The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Outcomes
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Studies Included in the Meta-Analysis
3.2. Resuscitation Characteristics in Pre- vs. Intra-COVID-19 Periods
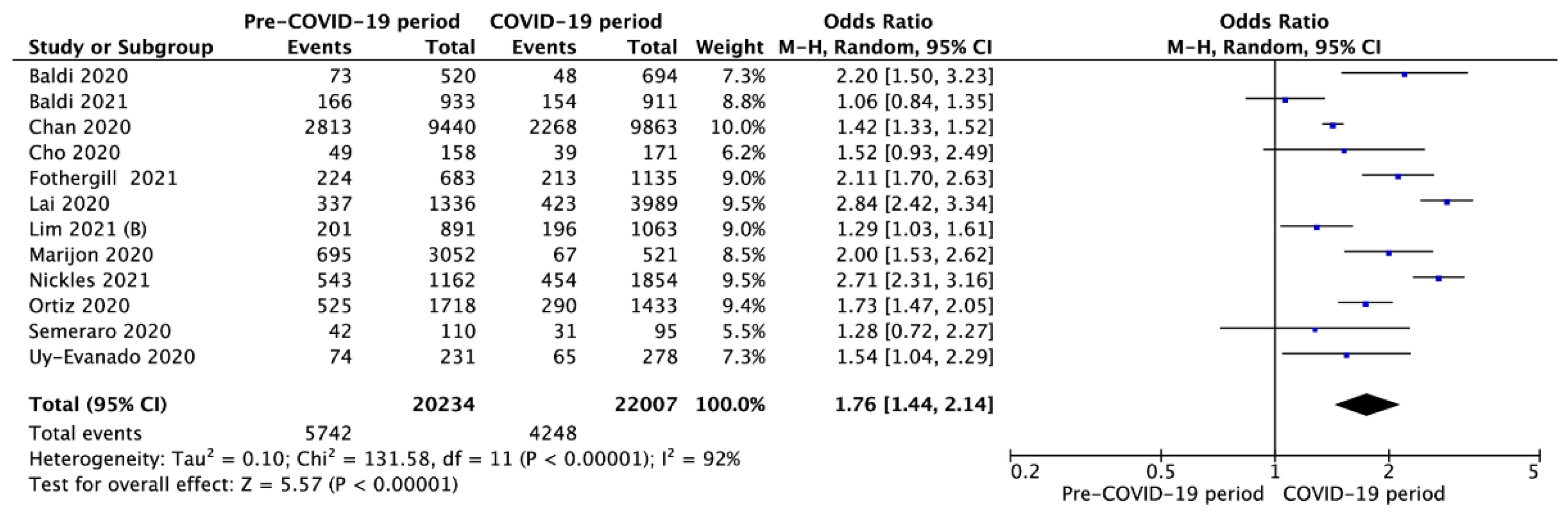
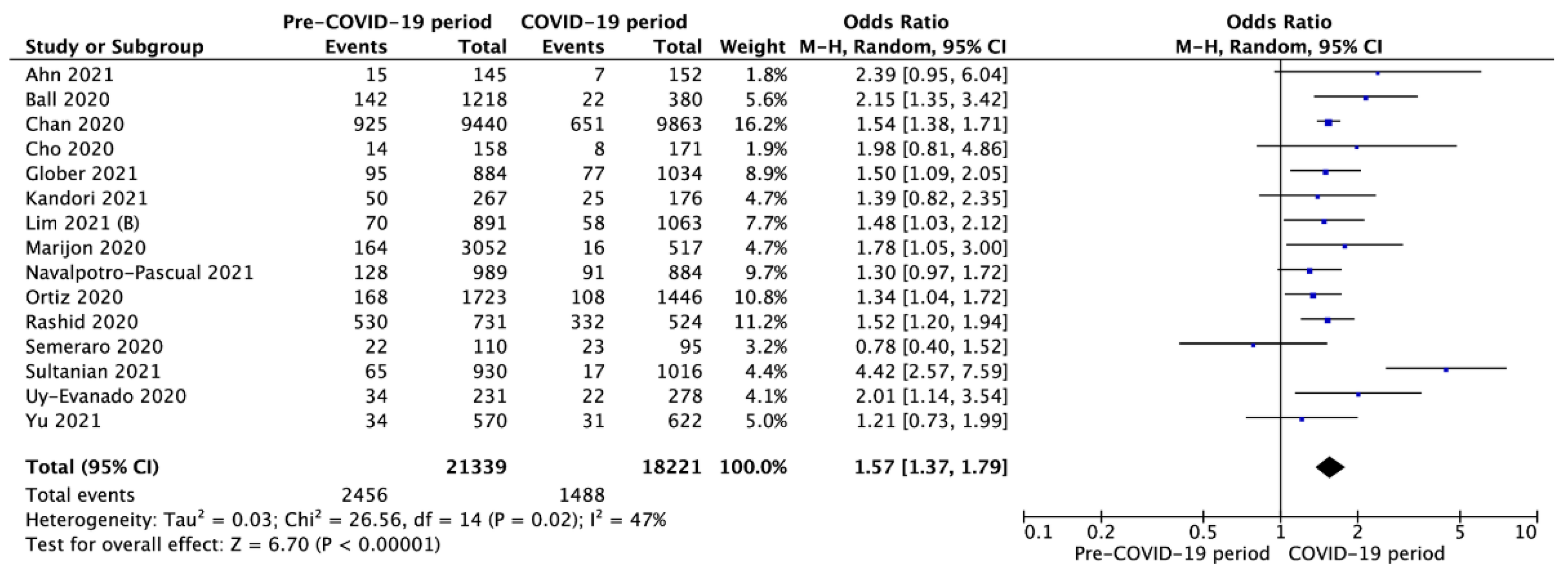
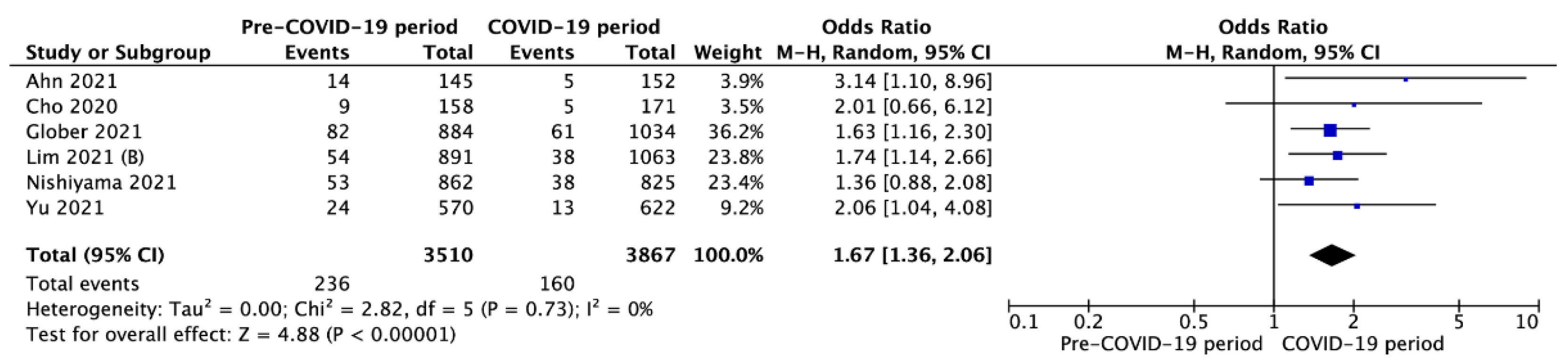
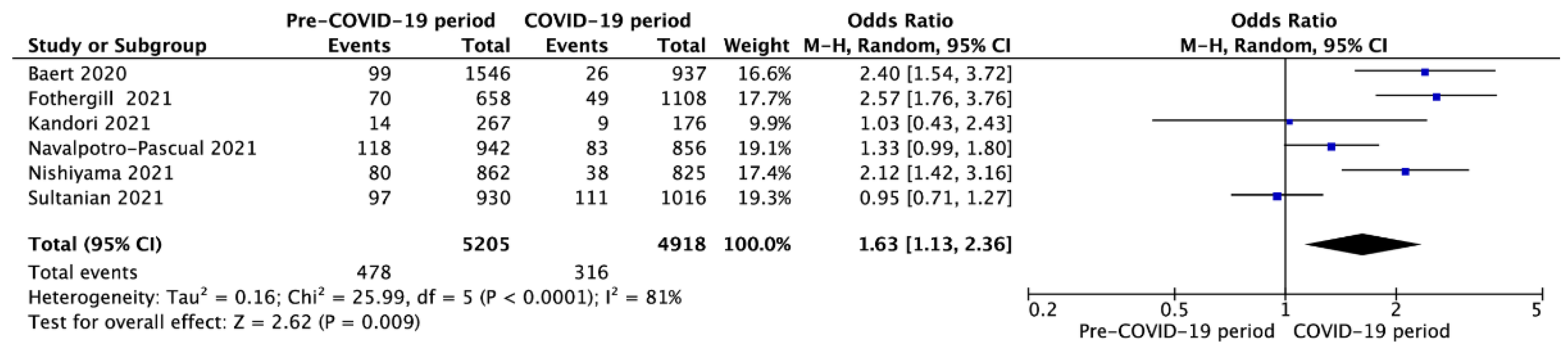
3.3. Outcomes in Pre- vs. Intra-COVID-19 Periods
3.4. Outcomes in SARS-CoV-2-Positive vs. -Negative Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef]
- Attila, K.; Ludwin, K.; Evrin, T.; Katipoglu, B.; Torlinski, T.; Pruc, M.; Szarpak, L. The impact of COVID-19 on airway management in prehospital resuscitation. Disaster Emerg. Med. J. 2020, 5, 216–217. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. COVID-19 Lombardy ICU Network. Risk Factors Associated with Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lin, H.Y.; Tseng, W.P.; Ma, M.H.; Tsai, M.S.; Chen, S.Y.; Huang, C.H. Resuscitation teamwork during the COVID-19 pandemic in the emergency department: Challenges and solutions. Resuscitation 2021, 160, 18–19. [Google Scholar] [CrossRef]
- Al-Jeabory, M.; Borkowska, G.O.; Olecka, A.; Goss, A.; Wieczorek, W.; Evrin, T. Mechanical chest compression devices as an option for out-of-hospital cardiac arrest in COVID-19 pandemic. Disaster Emerg. Med. J. 2021, 6, 50–51. [Google Scholar] [CrossRef]
- Playán-Escribano, J.; Gómez-Álvarez, Z.; Romero-Delgado, T.; Pérez-García, C.N.; Enríquez-Vázquez, D.; Vilacosta, I. Cardiovascular comorbidity and death from COVID-19: Prevalence and differential characteristics. Cardiol. J. 2021, 28, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Somri, M.; Gaitini, L.; Gat, M.; Sonallah, M.; Paz, A.; Gómez-Ríos, M.Á. Cardiopulmonary Resuscitation during the COVID-19 pandemic. Do supraglottic airways protect against aerosol-generation? Resuscitation 2020, 157, 123–125. [Google Scholar] [CrossRef]
- Kopar, P.K.; Brown, D.E.; Turnbull, I.R. Ethics of Codes and Codes of Ethics: When Is It Ethical to Provide Cardiopulmonary Resuscitation During the COVID-19 Pandemic? Ann. Surg. 2020, 272, 930–934. [Google Scholar] [CrossRef]
- Modes, M.E.; Lee, R.Y.; Curtis, J.R. Outcomes of Cardiopulmonary Resuscitation in Patients With COVID-19-Limited Data, but Further Reason for Action. JAMA Intern. Med. 2021, 181, 281–282. [Google Scholar] [CrossRef]
- Cabezón Villalba, G.; Amat-Santos, I.J.; Dueñas, C.; Lopez Otero, D.; Catala, P.; Aparisi, A.; López-Pais, J.; Cacho Antonio, C.E.; Candela, J.; Muiños, P.A.; et al. Impact of the presence of heart disease, cardiovascular medications and cardiac events on outcome in COVID-19. Cardiol. J. 2021, 28, 360–368. [Google Scholar] [CrossRef]
