Malignant Pleural Effusions—A Review of Current Guidelines and Practices
Abstract
:1. Introduction
2. Clinical Presentation
3. Diagnosis
3.1. Imaging
3.2. Pathological Diagnosis
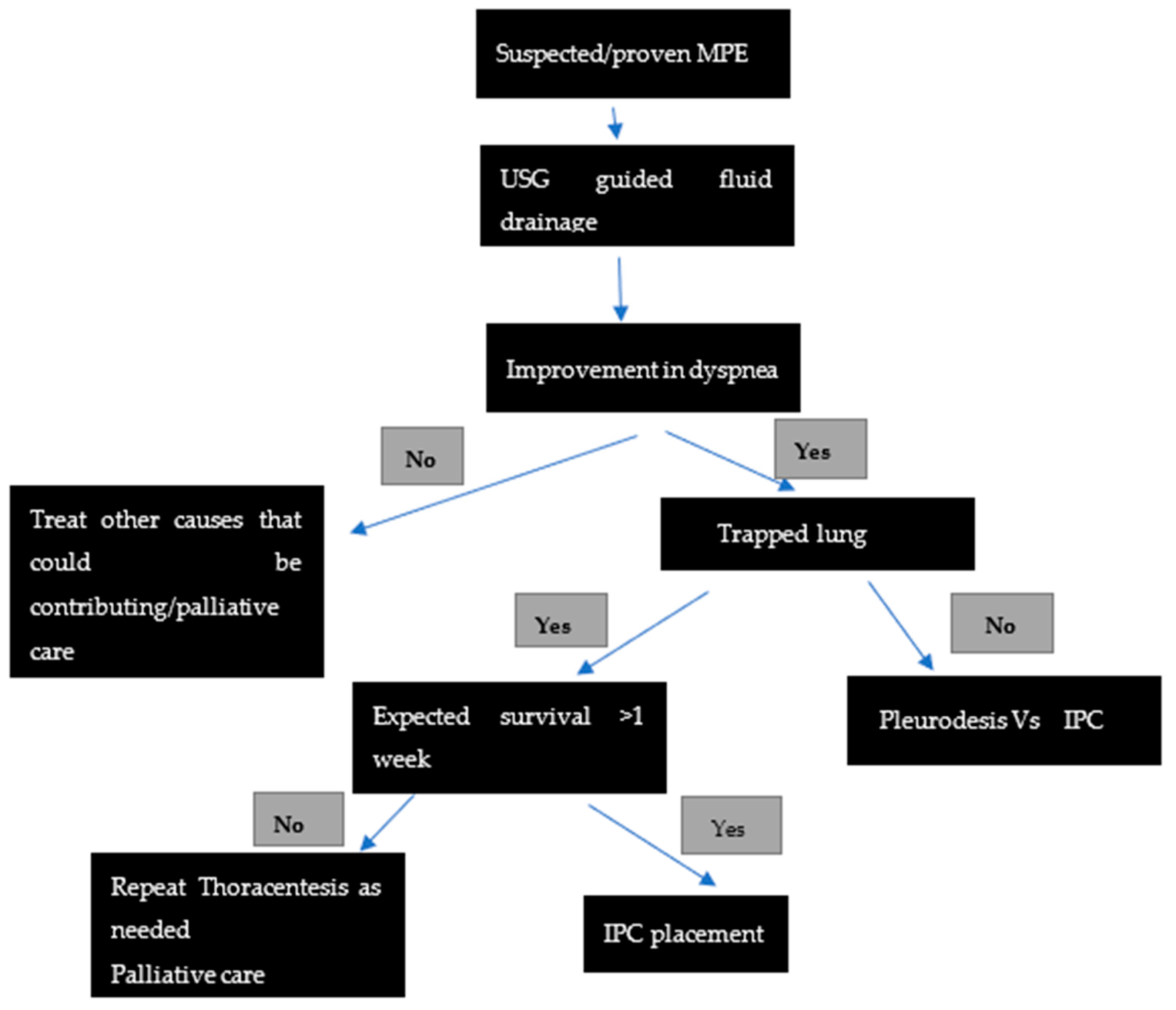
4. Management of MPE
4.1. Thoracic Drainage and Pleurodesis
4.2. A Thoracoscopy with Pleurodesis
4.3. Indwelling Pleural Catheters (IPC)
4.4. Other Interventions
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Feller-Kopman, D.J.; Reddy, C.B.; DeCamp, M.M.; Diekemper, R.L.; Gould, M.K.; Henry, T.; Iyer, N.P.; Lee, Y.G.; Lewis, S.Z.; Maskell, N.A.; et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849. [Google Scholar] [CrossRef]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. J. Cardiothorac. Surg. 2019, 55, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Penz, E.; Watt, K.N.; Hergott, C.A.; Rahman, N.M.; Psallidas, I. Management of malignant pleural effusion: Challenges and solutions. Cancer Manag. Res. 2017, 9, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estenne, M.; Yernault, J.C.; De Troyer, A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am. J. Med. 1983, 74, 813–819. [Google Scholar] [CrossRef]
- Lee, Y.C.; Light, R.W.; Musk, A.W. Management of malignant pleural mesothelioma: A critical review. Curr. Opin. Pulm. Med. 2000, 6, 267–274. [Google Scholar] [CrossRef]
- Thomas, R.; Jenkins, S.; Eastwood, P.R.; Lee, Y.C.; Singh, B. Physiology of breathlessness associated with pleural effusions. Curr. Opin. Pulm. Med. 2015, 21, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, N.R.; Rahman, N.M.; Gleeson, F.V. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009, 64, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.R.; Lee, H.J. Diagnosis and management of malignant pleural effusions: State of the art in 2017. J. Thorac. Dis. 2017, 9 (Suppl. S10), S1111–S1122. [Google Scholar] [CrossRef] [Green Version]
- Traill, Z.C.; Davies, R.J.; Gleeson, F.V. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin. Radiol. 2001, 56, 193–196. [Google Scholar] [CrossRef]
- Arnold, D.T.; De Fonseka, D.; Perry, S.; Morley, A.; Harvey, J.E.; Medford, A.; Brett, M.; Maskell, N.A. Investigating unilateral pleural effusions: The role of cytology. Eur. Respir. J. 2018, 52, 1801254. [Google Scholar] [CrossRef]
- Metintas, M.; Ak, G.; Dundar, E.; Yildirim, H.; Ozkan, R.; Kurt, E.; Erginel, S.; Alatas, F.; Metintas, S. Medical thoracoscopy vs. CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions. Chest 2010, 137, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Light, R.W. Diagnostic approach to pleural effusion in adults. Am. Fam. Physician 2006, 73, 1211–1220. [Google Scholar] [PubMed]
- Pereyra, M.F.; Ferreiro, L.; Valdés, L. Unexpandable lung. Arch. Bronconeumol. 2013, 49, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Clive, A.O.; Kahan, B.C.; Hooper, C.E.; Bhatnagar, R.; Morley, A.J.; Zahan-Evans, N.; Bintcliffe, O.J.; Boshuizen, R.C.; Fysh, E.T.; Tobin, C.L.; et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax 2014, 69, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, i61–i76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.G.; Baumann, M.H.; Maskell, N.A.; Waterer, G.W.; Eaton, T.E.; Davies, R.J.O.; Heffner, J.E.; Light, R.W. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003, 124, 2229–2238. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, R.; Piotrowska, H.E.; Laskawiec-Szkonter, M.; Kahan, B.C.; Luengo-Fernandez, R.; Pepperell, J.C.T.; Evison, M.D.; Holme, J.; Al-Aloul, M.; Psallidas, L.; et al. Effect of Thoracoscopic Talc Poudrage vs. Talc Slurry via Chest Tube on Pleurodesis Failure Rate among Patients with Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020, 323, 60–69. [Google Scholar] [CrossRef]
