Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Characteristics of Patients at Hospital Admission
3.2. Course of COVID-19 vs. eGFR
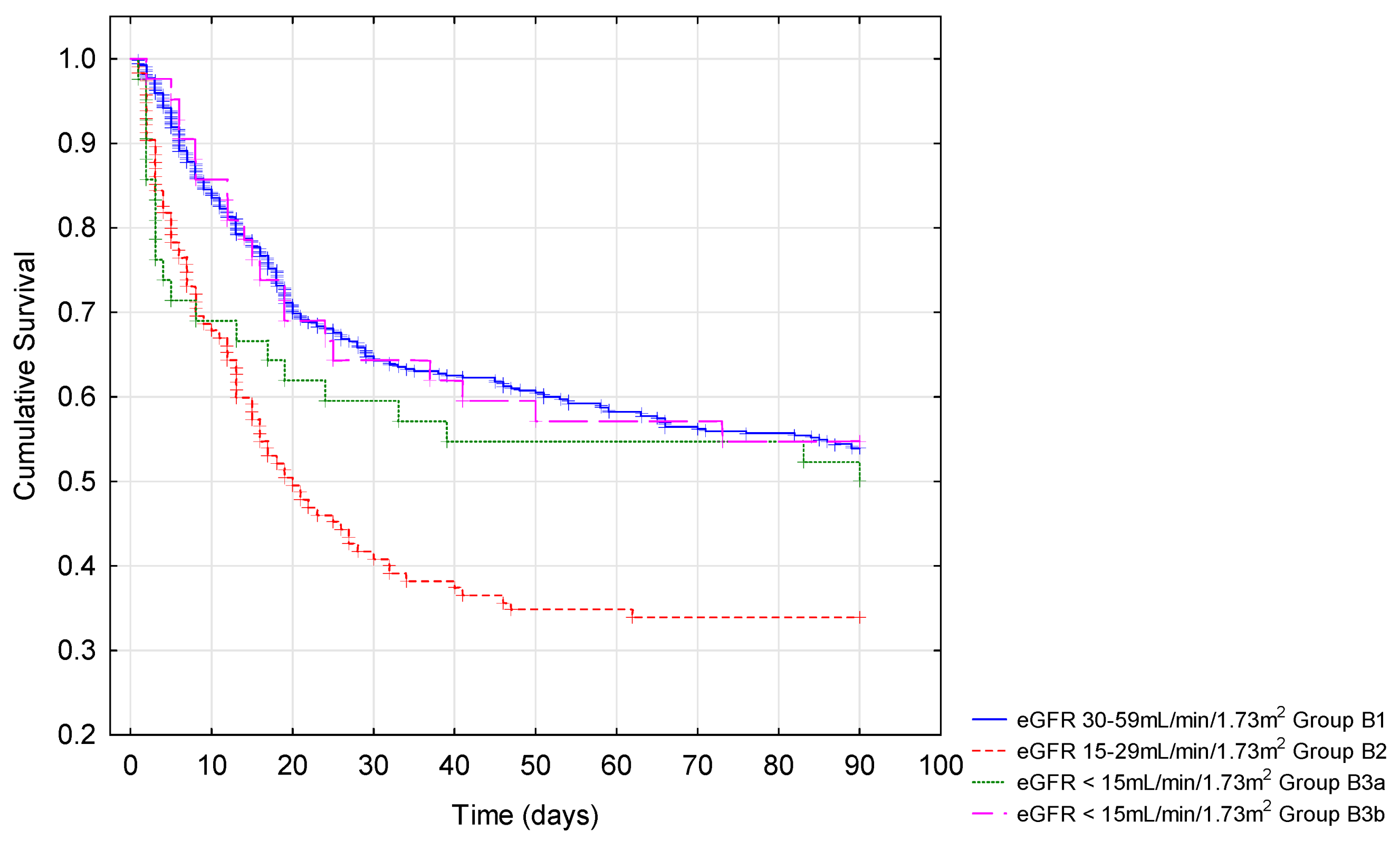
3.3. Outcomes Depending on eGFR
3.4. Changes of Creatinine Concentration during Hospitalization
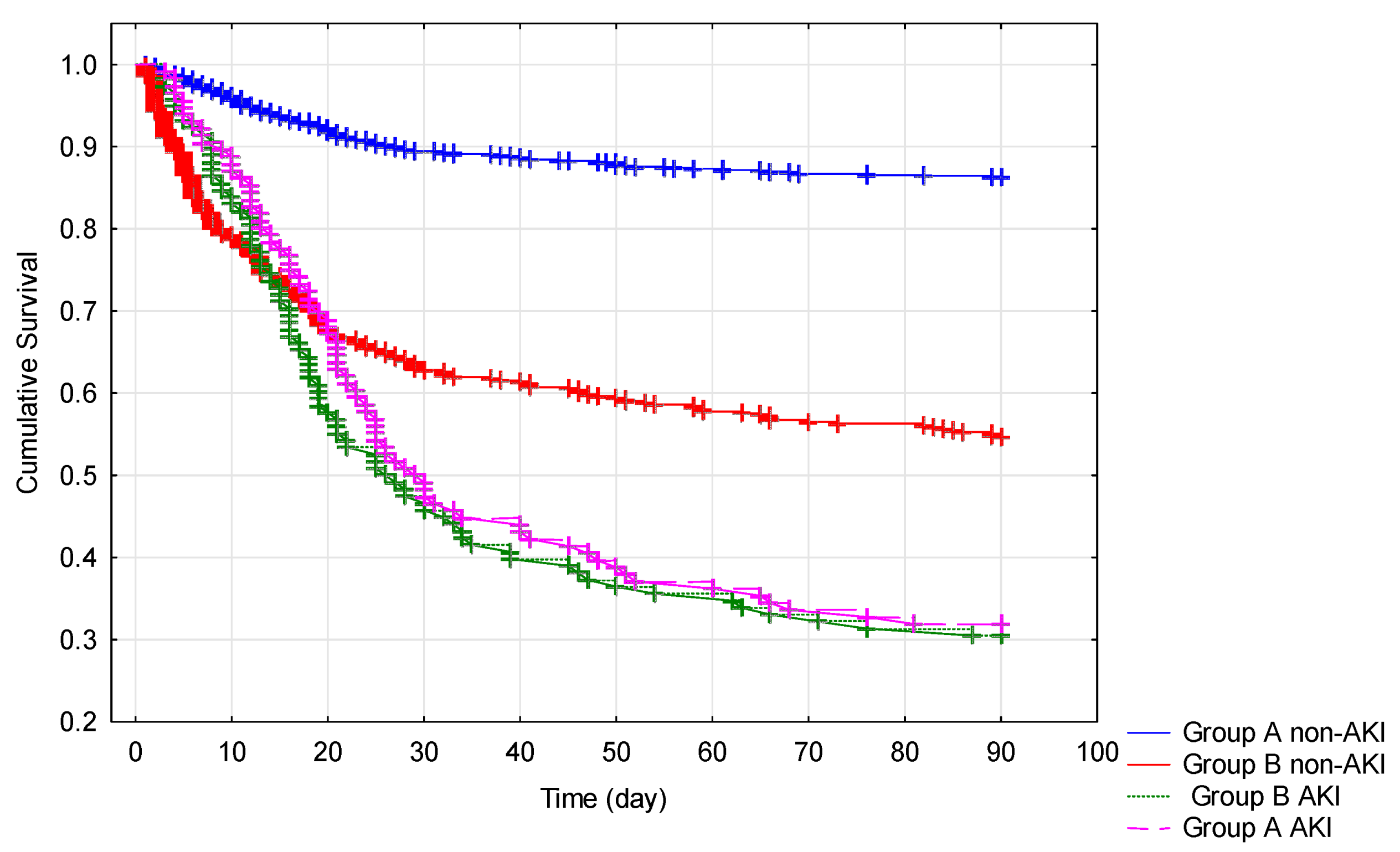
3.5. Occurrence of AKI during Hospitalization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farouk, S.S.; Fiaccadori, E.; Cravedi, P.; Campbell, K.N. COVID-19 and the kidney: What we think we know so far and what we don’t. J. Nephrol. 2020, 33, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Papachristou, S.; Papanas, N. COVID-19 and the kidney: Time to take a closer look. Int. Urol. Nephrol. 2021, 1–5. [Google Scholar] [CrossRef]
- Parmar, M.S. Acute kidney injury associated with COVID-19-Cumulative evidence and rationale supporting against direct kidney injury (infection). Nephrology 2021, 26, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE 2021, 16, e0250708. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; van Till, J.W.O.; Mulligan, G. Targeting acute kidney injury in COVID-19. Nephrol. Dial. Transplant. 2020, 35, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Arikan, H.; Ozturk, S.; Tokgoz, B.; Dursun, B.; Seyahi, N.; Trabulus, S.; Islam, M.; Ayar, Y.; Gorgulu, N.; Karadag, S.; et al. Characteristics and outcomes of acute kidney injury in hospitalized COVID-19 patients: A multicenter study by the Turkish society of nephrology. PLoS ONE 2021, 16, e0256023. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef]
- Bowe, B.; Cai, M.; Xie, Y.; Gibson, A.K.; Maddukuri, G.; Al-Aly, Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin. J. Am. Soc. Nephrol. 2020, 16, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Hu, H.; Wu, F.; Sha, T.; Zeng, Z.; Huang, Q.; Li, H.; Han, J.; Song, W.; Chen, Z.; et al. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: A single-center retrospective observational study. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 157–163. [Google Scholar] [CrossRef]
- Mehta, S.; Chauhan, K.; Patel, A.; Patel, S.; Pinotti, R.; Nadkarni, G.N.; Parikh, C.R.; Coca, S.G. The prognostic importance of duration of AKI: A systematic review and meta-analysis. BMC Nephrol. 2018, 19, 91. [Google Scholar] [CrossRef] [Green Version]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Gameiro, J.; Oliveira, J.; Fonseca, J.A.; Duarte, I.; Bernardo, J.; Branco, C.; Costa, C.; Carreiro, C.; Braz, S.; et al. Acute Kidney Disease and Mortality in Acute Kidney Injury Patients with COVID-19. J. Clin. Med. 2021, 10, 4599. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Hirsch, J.S.; Hazzan, A.; Wanchoo, R.; Shah, H.H.; Malieckal, D.A.; Ross, D.W.; Sharma, P.; Sakhiya, V.; Fishbane, S.; et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. Am. J. Kidney Dis. 2021, 77, 204–215. [Google Scholar] [CrossRef]
- Rahimzadeh, H.; Kazemian, S.; Rahbar, M.; Farrokhpour, H.; Montazeri, M.; Kafan, S.; Salimzadeh, A.; Talebpour, M.; Majidi, F.; Jannatalipour, A.; et al. The Risk Factors and Clinical Outcomes Associated with Acute Kidney Injury in Patients with COVID-19: Data from a Large Cohort in Iran. Kidney Blood Press. Res. 2021, 46, 620–628. [Google Scholar] [CrossRef]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrol. Dial. Transplant. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef] [Green Version]
- Brogan, M.; Ross, M.J. The Impact of Chronic Kidney Disease on Outcomes of Patients with COVID-19 Admitted to the Intensive Care Unit. Nephron 2021, 1–5. [Google Scholar] [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S. Defining AKD: The Spectrum of AKI, AKD, and CKD. Nephron 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; van Lente, F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance, 12 January 2020. Available online: https://apps.who.int/iris/handle/10665/332299 (accessed on 2 October 2021).
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef] [Green Version]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 5 November 2021).