- Borkowska, M.J.; Smereka, J.; Safiejko, K.; Nadolny, K.; Maslanka, M.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. Out-of-hospital cardiac arrest treated by emergency medical service teams during COVID-19 pandemic: A retrospective cohort study. Cardiol. J. 2021, 28, 15–22. [Google Scholar] [CrossRef]
- Nolan, J.P.; Monsieurs, K.G.; Bossaert, L.; Böttiger, B.W.; Greif, R.; Lott, C.; Madar, J.; Olasveengen, T.M.; Roehr, C.C.; Semeraro, F.; et al. European Resuscitation Council COVID-Guideline Writing Groups. European Resuscitation Council COVID-19 guidelines executive summary. Resuscitation 2020, 153, 45–55. [Google Scholar] [CrossRef]
- Borkowska, M.J.; Jaguszewski, M.J.; Koda, M.; Gasecka, A.; Szarpak, A.; Gilis-Malinowsk, N.; Safiejko, K.; Szarpak, L.; Filipiak, K.F.; Smereka, J. Impact of Coronavirus Disease 2019 on Out-of-Hospital Cardiac Arrest Survival Rate: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 1209. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021), Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 10 November 2021).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Ades, A.E.; Lu, G.; Higgins, J.P.T. The interpretation of random-effects meta-analysis in decision models. Med. Decis. Making 2005, 25, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Ryoo, H.W.; Cho, J.W.; Kim, J.H.; Lee, S.-H.; Jang, T.C. Impact of the COVID-19 outbreak on adult out-of-hospital cardiac arrest outcomes in Daegu, South Korea: An observational study. Clin. Exp. Emerg. Med. 2021, 8, 137–144. [Google Scholar] [CrossRef]
- Baert, V.; Jaeger, D.; Hubert, H.; Lascarrou, J.-B.; Debaty, G.; Chouihed, T.; Javaudin, F.; GR-RéAC. Assessment of changes in cardiopulmonary resuscitation practices and outcomes on 1005 victims of out-of-hospital cardiac arrest during the COVID-19 outbreak: Registry-based study. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 119. [Google Scholar] [CrossRef] [PubMed]
- Baert, V.; Beuscart, J.B.; Recher, M.; Javaudin, F.; Hugenschmitt, D.; Bony, T.; Revaux, F.; Mansouri, N.; Larcher, F.; Chazard, E.; et al. Coronavirus Disease 2019 and Out-of-Hospital Cardiac Arrest: No Survivors. Crit. Care Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Palo, A.; Contri, E.; Ronchi, V.; Beretta, G.; et al. Treatment of out-of-hospital cardiac arrest in the COVID-19 era: A 100 days experience from the Lombardy region. PLoS ONE 2020, 15, e0241028. [Google Scholar] [CrossRef]