- Janssen, J.P.; Collier, G.; Astoul, P.; Tassi, G.F.; Noppen, M.; Rodriguez-Panadero, F.; Loddenkemper, R.; Herth, F.J.; Gasparini, S.; Marquette, C.H.; et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: A prospective cohort study. Lancet 2007, 369, 1535–1539. [Google Scholar] [CrossRef]
- Shojaee, S.; Lee, H.J. Thoracoscopy: Medical versus surgical—In the management of pleural diseases. J. Thorac. Dis. 2015, 7 (Suppl. S4), S339–S351. [Google Scholar] [CrossRef]
- Tremblay, A.; Mason, C.; Michaud, G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur. Respir. J. 2007, 30, 759–762. [Google Scholar] [CrossRef]
- Thomas, R.; Fysh, E.T.; Smith, N.A.; Lee, P.; Kwan, B.C.; Yap, E.; Horwood, H.C.; Piccolo, F.; Lam, D.C.M.; Garske, L.A.; et al. Effect of an Indwelling Pleural Catheter vs. Talc Pleurodesis on Hospitalization Days in Patients with Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017, 318, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.E.; Mishra, E.K.; Kahan, B.C.; Wrightson, J.M.; Stanton, A.E.; Guhan, A.; Davies, C.W.H.; Grayez, J.; Harrison, R.; Prasad, A.; et al. Effect of an Indwelling Pleural Catheter vs. Chest Tube and Talc Pleurodesis for Relieving Dyspnea in Patients with Malignant Pleural Effusion: The TIME2 Randomized Controlled Trial. JAMA 2012, 307, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Genc, O.; Petrou, M.; Ladas, G.; Goldstraw, P. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur. J. Cardiothorac. Surg. 2000, 18, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, R.; Kahan, B.C.; Morley, A.J.; Keenan, E.K.; Miller, R.F.; Rahman, N.M.; A Maskell, N. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): Study protocol for a randomised controlled trial. Trials 2015, 16, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PICO 1 | RECOMMENDATIONS |
|---|---|
| Yes |
| No |
| Yes. Manometry if pleurodesis is contemplated to assess for lung re-expansion |
| Yes |
| Yes, there is no difference in the efficacy between the two |
| IPC is the preferred method of choice over chemical pleurodesis |
| Not unless infection does not improve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulandaisamy, P.C.; Kulandaisamy, S.; Kramer, D.; Mcgrath, C. Malignant Pleural Effusions—A Review of Current Guidelines and Practices. J. Clin. Med. 2021, 10, 5535. https://doi.org/10.3390/jcm10235535
Kulandaisamy PC, Kulandaisamy S, Kramer D, Mcgrath C. Malignant Pleural Effusions—A Review of Current Guidelines and Practices. Journal of Clinical Medicine. 2021; 10(23):5535. https://doi.org/10.3390/jcm10235535
Chicago/Turabian StyleKulandaisamy, Prarthna Chandar, Sakthidev Kulandaisamy, Daniel Kramer, and Christopher Mcgrath. 2021. "Malignant Pleural Effusions—A Review of Current Guidelines and Practices" Journal of Clinical Medicine 10, no. 23: 5535. https://doi.org/10.3390/jcm10235535
APA StyleKulandaisamy, P. C., Kulandaisamy, S., Kramer, D., & Mcgrath, C. (2021). Malignant Pleural Effusions—A Review of Current Guidelines and Practices. Journal of Clinical Medicine, 10(23), 5535. https://doi.org/10.3390/jcm10235535





