Retinal Diseases Regulated by Hypoxia—Basic and Clinical Perspectives: A Comprehensive Review
Abstract
:1. Introduction
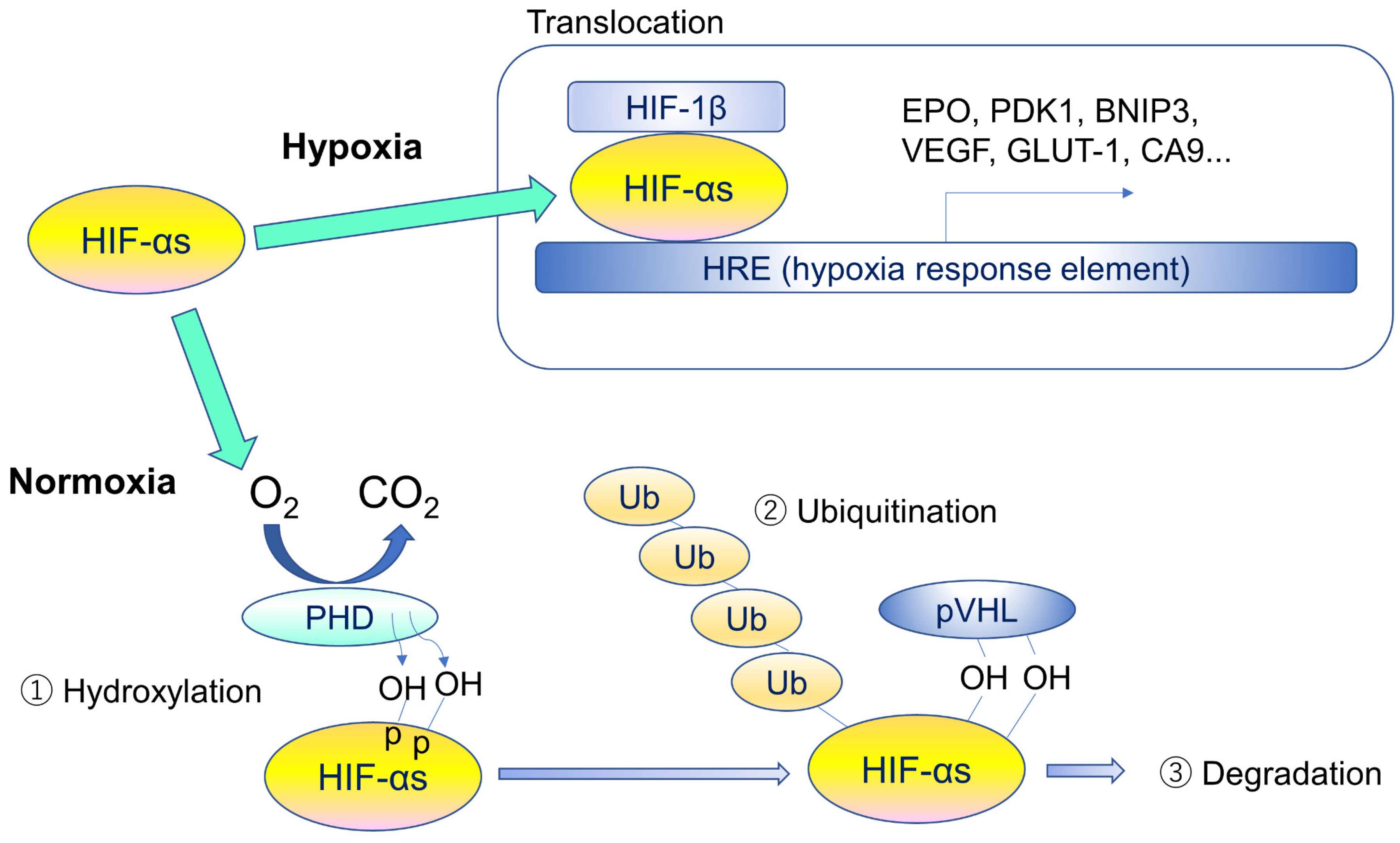
2. Hypoxia-Inducible Factors (HIFs) and Von Hippel-Lindau Disease
3. Types and Roles of HIFs