- Baldi, E.; Auricchio, A.; Klersy, C.; Burkart, R.; Benvenuti, C.; Vanetta, C.; Bärtschi, J.; SWISSRECA Researchers. Out-of-hospital cardiac arrests and mortality in Swiss Cantons with high and low COVID-19 incidence: A nationwide analysis. Resusc. Plus 2021, 6, 100105. [Google Scholar] [CrossRef]
- Ball, J.; Nehme, Z.; Bernard, S.; Stub, D.; Stephenson, M.; Smith, K. Collateral damage: Hidden impact of the COVID-19 pandemic on the out-of-hospital cardiac arrest system-of-care. Resuscitation 2020, 156, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Girotra, S.; Tang, Y.; Al-Araji, R.; Nallamothu, B.K.; McNally, B. Outcomes for Out-of-Hospital Cardiac Arrest in the United States During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2021, 6, 296–303. [Google Scholar] [CrossRef]
- Cho, J.W.; Jung, H.; Lee, M.J.; Lee, S.H.; Lee, S.H.; Mun, Y.H.; Chung, H.-S.; Kim, Y.H.; Kim, G.M.; Park, S.-Y.; et al. WinCOVID-19 consortium. Preparedness of personal protective equipment and implementation of new CPR strategies for patients with out-of-hospital cardiac arrest in the COVID-19 era. Resusc. Plus 2020, 3, 100015. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Okubo, M.; Guyette, F.X.; Martin-Gill, C. Indirect effects of COVID-19 on OHCA in a low prevalence region. Resuscitation 2020, 156, 282–283. [Google Scholar] [CrossRef]
- Fothergill, R.T.; Smith, A.L.; Wrigley, F.; Perkins, G.D. Out-of-Hospital Cardiac Arrest in London during the COVID-19 pandemic. Resusc. Plus 2021, 5, 100066. [Google Scholar] [CrossRef]
- Glober, N.K.; Supples, M.; Faris, G.; Arkins, T.; Christopher, S.; Fulks, T.; Rayburn, D.; Weinstein, E.; Liao, M.; O’Donnell, D.; et al. Out-of-hospital cardiac arrest volumes and characteristics during the COVID-19 pandemic. Am. J. Emerg. Med. 2021, 48, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.; Baert, V.; Beuscart, J.-B.; Chazard, E. Use of out-of-hospital cardiac arrest registries to assess COVID-19 home mortality. BMC Med. Res. Methodol. 2020, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Kandori, K.; Okada, Y.; Ishii, W.; Narumiya, H.; Iizuka, R. Evaluation of a revised resuscitation protocol for out-of-hospital cardiac arrest patients due to COVID-19 safety protocols: A single-center retrospective study in Japan. Sci. Rep. 2021, 11, 12985. [Google Scholar] [CrossRef]
- Kim, C.; Yeo, I.H.; Kim, J.K.; Cho, Y.; Lee, M.J.; Jung, H.; Cho, J.W.; Ham, J.Y.; Lee, S.H.; Chung, H.S.; et al. WinCOVID-19 consortium. Confirmation of COVID-19 in Out-of-Hospital Cardiac Arrest Patients and Postmortem Management in the Emergency Department during the COVID-19 Outbreak. Infect. Chemother. 2020, 52, 562–572. [Google Scholar] [CrossRef]