- Meena, P.; Bhargava, V.; Rana, D.S.; Bhalla, A.K.; Gupta, A. COVID-19 and the kidney: A matter of concern. Curr. Med. Res. Pract. 2020, 10, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals with Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.-X.; Fei, J.; Xiang, Y.; Xu, Z.; Zheng, L.; Li, X.-Y.; Fu, L.; Zhao, H. Renal dysfunction and prognosis of COVID-19 patients: A hospital-based retrospective cohort study. BMC Infect. Dis. 2021, 21, 158. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, S.W.; Kim, A.Y.; Cho, K.H.; Park, J.W.; Do, J.Y. Association between Chronic Kidney Disease or Acute Kidney Injury and Clinical Outcomes in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e434. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhang, Z.; Pan, X.-L.; Xing, G.-L.; Zhang, Y.; Liu, Z.-S.; Tu, S.-H. The chronic kidney disease and acute kidney injury involvement in COVID-19 pandemic: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0244779. [Google Scholar] [CrossRef]
- Öztürk, S.; Turgutalp, K.; Arıcı, M.; Çetinkaya, H.; Altıparmak, M.R.; Aydın, Z.; Soypaçacı, Z.; Bora, F.; Kara, E.; Cebeci, E.; et al. Impact of hospital-acquired acute kidney injury on Covid-19 outcomes in patients with and without chronic kidney disease: A multicenter retrospective cohort study. Turk. J. Med. Sci. 2021, 51, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, D.; Reno, C.; Rucci, P.; Fantini, M.P.; Buscaroli, A.; Mosconi, G.; Rigotti, A.; Giudicissi, A.; Mambelli, E.; Righini, M.; et al. COVID-19 incidence and mortality in non-dialysis chronic kidney disease patients. PLoS ONE 2021, 16, e0254525. [Google Scholar] [CrossRef]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Hilbrands, L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020, 16, 705–706. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Bonato, M.; Semenzato, U.; Tinè, M.; Bazzan, E.; Damin, M.; Biondini, D.; Casara, A.; Romagnoli, M.; Turato, G.; Cosio, M.G.; et al. Risk Factors for Development and Severity of COVID-19 in COPD Patients. Front. Med. 2021, 8, 714570. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Nuñez-Gil, I.J.; Abumayyaleh, M.; Estrada, V.; Manuel Becerra-Muñoz, V.; Uribarri, A.; Fernández-Rozas, I.; Feltes, G.; Arroyo-Espliguero, R.; Trabattoni, D.; et al. COVID-19 and the impact of arterial hypertension-An analysis of the international HOPE COVID-19 Registry (Italy-Spain-Germany). Eur. J. Clin. Investig. 2021, 51, e13582. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Keshmiri, Y.S.; Mirzaie, S.K.; Shahnaz, S.; Yadegarynia, D.; Abolghasemi, S.; Tehrani, S.; Zamani, A.; Derisi, M.M. Evaluation of COVID-19 Patients with Chronic Kidney Disease. J. Microbiol Infect. Dis 2021, 11, 152–158. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Naqvi, S.B.; Collins, A.J. Infectious complications in chronic kidney disease. Adv. Chronic Kidney Dis. 2006, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Mooiweer, E.; Luijk, B.; Bonten, M.J.M.; Ekkelenkamp, M.B. C-Reactive protein levels but not CRP dynamics predict mortality in patients with pneumococcal pneumonia. J. Infect. 2011, 62, 314–316. [Google Scholar] [CrossRef]
- Broman, N.; Rantasärkkä, K.; Feuth, T.; Valtonen, M.; Waris, M.; Hohenthal, U.; Rintala, E.; Karlsson, A.; Marttila, H.; Peltola, V.; et al. IL-6 and other biomarkers as predictors of severity in COVID-19. Ann. Med. 2021, 53, 410–412. [Google Scholar] [CrossRef]
- Uribarri, A.; Núñez-Gil, I.J.; Aparisi, A.; Becerra-Muñoz, V.M.; Feltes, G.; Trabattoni, D.; Fernández-Rozas, I.; Viana-Llamas, M.C.; Pepe, M.; Cerrato, E.; et al. Impact of renal function on admission in COVID-19 patients: An analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J. Nephrol. 2020, 33, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Neugarten, J.; Bellin, E.; Yunes, M.; Stahl, L.; Johns, T.S.; Abramowitz, M.K.; Levy, R.; Kumar, N.; Mokrzycki, M.H.; et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J. Am. Soc. Nephrol. 2020, 31, 2145–2157. [Google Scholar] [CrossRef]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 2020, 31, 1157–1165. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Altunok, E.S.; Satici, C.; Dinc, V.; Kamat, S.; Alkan, M.; Demirkol, M.A.; Toprak, I.D.; Kostek, M.E.; Yazla, S.; Esatoglu, S.N. Comparison of demographic and clinical characteristics of hospitalized COVID-19 patients with severe/critical illness in the first wave versus the second wave. J. Med. Virol. 2022, 94, 291–297. [Google Scholar] [CrossRef] [PubMed]