4. Expressions of HIF and VEGF in the Retina
5. Retinal Diseases and Anti-VEGF Drugs
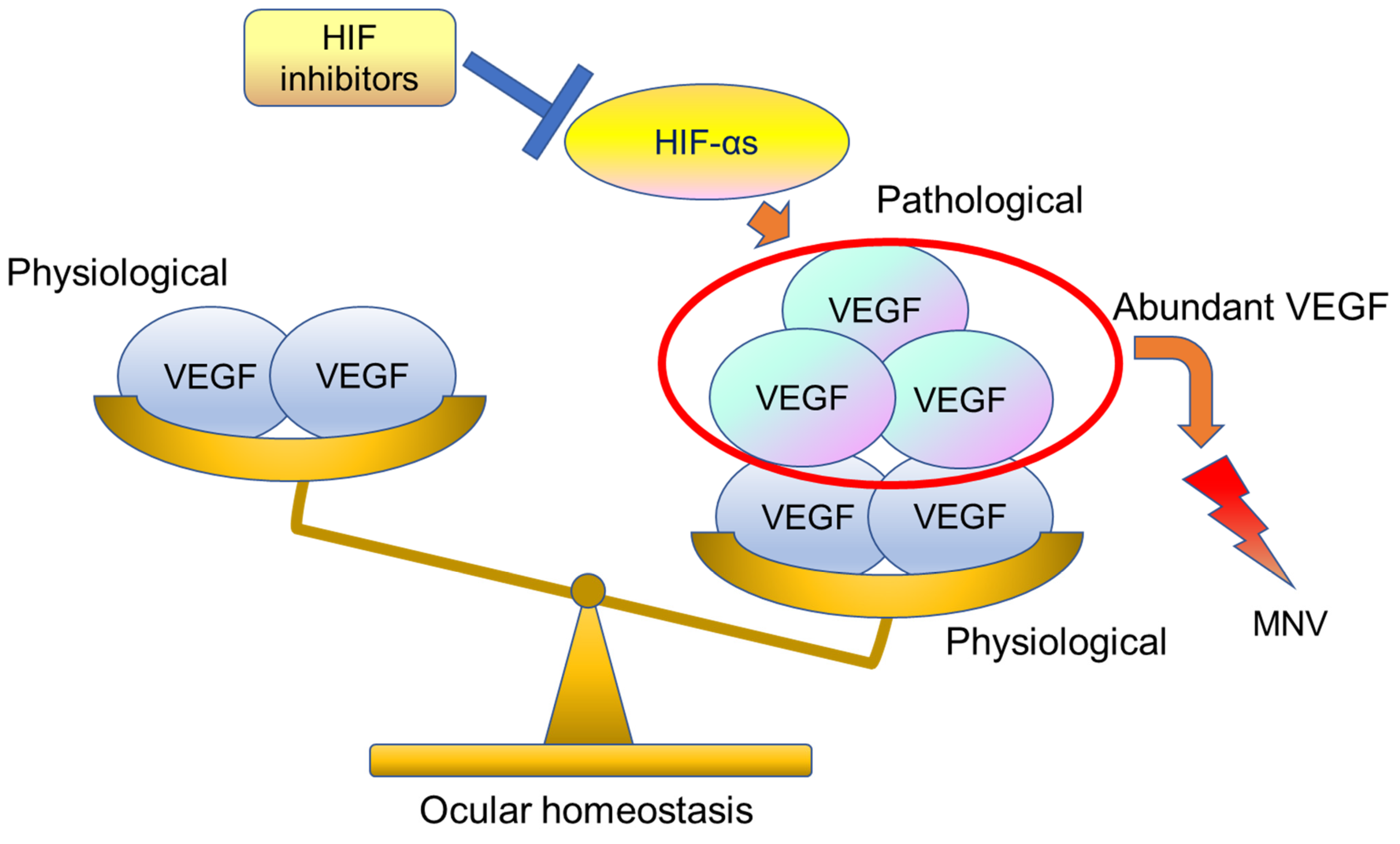
6. Potential for HIF Inhibitors
7. Age-Related Macular Degeneration (AMD)
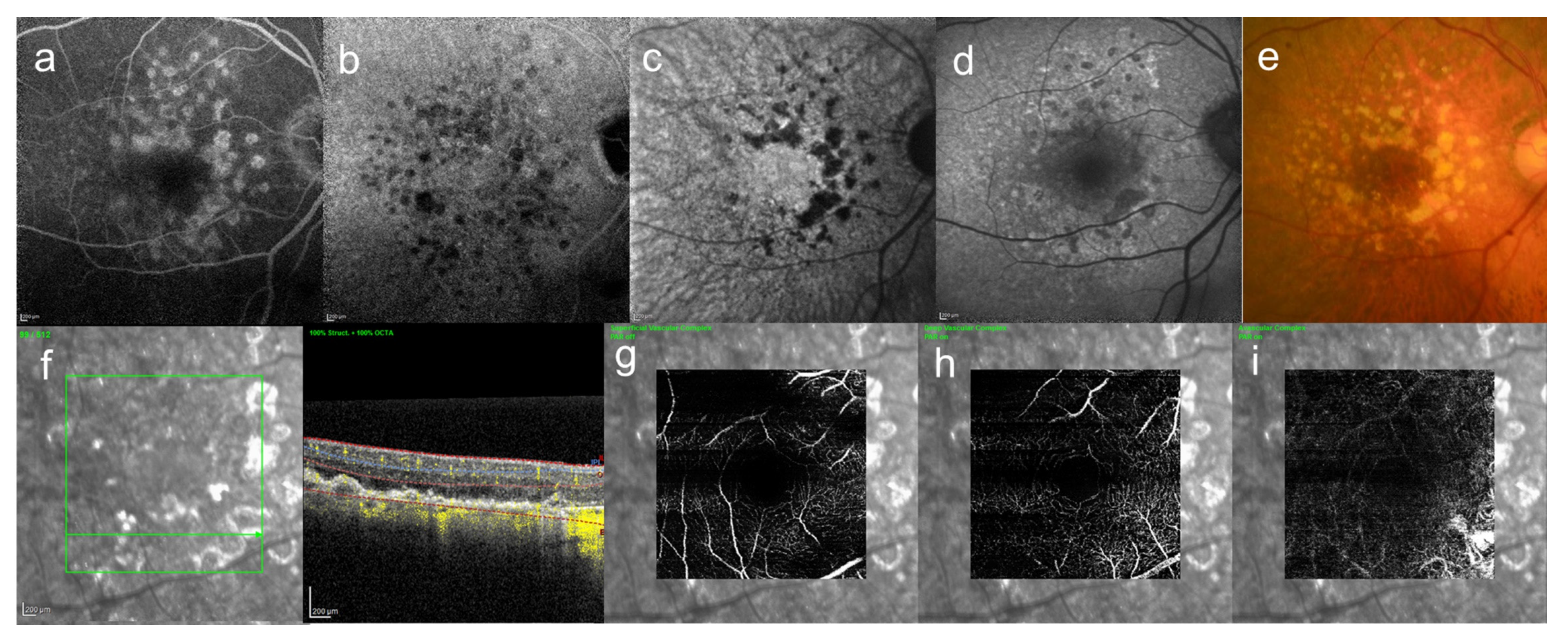