- Lai, P.H.; Lancet, E.A.; Weiden, M.D.; Webber, M.P.; Zeig-Owens, R.; Hall, C.B.; Prezant, D.J. Characteristics Associated With Out-of-Hospital Cardiac Arrests and Resuscitations During the Novel Coronavirus Disease 2019 Pandemic in New York City. JAMA Cardiol. 2020, 5, 1154–1163. [Google Scholar] [CrossRef]
- Lim, S.L.; Shahidah, N.; Saffari, S.E.; Ng, Q.X.; Ho, A.F.W.; Leong, B.S.; Arulanandam, S.; Siddiqui, F.J.; Ong, M.E.H. Impact of COVID-19 on Out-of-Hospital Cardiac Arrest in Singapore. Int. J. Environ. Res. Public Health 2021, 18, 3646. [Google Scholar] [CrossRef]
- Lim, D.; Park, S.Y.; Choi, B.; Kim, S.H.; Ryu, J.H.; Kim, Y.H.; Sung, A.J.; Bae, B.K.; Kim, H.B. The Comparison of Emergency Medical Service Responses to and Outcomes of Out-of-hospital Cardiac Arrest before and during the COVID-19 Pandemic in an Area of Korea. J. Korean Med. Sci. 2021, 36, e255. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: A population-based, observational study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef]
- Mathew, S.; Harrison, N.; Chalek, A.D.; Gorelick, D.; Brennan, E.; Wise, S.; Gandolfo, L.; O’Neil, B.; Dunne, R. Effects of the COVID-19 pandemic on out-of-hospital cardiac arrest care in Detroit. Am. J. Emerg. Med. 2021, 46, 90–96. [Google Scholar] [CrossRef]
- Navalpotro-Pascual, J.-M.; Monge-Martín, D.; González-León, M.J.; González-León, M.-J.; Neria, F.; Peinado-Vallejo, F.; Alonso-Blas, C.; Muñoz-Isabel, B.; Muñecas-Cuesta, Y.; Carrillo-Freire, A.; et al. Impact of Different Waves of COVID-19 on Emergency Medical Services and Out-of-Hospital Cardiopulmonary Arrest in Madrid, Spain. Research Square. 2021. Available online: https://www.researchsquare.com/article/rs-944651/v1 (accessed on 10 November 2021). [CrossRef]
- Navalpotro-Pascual, J.M.; Fernández Pérez, C.; Peinado Vallejo, F.A.; Carrillo Moya, A.; Muñecas Cuesta, Y.; Muñoz Isabel, B.; González León, M.J.; Les González, J.I. Caseload and cardiopulmonary arrest management by an out-of-hospital emergency service during the COVID-19 pandemic. Emergencias 2021, 33, 100–106. [Google Scholar] [PubMed]
- Ng, Q.X.; Lee, E.Z.; Tay, J.A.; Arulanandam, S. Impact of COVID-19 ‘circuit-breaker’ measures on emergency medical services utilisation and out-of-hospital cardiac arrest outcomes in Singapore. Emerg. Med. Australas 2021, 33, 149–151. [Google Scholar] [CrossRef]