| Variables | Group A (n = 1342) eGFR ≥ 60 mL/min/1.73 | Group B (n = 616) eGFR < 60 mL/min/1.73 | |||
|---|---|---|---|---|---|
| n | % | n | % | p-Value | |
| Gender | 0.319 | ||||
| women | 632 | 47.09 | 305 | 49.51 | |
| men | 710 | 52.91 | 311 | 50.49 | |
| Age (years) * | 58 ± 17.49 | 71.79 ± 13.56 | 0.01 | ||
| Vaccination 1 | 0.869 | ||||
| no | 917 | 95.62 | 411 | 95.80 | |
| one dose | 35 | 3.65 | 14 | 3.26 | |
| two | 7 | 0.73 | 4 | 0.93 | |
| Respiratory support | 0.001 | ||||
| no | 735 | 54.81 | 327 | 53.17 | |
| oxygen mustache cannula | 313 | 23.34 | 106 | 17.24 | |
| face mask | 89 | 6.64 | 54 | 8.78 | |
| Venturi mask | 9 | 0.67 | 8 | 1.30 | |
| passive oxygen therapy | 134 | 9.99 | 81 | 13.17 | |
| HFNC | 3 | 0.22 | 7 | 1.14 | |
| BiPAP/CPAP | 5 | 0.37 | 4 | 0.65 | |
| respiratory therapy | 53 | 3.95 | 28 | 4.55 | |
| Hypertension | <0.001 | ||||
| no | 776 | 57.82 | 192 | 31.17 | |
| yes | 566 | 42.18 | 424 | 68.83 | |
| Diabetes mellitus | <0.001 | ||||
| no | 1094 | 81.58 | 364 | 59.19 | |
| type 1 | 13 | 0.97 | 18 | 2.93 | |
| type 2 (oral therapy) | 158 | 11.78 | 148 | 24.07 | |
| type 2 (insulin therapy) | 50 | 3.73 | 73 | 11.87 | |
| prediabetes | 20 | 1.49 | 12 | 1.95 | |
| Cardiovascular disease | <0.001 | ||||
| no | 1245 | 92.77 | 462 | 75.00 | |
| yes | 97 | 7.23 | 154 | 25.00 | |
| Asthma | 0.356 | ||||
| no | 1282 | 95.53 | 594 | 96.43 | |
| yes | 60 | 4.47 | 22 | 3.57 | |
| COPD | <0.001 | ||||
| no | 1306 | 97.32 | 578 | 93.83 | |
| yes | 36 | 2.68 | 38 | 6.17 | |
| Liver diseases | 0.944 | ||||
| no | 1294 | 96.50 | 594 | 96.43 | |
| chronic hepatitis 2 | 17 | 1.27 | 8 | 1.30 | |
| cirrhosis with portal hypertension | 8 | 0.60 | 5 | 0.81 | |
| steatosis (NASH/NAFLD) | 22 | 1.64 | 9 | 1.46 | |
| Smoking | 0.082 | ||||
| no | 1223 | 91.27 | 541 | 88.11 | |
| in the past | 70 | 5.22 | 46 | 7.49 | |
| now | 47 | 3.51 | 27 | 4.40 | |
| Home medication | |||||
| ACEI | <0.001 | ||||
| no | 1147 | 85.47 | 461 | 74.84 | |
| yes | 195 | 14.53 | 155 | 25.16 | |
| ARB | 0.798 | ||||
| no | 1244 | 92.70 | 573 | 93.02 | |
| yes | 98 | 7.30 | 43 | 6.98 | |
| Beta-blocker | <0.001 | ||||
| no | 1070 | 79.73 | 366 | 59.42 | |
| yes | 272 | 20.27 | 250 | 40.58 | |
| CCB | <0.001 | ||||
| no | 1221 | 90.98 | 480 | 77.92 | |
| yes | 121 | 9.02 | 136 | 22.08 | |
| Alfa-blocker | <0.001 | ||||
| no | 1288 | 95.98 | 553 | 89.77 | |
| yes | 54 | 4.02 | 63 | 10.23 | |
| Statin | <0.001 | ||||
| no | 1163 | 86.66 | 446 | 72.40 | |
| yes | 179 | 13.34 | 170 | 27.60 | |
| Loop diuretic | <0.001 | ||||
| no | 1277 | 95.16 | 497 | 80.68 | |
| yes | 65 | 4.84 | 119 | 19.32 | |
| Thiazides or thiazide-like diuretics | 0.019 | ||||
| no | 1254 | 93.44 | 557 | 90.42 | |
| yes | 88 | 6.56 | 59 | 9.58 | |
| A Group (n = 1342) eGFR ≥ 60 mL/min/1.73 m2 | B Group (n = 616) eGFR < 60 mL/min/1.73 m2 | p-Value | |
|---|---|---|---|
| HGB, g/dL | 13.5 (2.5) | 12.50(3.45) | ≤0.0001 |
| WBC, 103/µL | 7.09 (4.56) | 8.06 (6.03) | ≤0.0001 |
| Lymphocytes, 103/µL | 1.01 (0.78) | 0.88 (0.78) | ≤0.0001 |
| PLT, 103/µL | 215.00 (120.00) | 200.50 (119.00) | 0.0004 |
| Albumin, g/dL | 3.20 (0.80) | 3.00 (0.80) | ≤0.0001 |
| Total protein, g/dL | 6.10 (1.10) | 5.80 (1.10) | 0.0001 |
| CRP, mg/dL | 44.23 (94.98) | 67.89 (120.94) | ≤0.0001 |
| D-dimer, µg/mL | 1.03 (1.99) | 1.79 (4.36) | ≤0.0001 |
| Fibrinogen, g/L | 4.76 (2.30) | 4.60 (2.64) | 0.492 |
| IL-6, pg/mL | 15.30 (36.52) | 24.70 (50.26) | 0.0002 |
| Uric acid, mg/dL | 4.80 (2.10) | 7.05 (3.35) | ≤0.0001 |
| Creatinine, mg/dL | 0.82 (0.24) | 1.70 (1.16) | ≤0.0001 |
| Urea, mg/dL | 31.00 (20.00) | 76.00 (57.00) | ≤0.0001 |
| Potassium, mmol/L | 4.00 (0.67) | 4.30 (0.93) | ≤0.0001 |
| PCT, ng/mL | 0.06 (0.13) | 0.27 (0.84) | ≤0.0001 |
| Sodium, mmol/L | 138.00 (5.00) | 138.00 (6.00) | 0.361 |
| INR | 1.10 (0.19) | 1.17 (0.27) | ≤0.0001 |
| APTT | 32.00 (8.20) | 33.10 (9.90) | 0.0003 |
| Variables | Group B1 eGFR 59-30 (n = 409) | Group B2 eGFR 29-15 (n = 122) | a Group B3a eGFR < 15 m2 (n = 43) | b Group B3b eGFR < 15 (n = 42) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Gender | |||||||||
