Dutka, A.; Tim, S.; Mazur-Bialy, A. Functional Changes of the Genitourinary and Gastrointestinal Systems before and after the Treatment of Endometrial Cancer—A Systematic Review. J. Clin. Med. 2021, 10, 5579. https://doi.org/10.3390/jcm10235579
Oplawski M, Średnicka A, Dutka A, Tim S, Mazur-Bialy A. Functional Changes of the Genitourinary and Gastrointestinal Systems before and after the Treatment of Endometrial Cancer—A Systematic Review. Journal of Clinical Medicine. 2021; 10(23):5579. https://doi.org/10.3390/jcm10235579
Chicago/Turabian StyleOplawski, Marcin, Agata Średnicka, Aleksandra Dutka, Sabina Tim, and Agnieszka Mazur-Bialy. 2021. "Functional Changes of the Genitourinary and Gastrointestinal Systems before and after the Treatment of Endometrial Cancer—A Systematic Review" Journal of Clinical Medicine 10, no. 23: 5579. https://doi.org/10.3390/jcm10235579
APA StyleOplawski, M., Średnicka, A., Dutka, A., Tim, S., & Mazur-Bialy, A. (2021). Functional Changes of the Genitourinary and Gastrointestinal Systems before and after the Treatment of Endometrial Cancer—A Systematic Review. Journal of Clinical Medicine, 10(23), 5579. https://doi.org/10.3390/jcm10235579