- Nickles, A.V.; Oostema, A.; Allen, J.; O’Brien, S.L.; Demel, S.L.; Reeves, M.J. Comparison of Out-of-Hospital Cardiac Arrests and Fatalities in the Metro Detroit Area During the COVID-19 Pandemic With Previous-Year Events. JAMA Netw. Open 2021, 4, e2032331. [Google Scholar] [CrossRef]
- Nishiyama, C.; Kiyohara, K.; Iwami, T.; Hayashida, S.; Kiguchi, T.; Matsuyama, T.; Katayama, Y.; Shimazu, T.; Kitamura, T. Influence of COVID-19 pandemic on bystander interventions, emergency medical service activities, and patient outcomes in out-of-hospital cardiac arrest in Osaka City, Japan. Resusc. Plus 2021, 5, 100088. [Google Scholar] [CrossRef]
- Ortiz, F.R.; Del Valle, P.F.; Knox, E.C.; Fábrega, X.J.; Navalpotro Pascual, J.M.; Rodríguez, I.M.; José Azpiazu, J.I.; Iglesias Vázquez, J.I.; Echarri Sucunza, A.; Moreno, D.F.A.; et al. OHSCAR investigators. Influence of the Covid-19 pandemic on out-of-hospital cardiac arrest. A Spanish nationwide prospective cohort study. Resuscitation 2020, 157, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Brischigliaro, L.; Scquizzato, T.; Favaretto, A.; Spagna, A. Out-of-hospital cardiac arrest during the COVID-19 pandemic in the Province of Padua, Northeast Italy. Resuscitation 2020, 154, 47–49. [Google Scholar] [CrossRef]
- Rashid, M.; Gale Hons, C.P.; Hons, N.C.; Ludman Hons, P.; De Belder Hons, M.; Timmis Hons, A.; Mohamed Hons, M.O.; Lüscher Hons, T.F.; Hains Hons, J.; Wu, J.; et al. Impact of Coronavirus Disease 2019 Pandemic on the Incidence and Management of Out-of-Hospital Cardiac Arrest in Patients Presenting With Acute Myocardial Infarction in England. J. Am. Heart Assoc. 2020, 9, e018379. [Google Scholar]
- Semeraro, F.; Gamberini, L.; Tartaglione, M.; Iarussi, B.; Descovich, C.; Picoco, C.; Gordini, G. Out-of-hospital cardiac arrest during the COVID-19 era in Bologna: System response to preserve performances. Resuscitation 2020, 157, 1–2. [Google Scholar] [CrossRef]
- Sultanian, P.; Lundgren, P.; Strömsöe, A.; Aune, S.; Bergström, G.; Hagberg, E.; Hollenberg, J.; Lindqvist, J.; Djärv, T.; Castelheim, A.; et al. Cardiac arrest in COVID-19: Characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur. Heart J. 2021, 42, 1094–1106. [Google Scholar] [CrossRef]
- Uy-Evanado, A.; Chugh, H.S.; Sargsyan, A.; Nakamura, K.; Mariani, R.; Hadduck, K.; Salvucci, A.; Jui, J.; Chugh, S.S.; Reinier, K. Out-of-Hospital Cardiac Arrest Response and Outcomes During the COVID-19 Pandemic. JACC Clin. Electrophysiol. 2021, 7, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Liu, C.-Y.; Chen, W.-K.; Yu, S.-H.; Huang, F.-W.; Yang, M.-T.; Chen, C.-Y.; Shih, H.