| women | 199 | 48.66 | 67 | 54.92 | 24 | 55.81 | 15 | 35.71 | 0.143 |
| men | 210 | 51.34 | 55 | 45.08 | 19 | 44.19 | 27 | 64.29 | |
| Age (years) * | 72.48 ± 13.07 | 73.38 ± 12.65 | 66.81 ± 17.49 | 65.55 ± 14.00 | 0.01 | ||||
| Vaccination 1 | 0.732 | ||||||||
| no | 270 | 95.41 | 83 | 95.40 | 30 | 100.00 | 28 | 96.55 | |
| one dose | 9 | 3.18 | 4 | 4.60 | 0 | 0.00 | 1 | 3.45 | |
| two | 4 | 1.41 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Respiratory support | 0.227 | ||||||||
| no | 208 | 50.98 | 62 | 50.82 | 31 | 72.09 | 26 | 61.90 | |
| oxygen mustache cannula | 78 | 19.12 | 17 | 13.93 | 2 | 4.65 | 9 | 21.43 | |
| face mask | 35 | 8.58 | 14 | 11.48 | 2 | 4.65 | 3 | 7.14 | |
| Venturi mask | 4 | 0.98 | 3 | 2.46 | 0 | 0.00 | 1 | 2.38 | |
| passive oxygen therapy | 51 | 12.50 | 20 | 16.39 | 7 | 16.28 | 3 | 7.14 | |
| HFNC | 6 | 1.47 | 0 | 0.00 | 1 | 2.33 | 0 | 0.00 | |
| BiPAP/CPAP | 3 | 0.74 | 1 | 0.82 | 0 | 0.00 | 0 | 0.00 | |
| respiratory therapy | 23 | 5.64 | 5 | 4.10 | 0 | 0.00 | 0 | 0.00 | |
| Hypertension | 0.082 | ||||||||
| no | 133 | 32.52 | 37 | 30.33 | 16 | 37.21 | 6 | 14.29 | |
| yes | 276 | 67.48 | 85 | 69.67 | 27 | 62.79 | 36 | 85.71 | |
| Diabetes mellitus | 0.405 | ||||||||
| no | 250 | 61.27 | 66 | 54.10 | 25 | 58.14 | 23 | 54.76 | |
| type 1 | 7 | 1.72 | 6 | 4.92 | 3 | 6.98 | 2 | 4.76 | |
| type 2 (oral therapy) | 98 | 24.02 | 34 | 27.87 | 9 | 20.93 | 7 | 16.67 | |
| type 2 (insulin therapy) | 43 | 10.54 | 15 | 12.30 | 6 | 13.95 | 9 | 21.43 | |
| prediabetes | 10 | 2.45 | 1 | 0.82 | 0 | 0.00 | 1 | 2.38 | |
| Cardiovascular disease | 0.007 | ||||||||
| no | 319 | 78.00 | 80 | 65.57 | 36 | 83.72 | 27 | 64.29 | |
| yes | 90 | 22.00 | 42 | 34.43 | 7 | 16.28 | 15 | 36.71 | |
| Asthma: | 0.750 | ||||||||
| no | 392 | 95.84 | 119 | 97.54 | 42 | 97.67 | 41 | 97.62 | |
| yes | 17 | 4.16 | 3 | 2.46 | 1 | 2.33 | 1 | 2.38 | |
| COPD | 0.358 | ||||||||
| no | 383 | 93.64 | 113 | 92.62 | 43 | 100.00 | 39 | 92.86 | |
| yes | 26 | 6.36 | 9 | 7.38 | 0 | 0.00 | 3 | 7.14 | |
| Liver diseases | 0.630 | ||||||||
| no | 394 | 96.33 | 118 | 96.72 | 42 | 97.67 | 40 | 95.24 | |
| chronic hepatitis 2 | 4 | 0.98 | 2 | 1.64 | 0 | 0.00 | 2 | 4.76 | |
| cirrhosis with portal hypertension | 4 | 0.98 | 1 | 0.82 | 0 | 0.00 | 0 | 0.00 | |
| steatosis (NASH/NAFLD) | 7 | 1.71 | 1 | 0.82 | 1 | 2.33 | 0 | 0.00 | |
| Smoking | 0.876 | ||||||||
| no | 358 | 87.96 | 106 | 86.89 | 40 | 93.02 | 37 | 88.10 | |
| in the past | 32 | 7.86 | 10 | 8.20 | 2 | 4.65 | 2 | 4.76 | |
| now | 17 | 4.18 | 6 | 4.92 | 1 | 2.33 | 3 | 7.14 | |
| Home medication | |||||||||
| ACEI | 0.022 | ||||||||
| no | 291 | 71.15 | 103 | 84.43 | 34 | 79.07 | 33 | 78.57 | |
| yes | 118 | 28.85 | 19 | 15.57 | 9 | 20.93 | 9 | 21.43 | |
| ARB | 0.01 | ||||||||
| no | 371 | 90.71 | 117 | 95.90 | 43 | 100.00 | 42 | 100.00 | |
| yes | 38 | 9.29 | 5 | 4.10 | 0 | 0.00 | 0 | 0.00 | |
| Beta-blocker | <0.001 | ||||||||
| no | 251 | 61.37 | 73 | 59.84 | 30 | 69.77 | 12 | 28.57 | |
| yes | 158 | 38.63 | 49 | 40.16 | 13 | 30.23 | 30 | 71.43 | |
| CCB | <0.001 | ||||||||
| no | 323 | 78.97 | 103 | 84.43 | 34 | 79.07 | 20 | 47.62 | |
| yes | 86 | 21.03 | 19 | 15.57 | 9 | 20.93 | 22 | 52.38 | |
| Alfa-blocker | <0.001 | ||||||||
| no | 383 | 93.64 | 109 | 89.34 | 39 | 90.70 | 22 | 52.38 | |
| yes | 26 | 6.36 | 13 | 10.66 | 4 | 9.30 | 20 | 47.62 | |
| Statin | 0.094 | ||||||||
| no | 292 | 71.39 | 88 | 72.13 | 38 | 88.37 | 28 | 66.67 | |
| yes | 117 | 28.61 | 34 | 27.87 | 5 | 11.63 | 14 | 33.33 | |
| Loop diuretic | <0.001 | ||||||||
| no | 342 | 83.62 | 95 | 77.87 | 38 | 88.37 | 22 | 52.38 | |
| yes | 67 | 16.38 | 27 | 22.13 | 5 | 11.63 | 20 | 47.62 | |
| Thiazides or thiazide-like diuretics | 0.036 | ||||||||
| no | 361 | 88.26 | 113 | 92.62 | 41 | 95.35 | 42 | 100.00 | |
| yes | 48 | 11.74 | 9 | 7.38 | 2 | 4.65 | 0 | 0.00 | |
| Group B1 eGFR 59-30 (n = 409) | Group B2 eGFR 29-15 (n = 122) | a Group B3a eGFR < 15 (n = 43) | b Group B3b eGFR < 15 (n = 42) | p-Value | |
|---|---|---|---|---|---|
| HGB, g/dL | 12.80 (3.10) | 12.10 (3.50) | 10.20 (3.70) | 10.00 (2.70) | ≤0.0001 |
| WBC, 103/µL | 7.95 (5.18) | 10.07 (7.68) | 10.93 (8.20) | 5.14 (3.47) | ≤0.0001 |
| Lymphocytes, 103/µL | 0.90 (0.77) | 0.90 (0.78) | 0.83 (0.98) | 0.70 (0.52) | 0.118 |
| PLT, 103/µL | 208.00 (118.00) | 199.00 (130.00) | 224.00 (179.00) | 163.00 (67.00) | 0.0032 |
| Albumin, g/dL | 3.00 (0.70) | 2.80 (0.70) | 3.00 (0.70) | 3.15 (0.65) | 0.0424 |
| Total protein, g/dL | 5.95 (1.20) | 5.70 (1.10) | 5.80 (1.15) | 5.95 (1.35) | 0.481 |
| CRP, mg/dL | 66.52 (116.13) | 90.16 (142.53) | 87.30 (117.91) | 37.90 (79.45) | 0.0011 |
| D-dimer, µg/mL | 1.77 (4.33) | 1.40 (5.57) | 0.94 (1.83) | 2.68 (11.24) | 0.494 |
| Fibrinogen, g/L | 4.66 (2.43) | 4.83 (3.04) | 3.44 (1.01) | 5.27 (2.85) | 0.425 |
| IL-6, pg/mL | 22.05 (46.50) | 28.35 (51.90) | 66.25 (499.90) | 39.80 (42.15) | 0.069 |
| Uric acid, mg/dL | 7.00 (3.10) | 7.00 (3.60) | 10.10 (4.70) | 5.80 (2.10) | 0.0004 |
| Creatinine, mg/dL | 1.40 (0.50) | 2.52 (0.94) | 5.16 (2.19) | 5.72 (2.31) | ≤0.0001 |
| Urea, mg/dL | 64.00 (38.00) | 118.00 (60.00) | 180.00 (91.00) | 109.00 (80.00) | ≤0.0001 |
| Potassium, mmol/L | 4.20 (0.80) | 4.40 (1.00) | 4.62 (1.10) | 5.09 (1.10) | ≤0.0001 |
| PCT, ng/mL | 0.17 (0.51) | 0.53 (2.32) | 0.68 (1.03) | 0.56 (1.42) | ≤0.0001 |
| Sodium, mmol/L | 138.00 (6.00) | 138.00 (7.00) | 138.50 (9.00) | 136.50 (4.00) | 0.092 |
| INR | 1.15 (0.24) | 1.21 (0.40) | 1.24 (0.33) | 1.13 (0.21) | 0.0010 |
| APTT | 32.90 (10.10) | 32.90 (8.90) | 35.50 (8.20) | 33.40 (12.00) | 0.56 |
| Variables | Group A (n = 1342) eGFR ≥ 60 mL/min/1.73 | Group B (n = 616) eGFR < 60 mL/min/1.73 | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Pneumonia | 0.004 | ||||
| no | 657 | 48.96 | 259 | 42.05 | |
| yes | 685 | 51.04 | 357 | 57.95 | |
| Deterioration of patient’s condition | <0.001 | ||||
| no | 1030 | 76.75 | 375 | 60.88 | |
| yes | 312 | 23.25 | 241 | 39.12 | |
| The most aggressive respiratory support | 0.003 | ||||
| without oxygen therapy | 585 | 43.59 | 242 | 39.48 | |
| passive low-flow oxygen therapy | 523 | 38.97 | 223 | 36.38 | |
| passive high-flow oxygen therapy | 87 | 6.48 | 44 | 7.18 | |
| non-invasive ventilation (BiPAP/CPAP) | 21 | 1.56 | 21 | 3.43 | |
| respiratory therapy | 126 | 9.39 | 83 | 13.54 | |
| Transfer to ICU | 0.009 | ||||
| no | 1214 | 90.46 | 533 | 86.53 | |
| yes | 128 | 9.54 | 83 | 13.47 | |
| Antibiotics | <0.001 | ||||
| no | 577 | 43.00 | 188 | 30.52 | |
| yes | 765 | 57.00 | 428 | 69.48 | |
| Loop diuretics i.v. | <0.001 | ||||
| no | 1171 | 87.26 | 455 | 73.86 | |
| yes | 171 | 12.74 | 161 | 26.14 | |
| Catecholamines | <0.001 | ||||
| no | 1225 | 96.61 | 517 | 90.86 | |
| yes | 43 | 3.39 | 52 | 9.14 | |
| Variables | Group B1 eGFR 59-30 (n = 409) | Group B2 eGFR 29-15 (n = 122) | a Group B3a eGFR < 15 (n = 43) | b Group B3b eGFR < 15 (n = 42) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Pneumonia | 0.04 | ||||||||
| no | 159 | 38.88 | 55 | 45.08 | 26 | 60.47 | 19 | 45.24 | |
| yes | 250 | 61.12 | 67 | 54.92 | 17 | 39.53 | 23 | 54.76 | |
| Deterioration of patient’s condition | 0.058 | ||||||||
| no | 259 | 63.33 | 63 | 51.64 | 30 | 69.77 | 23 | 54.76 | |
| yes | 150 | 36.67 | 59 | 48.36 | 13 | 30.23 | 19 | 45.24 | |
| The most aggressive respiratory support | 0.256 | ||||||||
| without oxygen therapy | 158 | 38.82 | 46 | 37.70 | 23 | 53.49 | 15 | 36.59 | |
| passive low-flow oxygen therapy | 148 | 36.36 | 40 | 32.79 | 14 | 32.56 | 21 | 51.22 | |
| passive high-flow oxygen therapy | 29 | 7.13 | 11 | 9.02 | 2 | 4.65 | 2 | 4.88 | |
| non-invasive ventilation (BiPAP/CPAP) | 12 | 2.95 | 7 | 5.74 | 2 | 4.65 | 0 | 0.00 | |
| respiratory therapy | 60 | 14.74 | 18 | 14.75 | 2 | 4.65 | 3 | 7.32 | |
| Transfer to ICU | 0.115 | ||||||||
| no | 350 | 85.57 | 103 | 84.43 | 42 | 97.67 | 38 | 90.48 | |
| yes | 59 | 14.43 | 19 | 15.57 | 1 | 2.33 | 4 | 9.52 | |
| Antibiotics | 0.071 | ||||||||
| no | 135 | 33.01 | 28 | 22.95 | 16 | 37.21 | 9 | 21.43 | |
| yes | 274 | 66.99 | 94 | 77.05 | 27 | 62.78 | 33 | 78.57 | |
| Loop diuretics i.v. | 0.277 | ||||||||
| no | 309 | 75.55 | 83 | 68.03 | 34 | 79.07 | 29 | 69.05 | |
| yes | 100 | 24.45 | 39 | 31.97 | 9 | 20.93 | 13 | 30.95 | |
| Catecholamines | 0.286 | ||||||||
| no | 344 | 84.11 | 97 | 79.51 | 39 | 90.70 | 37 | 88.10 | |
| yes | 65 | 15.89 | 25 | 20.49 | 4 | 9.30 | 5 | 11.90 | |
| Variables | Group A (n = 1342) eGFR ≥ 60 mL/min/1.73 | Group B (n = 616) eGFR < 60 mL/min/1.73 | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| In-hospital mortality | <0.001 | ||||