-M. Impact of the COVID-19 pandemic on emergency medical service response to out-of-hospital cardiac arrests in Taiwan: A retrospective observational study. Emerg. Med. J. 2021, 38, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.S.; Fahrenbruch, C.E.; Rea, T.D.; Eisenberg, M.S. The relationship between time to arrival of emergency medical services (EMS) and survival from out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation 2010, 81, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, S.; Matsuyama, T.; Kitamura, T.; Kiyohara, K.; Kiguchi, T.; Nishiyama, C.; Kobayashi, D.; Shimamoto, T.; Sado, J.; Kawamura, T.; et al. Outcomes of patients 65 years or older after out-of-hospital cardiac arrest based on the location of cardiac arrest in Japan. JAMA Netw. Open 2019, 2, e191011. [Google Scholar] [CrossRef] [Green Version]
- Blewer, A.L.; Ho, A.F.W.; Shahidah, N.; White, A.E.; Pek, P.P.; Ng, Y.Y.; Mao, D.R.; Tiah, L.; Chia, M.Y.; Leong, B.S.; et al. Impact of bystander-focused public health interventions on cardiopulmonary resuscitation and survival: A cohort study. Lancet Public Health 2020, 5, e428–e436. [Google Scholar] [CrossRef]
- Grunau, B.; Reynolds, J.C.; Scheuermeyer, F.X.; Stenstrom, R.; Pennington, S.; Cheung, C.; Li, J.; Habibi, M.; Ramanathan, K.; Barbic, D.; et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: Informing minimum duration of resuscitation. Resuscitation 2016, 101, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Szarpak, Ł.; Smereka, J.; Filipiak, K.J.; Jaguszewski, M. Should we resuscitate COVID-19 patients with non-shockable rhythms? Med. Res. J. 2020, 5, 134. [Google Scholar] [CrossRef]
- Orser, B.A. Recommendations for Endotracheal Intubation of COVID-19 Patients. Anesth Analg. 2020, 130, 1109–1110. [Google Scholar] [CrossRef]
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef]
- Brown, J.; Gregson, F.K.A.; Shrimpton, A.; Cook, T.M.; Bzdek, B.R.; Reid, J.P.; Pickering, A.E. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia 2021, 76, 174–181. [Google Scholar] [CrossRef]
- Dhillon, R.S.; Rowin, W.A.; Humphries, R.S.; Kevin, K.; Ward, J.D.; Phan, T.D.; Nguyen, L.V.; Wynne, D.D.; Scott, D.A.; Clinical Aerosolisation Study Group. Aerosolisation during tracheal intubation and extubation in an operating theatre setting. Anaesthesia 2021, 76, 182–188. [Google Scholar] [CrossRef]
- Feldman, O.; Samuel, N.; Kvatinsky, N.; Idelman, R.; Diamand, R.; Shavit, I. Endotracheal intubation of COVID-19 patients by paramedics using a box barrier: A randomized crossover manikin study. PLoS ONE 2021, 16, e0248383. [Google Scholar] [CrossRef]