| no | 1203 | 89.64 | 435 | 70.62 | |
| yes | 139 | 10.36 | 181 | 29.38 | |
| Death within 90 days of admission | <0.001 | ||||
| no | 1037 | 81.40 | 296 | 49.92 | |
| yes | 237 | 18.60 | 297 | 50.08 | |
| Death within 180 days of admission | <0.001 | ||||
| no | 306 | 54.64 | 144 | 31.65 | |
| yes | 254 | 45.36 | 311 | 68.35 | |
| End of hospitalization | <0.001 | ||||
| discharge home | 874 | 65.13 | 235 | 38.15 | |
| emergency transfer to other centers | 150 | 11.18 | 125 | 20.29 | |
| transfer to other centers for rehabilitation | 179 | 13.34 | 75 | 12.18 | |
| death | 139 | 10.36 | 181 | 29.38 | |
| Number of hospitalization days 1 | 10 (2–16) | 10 (2–19) | 0.36 | ||
| Variables | Group B1 eGFR 59-30 (n = 409) | Group B2 eGFR 29-15 (n = 122) | a Group B3a eGFR < 15 (n = 43) | b Group B3b GFR < 15 (n = 42) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| In-hospital mortality | 0.001 | ||||||||
| no | 306 | 74.82 | 69 | 56.56 | 29 | 67.44 | 31 | 73.81 | |
| yes | 103 | 25.18 | 53 | 53.44 | 14 | 32.56 | 11 | 26.19 | |
| Death within 90 days of admission | 0.002 | ||||||||
| no | 213 | 53.92 | 39 | 33.91 | 21 | 50.00 | 23 | 54.76 | |
| yes | 182 | 46.08 | 76 | 66.09 | 21 | 50.00 | 19 | 45.24 | |
| Death within 180 days of admission | 0.002 | ||||||||
| no | 98 | 34.03 | 16 | 16.67 | 14 | 38.89 | 16 | 44.44 | |
| yes | 190 | 65.97 | 80 | 83.33 | 22 | 61.11 | 20 | 55.56 | |
| End of hospitalization | 0.007 | ||||||||
| discharge home | 173 | 42.30 | 30 | 24.59 | 14 | 32.56 | 18 | 42.86 | |
| emergency transfer to other centers | 83 | 20.29 | 28 | 22.95 | 8 | 18.60 | 6 | 14.29 | |
| transfer to other centers for rehabilitation | 50 | 12.22 | 11 | 9.02 | 7 | 16.28 | 7 | 16.67 | |
| death | 103 | 25.18 | 53 | 43.44 | 14 | 32.56 | 11 | 26.19 | |
| Number of hospitalization days 1 | 10 (2–19) | 8 (2–18) | 9 (2–14) | 14.5 (6–20) | 0.119 | ||||
| Variables | Group A (eGFR ≥ 60) | Group B (eGFR < 60) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-AKI (n = 1224) | AKI (n = 118) | p-Value | Non-AKI (n = 497) | AKI (n = 119) | p-Value | |||||
| n | % | n | % | n | % | n | % | |||
| Baseline characteristics at admission | ||||||||||
| Gender | 0.009 | 0.315 | ||||||||
| women | 590 | 48.20 | 42 | 35.59 | 251 | 50.50 | 54 | 45.38 | ||
| men | 634 | 51.80 | 76 | 64.41 | 246 | 49.50 | 65 | 54.62 | ||
| Age (years) * | 57.32 ± 17.65 | 65.13± 17.65 | 0.001 | 70.14 ± 13.28 | 70.32 ± 14.66 | 0.3 | ||||
| Vaccination 1 | 0.460 | 0.485 | ||||||||
| no | 823 | 95.37 | 94 | 97.92 | 324 | 95.29 | 87 | 97.75 | ||
| one dose | 33 | 3.82 | 2 | 2.08 | 12 | 3.53 | 2 | 2.25 | ||
| two | 7 | 0.81 | 0 | 0 | 4 | 1.18 | 0 | 0.00 | ||
| Respiratory support | <0.001 | <0.001 | ||||||||
| no | 704 | 57.56 | 31 | 26.27 | 260 | 52.42 | 67 | 56.30 | ||
| oxygen mustache cannula | 290 | 23.71 | 23 | 19.49 | 95 | 19.15 | 11 | 9.24 | ||
| face mask | 76 | 6.21 | 13 | 11.02 | 45 | 9.07 | 9 | 7.56 | ||
| Venturi mask | 8 | 0.65 | 1 | 0.85 | 8 | 1.61 | 0 | 0.00 | ||
| passive oxygen therapy | 111 | 9.08 | 23 | 19.49 | 67 | 13.51 | 14 | 11.76 | ||
| HFNC | 1 | 0.08 | 2 | 1.96 | 5 | 1.01 | 2 | 1.68 | ||
| BiPAP/CPAP | 1 | 0.08 | 4 | 3.39 | 2 | 0.40 | 2 | 1.68 | ||
| respiratory therapy | 32 | 2.62 | 21 | 17.80 | 14 | 2.82 | 14 | 11.76 | ||
| Hypertension | <0.001 | 0.367 | ||||||||
| no | 738 | 60.29 | 38 | 32.20 | 159 | 31.99 | 33 | 27.73 | ||
| yes | 486 | 39.71 | 80 | 67.80 | 338 | 68.01 | 86 | 72.27 | ||
| Diabetes mellitus | <0.001 | 0.741 | ||||||||
| no | 1014 | 82.91 | 80 | 67.80 | 299 | 60.28 | 65 | 54.62 | ||
| type 1 | 13 | 1.06 | 0 | 0.00 | 13 | 2.62 | 5 | 4.20 | ||
| type 2 (oral therapy) | 130 | 10.63 | 28 | 23.73 | 13 | 2.62 | 5 | 4.20 | ||
| type 2 (insulin therapy) | 43 | 3.52 | 7 | 5.93 | 118 | 23.79 | 30 | 25.21 | ||
| prediabetes | 17 | 1.39 | 3 | 2.54 | 57 | 11.49 | 16 | 13.45 | ||
| Cardiovascular disease | 0.196 | <0.001 | ||||||||
| no | 1139 | 93.06 | 106 | 89.83 | 387 | 77.87 | 75 | 63.03 | ||
| yes | 85 | 6.94 | 12 | 10.17 | 110 | 22.13 | 44 | 36.97 | ||
| Asthma | 0.898 | 0.680 | ||||||||
| no | 1169 | 95.51 | 113 | 95.76 | 478 | 96.18 | 116 | 97.48 | ||
| yes | 55 | 4.49 | 5 | 4.24 | 19 | 3.82 | 3 | 2.52 | ||
| COPD | 0.09 | 0.780 | ||||||||
| no | 1194 | 97.55 | 112 | 94.92 | 467 | 93.96 | 111 | 93.28 | ||
| yes | 30 | 2.45 | 6 | 5.08 | 30 | 6.04 | 8 | 6.72 | ||
| Liver diseases | 0.146 | 0.114 | ||||||||
| no | 1181 | 96.57 | 113 | 95.76 | 480 | 96.58 | 114 | 95.80 | ||
| chronic hepatitis 2 | 15 | 1.23 | 2 | 1.69 | 7 | 1.41 | 1 | 0.84 | ||
| cirrhosis with portal hypertension | 5 | 0.41 | 3 | 2.54 | 2 | 0.40 | 3 | 2.52 | ||
| steatosis (NASH/NAFLD) | 22 | 1.80 | 0 | 0.00 | 8 | 1.61 | 1 | 0.84 | ||
| Smoking | 0.251 | 0.422 | ||||||||
| no | 1119 | 91.57 | 104 | 88.14 | 438 | 88.48 | 103 | 86.55 | ||
| in the past | 60 | 4.91 | 10 | 8.47 | 34 | 6.87 | 12 | 10.08 | ||
| now | 43 | 3.52 | 4 | 3.39 | 23 | 4.65 | 4 | 3.36 | ||
| Home medication | ||||||||||
| ACEI | 0.184 | 0.018 | ||||||||
| no | 1051 | 85.87 | 96 | 81.36 | 382 | 76.86 | 79 | 66.39 | ||
| yes | 173 | 14.13 | 22 | 18.64 | 115 | 23.14 | 40 | 33.61 | ||
| ARB: | 0.377 | 0.356 | ||||||||
| no | 1137 | 92.89 | 107 | 90.68 | 460 | 92.56 | 113 | 94.96 | ||
| yes | 87 | 7.11 | 11 | 9.32 | 37 | 7.44 | 6 | 5.04 | ||
| Beta-blocker | 0.029 | 0.026 | ||||||||
| no | 985 | 80.47 | 85 | 72.03 | 306 | 61.57 | 60 | 50.42 | ||
| yes | 239 | 19.53 | 33 | 27.97 | 191 | 38.43 | 59 | 49.58 | ||
| CCB | 0.465 | 0.858 | ||||||||
| no | 1114 | 91.01 | 105 | 88.98 | 388 | 78.07 | 92 | 77.31 | ||
| yes | 110 | 8.99 | 13 | 11.02 | 109 | 21.93 | 27 | 22.69 | ||
| Alfa-blocker | 0.903 | 0.082 | ||||||||
| no | 1175 | 96.00 | 113 | 95.76 | 441 | 88.73 | 112 | 94.12 | ||
| yes | 49 | 4.00 | 5 | 4.24 | 56 | 11.27 | 7 | 5.88 | ||
| Statin | 0.522 | 0.062 | ||||||||
| no | 1063 | 86.85 | 100 | 84.75 | 368 | 74.04 | 78 | 65.55 | ||
| yes | 161 | 13.15 | 18 | 15.25 | 129 | 25.96 | 41 | 34.45 | ||
| Loop diuretic | 0.14 | 0.12 | ||||||||
| no | 1168 | 95.42 | 109 | 92.37 | 407 | 81.89 | 90 | 75.63 | ||
| yes | 56 | 4.58 | 9 | 7.63 | 90 | 18.11 | 29 | 24.37 | ||
| Thiazides or thiazide-like diuretics | 0.623 | 0.367 | ||||||||
| no | 1145 | 93.55 | 109 | 92.37 | 452 | 90.95 | 105 | 88.24 | ||
| yes | 79 | 6.45 | 9 | 7.63 | 45 | 9.05 | 14 | 11.76 | ||
| Course of hospitalization | ||||||||||
| Pneumonia | <0.001 | <0.001 | ||||||||
| no | 628 | 51.31 | 29 | 24.58 | 225 | 45.27 | 34 | 28.57 | ||
| yes | 596 | 48.69 | 89 | 75.42 | 272 | 54.73 | 85 | 71.43 | ||
| The most aggressive respiratory support | <0.001 | <0.001 | ||||||||
| without oxygen therapy | 574 | 46.90 | 11 | 9.32 | 214 | 43.23 | 28 | 23.73 | ||
| passive low-flow oxygen therapy | 497 | 40.60 | 26 | 22.03 | 190 | 38.38 | 33 | 27.97 | ||
| passive high-flow oxygen therapy | 71 | 5.80 | 16 | 13.56 | 37 | 7.47 | 7 | 5.93 | ||
| non-invasive ventilation (BiPAP/CPAP) | 15 | 1.23 | 6 | 5.08 | 17 | 3.43 | 4 | 3.39 | ||
| respiratory therapy | 67 | 5.47 | 59 | 50.00 | 37 | 7.47 | 46 | 38.98 | ||
| Transfer to ICU | <0.001 | <0.001 | ||||||||
| no | 1153 | 94.20 | 61 | 51.69 | 458 | 92.15 | 75 | 63.03 | ||
| yes | 71 | 5.80 | 57 | 48.31 | 75 | 7.85 | 44 | 36.97 | ||
| Antibiotics | <0.001 | <0.001 | ||||||||
| No | 570 | 46.57 | 7 | 5.93 | 178 | 35.81 | 10 | 8.40 | ||
| yes | 654 | 53.43 | 111 | 94.07 | 319 | 64.19 | 109 | 91.60 | ||
| Loop diuretics i.v. | 0.14 | 0.12 | ||||||||
| No | 1168 | 95.42 | 109 | 92.37 | 407 | 81.89 | 90 | 75.63 | ||
| yes | 56 | 4.58 | 9 | 7.63 | 90 | 18.11 | 29 | 24.37 | ||
| Catecholamines | <0.001 | <0.001 | ||||||||
| No | 1169 | 95.51 | 56 | 47.46 | 446 | 89.74 | 71 | 59.66 | ||
| yes | 55 | 4.49 | 62 | 52.54 | 51 | 10.26 | 48 | 40.34 | ||
| Group A eGFR ≥ 60 Non-AKI (n = 1224) | Group A eGFR ≥ 60 AKI (n = 118) | p-Value | |
|---|---|---|---|
| HGB, g/dL | 13.50 (2.40) | 12.55 (2.90) | ≤0.0001 |
| WBC, 103/µL | 6.90 (4.25) | 8.97 (6.90) | ≤0.0001 |
| Lymphocytes, 103/µL | 1.02 (0.79) | 0.88 (0.71) | 0.0418 |
| PLT, 103/µL | 215.00 (119.00) | 224.00 (129.00) | 0.573 |
| Albumin, g/dL | 3.30 (0.80) | 2.90 (0.65) | ≤0.0001 |
| Total protein, g/dL | 6.20 (6.70) | 5.60 (1.10) | ≤0.0001 |
| CRP, mg/dL | 40.71 (90.37) | 95.29 (107.04) | ≤0.0001 |
| D-dimer, µg/mL | 0.97 (1.68) | 1.30 (4.37) | 0.0133 |
| Fibrinogen, g/L | 4.65 (2.36) | 5.09 (2.52) | 0.125 |
| IL-6, pg/mL | 13.80 (30.23) | 48.00 (90.60) | ≤0.0001 |
| Uric acid, mg/dL | 4.80 (2.10) | 4.55 (2.80) | 0.847 |