- Malysz, M.; Dabrowski, M.; Böttiger, B.W.; Smereka, J.; Kulak, K.; Szarpak, A.; Jaguszewski, M.; Filipiak, K.J.; Ladny, J.R.; Ruetzler, K.; et al. Resuscitation of the patient with suspected/confirmed COVID-19 when wearing personal protective equipment: A randomized multicenter crossover simulation trial. Cardiol. J. 2020, 27, 497–506. [Google Scholar] [CrossRef]
- Malysz, M.; Jaguszewski, M.J.; Szarpak, L.; Telecka-Gadek, D.; Bielski, K.; Dabrowska, A.; Smereka, J.; Filipiak, K.J. Comparison of different chest compression positions for use while wearing CBRN-PPE: A randomized crossover simulation trial. Disaster Emerg. Med. J. 2020, 5, 127–133. [Google Scholar] [CrossRef]
- Scquizzato, T.; Landoni, G.; Paoli, A.; Lembo, R.; Fominskiy, E.; Kuzovlev, A.; Likhvantsev, V.; Zangrillo, A. Effects of COVID-19 pandemic on out-of-hospital cardiac arrests: A systematic review. Resuscitation 2020, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Al-Jeabory, M.; Safiejko, K.; Bialka, S.; Pruc, M.; Gasecka, A.; Szarpak, L. Impact of COVID-19 on bystander cardiopulmonary resuscitation in out-of-hospital cardiac arrest: Is it as bad as we think? Cardiol. J. 2020, 27, 884–885. [Google Scholar] [CrossRef]
- Yılmaz, E.; Arsava, E.M.; Topcuoglu, M.A. Resuscitation in COVID-19 patients: What do we know and what should we do? Cardiol. J. 2020, 27, 656–657. [Google Scholar] [PubMed]
- Solomon, M.D.; McNulty, E.J.; Rana, J.S.; Leong, T.K.; Lee, C.; Sung, S.H.; Ambrosy, A.P.; Sidney, S.; Go, A.S. The covid-19 pandemic and the incidence of acute myocardial infarction. N. Engl. J. Med. 2020, 383, 691–693. [Google Scholar] [CrossRef]
- Blondin, N.A.; Greer, D.M. Neurologic prognosis in cardiac arrest patients treated with therapeutic hypothermia. Neurologist 2011, 17, 241–248. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hanif, M.; Ali, M.J.; Haider, M.A.; Kherani, D.; Memon, G.M.; Karim, A.H.; Sattar, A. Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front. Neurol. 2020, 11, 518. [Google Scholar] [CrossRef]



| Outcome | No. of Studies | Events/Participants | Events | Heterogeneity between Trials | p-Value for Differences across Groups | |||
|---|---|---|---|---|---|---|---|---|
| Pre-COVID-19 Period | COVID-19 Period | OR | 95%CI | p-Value | I2 Statistic | |||
| Cardiac arrest location at home | 17 | 19,493/26,948 (73.3%) | 19,860/25,625 (77.5%) | 0.74 | 0.65 to 0.84 | <0.001 | 86% | <0.001 |
| Witnessed arrest | 21 | 16,798/37,960 (44.3%) | 12,416/26,994 (46.0%) | 1.04 | 0.96 to 1.11 | <0.001 | 62% | 0.34 |
| Bystander CPR | 23 | 17,092/38,741 (44.1%) | 12,586/27,248 (46.2%) | 1.00 | 0.88 to 1.14 | <0.001 | 90% | 1.0 |