| Creatinine, mg/dL | 0.82 (0.25) | 0.82 (0.26) | 0.491 |
| Urea, mg/dL | 31.00 (19.00) | 38.00 (25.00) | ≤0.0001 |
| Potassium, mmol/L | 4.00 (0.67) | 4.10 (0.88) | 0.0462 |
| PCT, ng/mL | 0.06 (0.10) | 0.15 (0.44) | ≤0.0001 |
| Sodium, mmol/L | 138.00 (5.00) | 138.00 (6.00) | 0.227 |
| INR | 1.09 (0.18) | 1.18 (0.21) | ≤0.0001 |
| APTT | 31.80 (8.00) | 33.20 (11.70) | 0.0438 |
| Group A eGFR ≥ 60 (n = 1342) | Group B eGFR < 60 (n = 616) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Non-AKI (n = 1224) | AKI (n = 118) | Non-AKI (n = 497) | AKI (n = 119) | ||||||
| n | % | n | % | p-Value | n | % | n | % | p-Value | |
| In-hospital mortality | <0.001 | <0.001 | ||||||||
| no | 1159 | 94.69 | 44 | 37.29 | 388 | 78.07 | 47 | 39.5 | ||
| yes | 65 | 5.31 | 74 | 62.71 | 109 | 21.93 | 72 | 60.5 | ||
| Death within 90 days of admission | <0.001 | <0.001 | ||||||||
| no | 1000 | 86.36 | 37 | 31.90 | 260 | 54.62 | 36 | 30.51 | ||
| yes | 158 | 13.64 | 79 | 68.10 | 216 | 45.38 | 82 | 69.49 | ||
| Death within 180 days of admission | <0.001 | <0.001 | ||||||||
| no | 286 | 62.17 | 20 | 20.00 | 126 | 35.80 | 18 | 17.31 | ||
| yes | 174 | 37.83 | 80 | 80.00 | 226 | 64.20 | 86 | 82.69 | ||
| End of hospitalization | <0.001 | <0.001 | ||||||||
| discharge home | 858 | 70.1 | 16 | 13.56 | 205 | 41.25 | 30 | 25.21 | ||
| emergency transfer to other centers | 144 | 11.76 | 6 | 5.08 | 121 | 24.35 | 4 | 3.36 | ||
| transfer to other centers for rehabilitation | 157 | 12.83 | 22 | 18.64 | 62 | 12.47 | 13 | 10.92 | ||
| death | 65 | 5.31 | 74 | 62.71 | 109 | 21.93 | 72 | 60.50 | ||
| Number of hospitalization days 1 | 11 (2–15) | 28 (13–32) | 0.001 | 11 (2–15) | 26 (12–34) | 0.001 | ||||
| Group B eGFR < 60 Non-AKI (n = 497) | Group B eGFR < 60 AKI (n = 119) | p-Value | |
|---|---|---|---|
| HGB, g/dL | 12.50 (3.50) | 12.50 (3.60) | 0.412 |
| WBC, 103/µL | 7.83 (6.14) | 8.75 (4.97) | 0.022 |
| Lymphocytes, 103/µL | 0.88 (0.77) | 0.86 (0.80) | 0.19 |
| PLT, 103/µL | 201.00 (125.00) | 196.00 (101.00) | 0.641 |
| Albumin, g/dL | 3.00 (0.80) | 2.90 (0.70) | 0.135 |
| Total protein, g/dL | 6.00 (1.10) | 5.50 (1.30) | 0.0003 |
| CRP, mg/dL | 67.16 (121.87) | 73.46 (121.15) | 0.911 |
| D-dimer, µg/mL | 1.71 (2.70) | 2.37 (5.20) | 0.287 |
| Fibrinogen, g/L | 4.55 (2.99) | 4.60 (2.34) | 0.48 |
| IL-6, pg/mL | 23.00 (44.29) | 30.85 (73.05) | 0.172 |
| Uric acid, mg/dL | 6.90 (3.30) | 7.30 (3.50) | 0.516 |
| Creatinine, mg/dL | 1.62 (1.17) | 1.83 (1.09) | 0.022 |
| Urea, mg/dL | 74.00 (63.00) | 80.00 (47.00) | 0.171 |
| Potassium, mmol/L | 4.30 (0.91) | 4.40 (1.04) | 0.063 |
| PCT, ng/mL | 0.22 (0.64) | 0.51 (1.03) | 0.0001 |
| Sodium, mmol/L | 138.00 (6.00) | 138.00 (6.00) | 0.897 |
| INR | 1.17 (0.27) | 1.18 (0.24) | 0.855 |
| APTT | 33.00 (9.90) | 33.40 (9.05) | 0.835 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilis-Pstrusinska, K.; Akutko, K.; Braksator, J.; Dancewicz, A.; Grosman-Dziewiszek, P.; Jamer, T.; Juszczyńska, K.; Konikowska, K.; Koruba, M.; Pupek, M.; et al. Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. J. Clin. Med. 2021, 10, 5522. https://doi.org/10.3390/jcm10235522
Kilis-Pstrusinska K, Akutko K, Braksator J, Dancewicz A, Grosman-Dziewiszek P, Jamer T, Juszczyńska K, Konikowska K, Koruba M, Pupek M, et al. Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. Journal of Clinical Medicine. 2021; 10(23):5522. https://doi.org/10.3390/jcm10235522
Chicago/Turabian StyleKilis-Pstrusinska, Katarzyna, Katarzyna Akutko, Joanna Braksator, Anna Dancewicz, Patrycja Grosman-Dziewiszek, Tatiana Jamer, Katarzyna Juszczyńska, Klaudia Konikowska, Marta Koruba, Małgorzata Pupek, and et al. 2021. "Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes" Journal of Clinical Medicine 10, no. 23: 5522. https://doi.org/10.3390/jcm10235522
APA StyleKilis-Pstrusinska, K., Akutko, K., Braksator, J., Dancewicz, A., Grosman-Dziewiszek, P., Jamer, T., Juszczyńska, K., Konikowska, K., Koruba, M., Pupek, M., Rusiecka, A., Kujawa, K., Adamik, B., Doroszko, A., Kaliszewski, K., Matera-Witkiewicz, A., Pomorski, M., Protasiewicz, M., Sokołowski, J., ... Jankowska, E. A. (2021). Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. Journal of Clinical Medicine, 10(23), 5522. https://doi.org/10.3390/jcm10235522