| Bystander AED use | 14 | 1704/21,089 (8.1%) | 1221/19,964 (6.1%) | 1.35 | 1.25 to 1.46 | <0.001 | 73% | <0.001 |
| Advanced airway management | 10 | 9707/20,839 (46.6%) | 8166/12,549 (65.1%) | 1.20 | 0.82 to 1.76 | <0.001 | 97% | 0.34 |
| Endotracheal intubation | 8 | 6605/20,058 (32.9%) | 3838/10,277 (37.3%) | 1.91 | 1.37 to 2.68 | <0.001 | 95% | <0.001 |
| Supraglottic airway devices | 8 | 2926/19,410 (15.1%) | 3743/10,519 (35.6%) | 0.67 | 0.42 to 1.05 | <0.001 | 97% | 0.08 |
| Mechanical chest compression | 3 | 486/2629 (18.5%) | 557/2137 (26.1%) | 0.97 | 0.50 to 1.88 | <0.001 | 92% | 0.93 |
| Targeted temperature management | 3 | 81/2920 (2.8%) | 44/2638 (1.7%) | 1.62 | 0.85 to 3.07 | 0.07 | 63% | 0.14 |
| Outcome | No of Studies | Events/Participants | Events | Heterogeneity between Trials | p-Value for Differences across Groups | |||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 (+) | SARS-CoV-2 (−) | OR | 95%CI | p-Value | I2 Statistic | |||
| ROSC | 6 | 173/757 (22.9%) | 582/2058 (28.3%) | 0.69 | 0.52 to 0.92 | 0.23 | 27% | 0.01 |
| SHA | 4 | 51/582 (8.8%) | 277/1498 (18.5%) | 0.44 | 0.22 to 0.88 | 0.07 | 58% | 0.02 |
| SHD | 4 | 2/115 (1.7%) | 25/591 (4.2%) | 0.98 | 0.25 to 3.83 | 0.37 | 5% | 0.97 |
| SHD with CPC 1-2 | 2 | 2/18 (11.1%) | 7/186 (3.8%) | 2.67 | 0.47 to 15.28 | 0.58 | 0% | 0.27 |
| 30-day survival | 3 | 4/606 (0.7%) | 299/7055 (4.2%) | 0.12 | 0.05 to 0.31 | 0.63 | 0% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielski, K.; Szarpak, A.; Jaguszewski, M.J.; Kopiec, T.; Smereka, J.; Gasecka, A.; Wolak, P.; Nowak-Starz, G.; Chmielewski, J.; Rafique, Z.; et al. The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5573. https://doi.org/10.3390/jcm10235573
Bielski K, Szarpak A, Jaguszewski MJ, Kopiec T, Smereka J, Gasecka A, Wolak P, Nowak-Starz G, Chmielewski J, Rafique Z, et al. The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(23):5573. https://doi.org/10.3390/jcm10235573
Chicago/Turabian StyleBielski, Karol, Agnieszka Szarpak, Miłosz Jaroslaw Jaguszewski, Tomasz Kopiec, Jacek Smereka, Aleksandra Gasecka, Przemysław Wolak, Grazyna Nowak-Starz, Jaroslaw Chmielewski, Zubaid Rafique, and et al. 2021. "The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 23: 5573. https://doi.org/10.3390/jcm10235573
APA StyleBielski, K., Szarpak, A., Jaguszewski, M. J., Kopiec, T., Smereka, J., Gasecka, A., Wolak, P., Nowak-Starz, G., Chmielewski, J., Rafique, Z., Peacock, F. W., & Szarpak, L. (2021). The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(23), 5573. https://doi.org/10.3390/jcm10235573