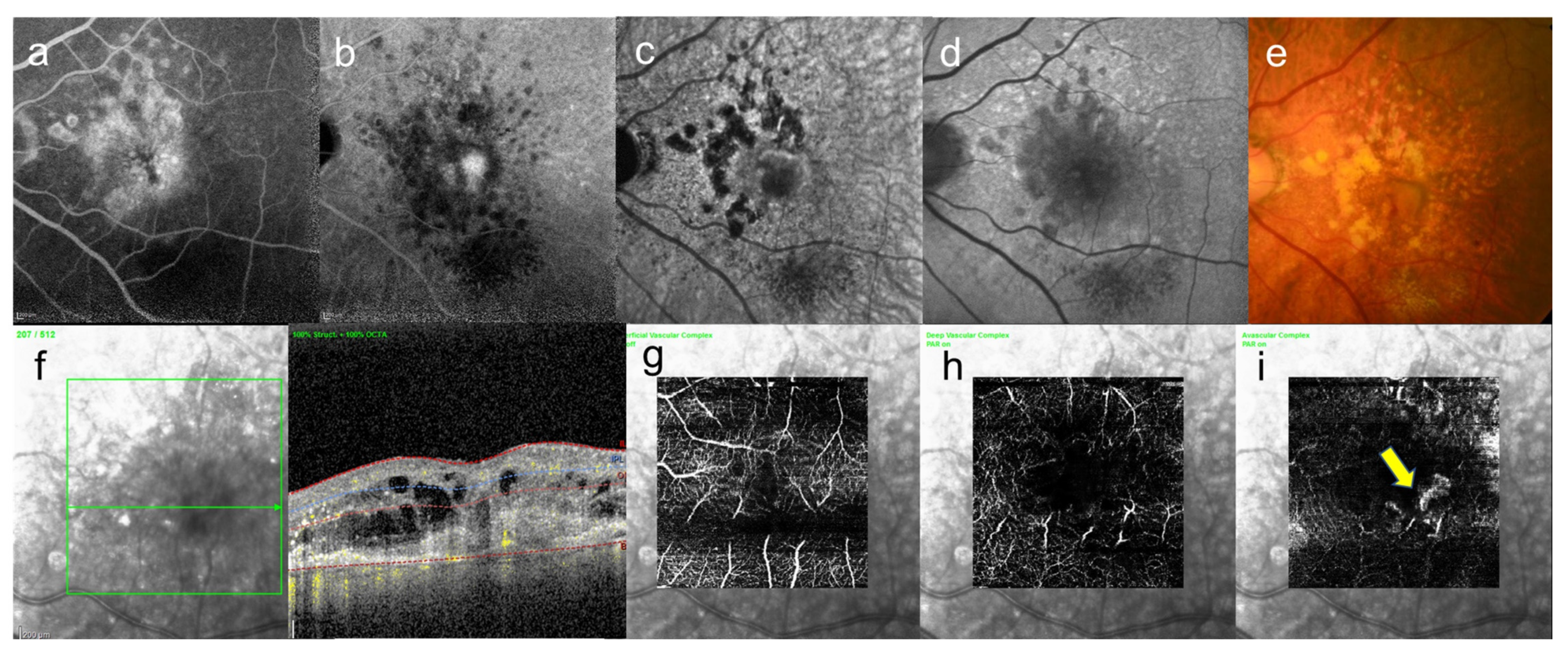
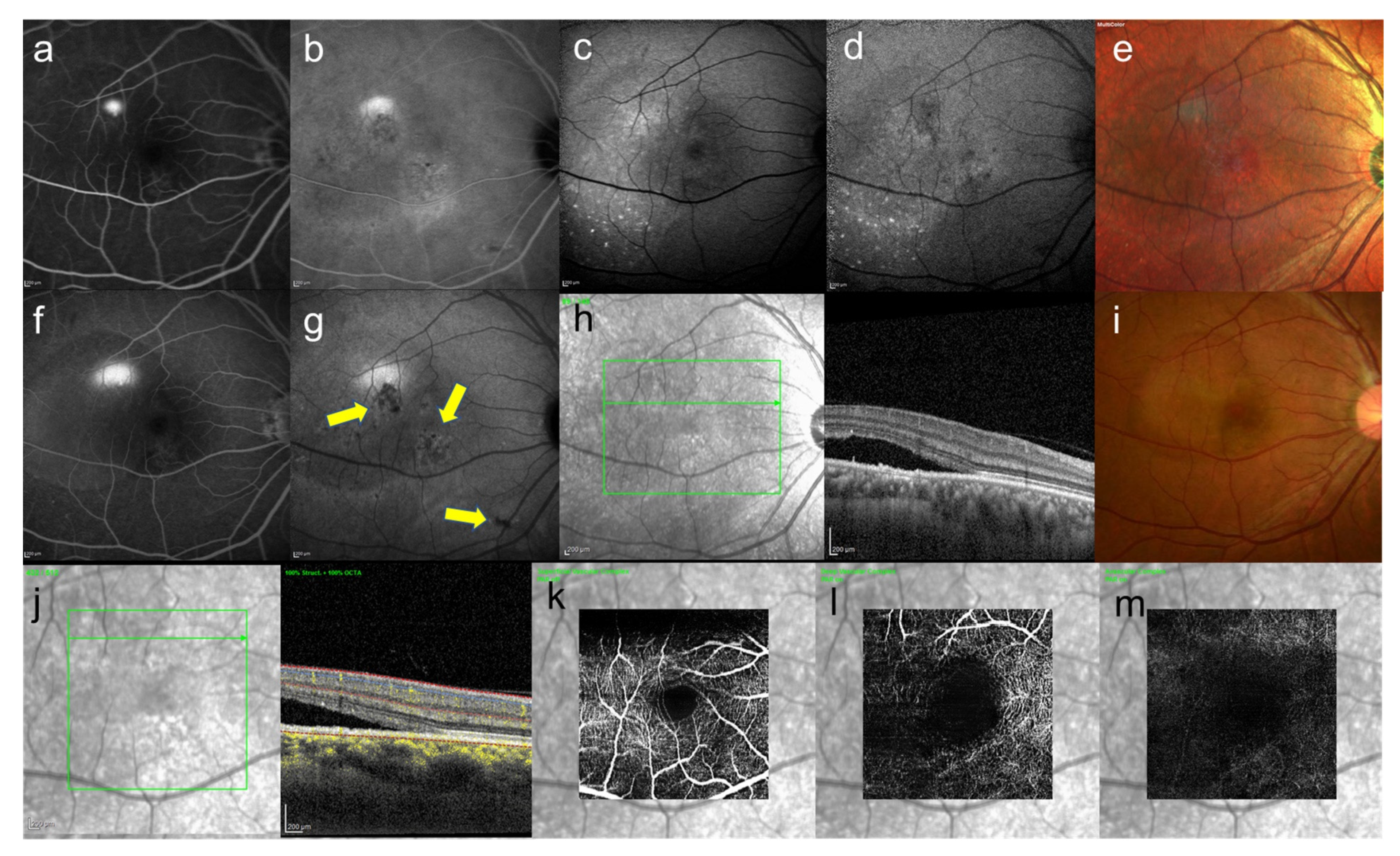
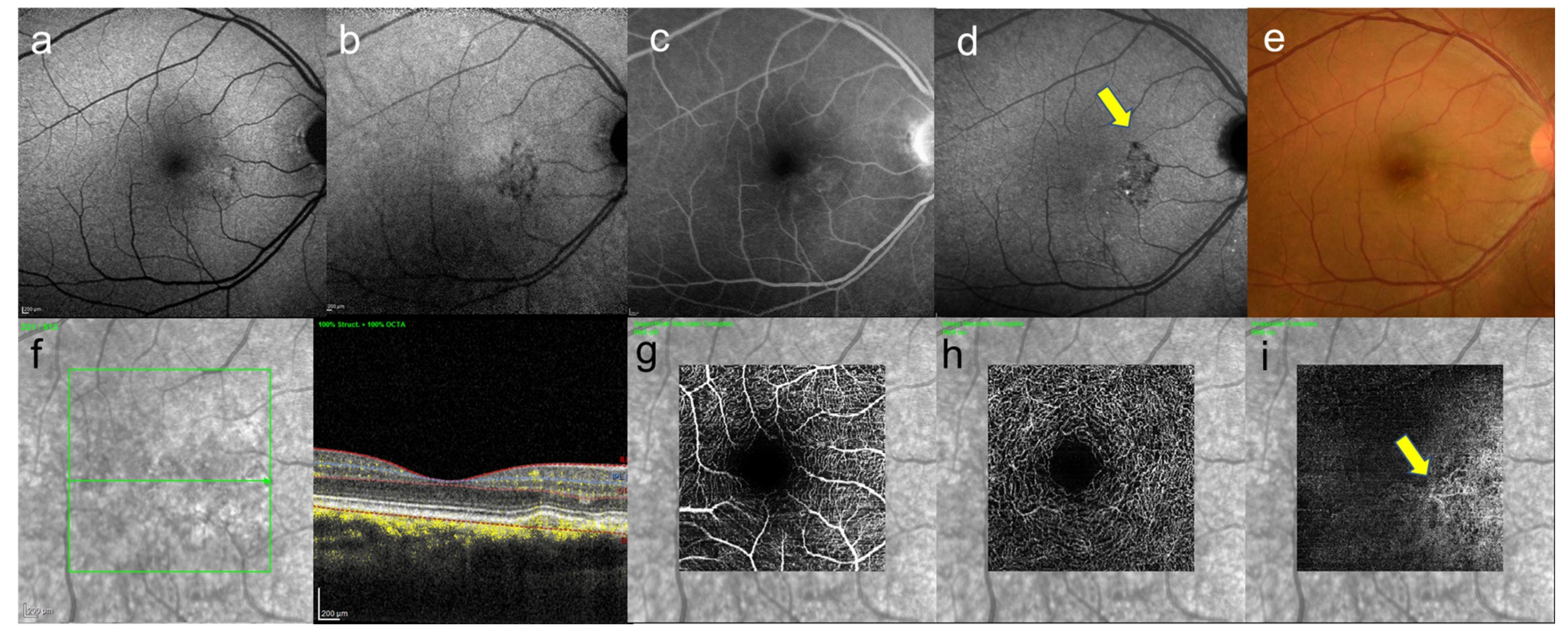
8. Central Serous Chorioretinopathy
9. Pachychoroid Neovasculopathy
10. VEGF and the Choroid
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurihara, T. The retina regulated by hypoxia response—The development to the pathophysiology. Ganka Ophthalmol. 2015, 57, 1027–1036. (In Japanese) [Google Scholar]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Pawlus, M.R.; Hu, C.-J. Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell Signal. 2013, 25, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, K.; Yamanaka, K.; Kamura, T.; Minato, N.; Conaway, R.C.; Conaway, J.; Klausner, R.D.; Pause, A. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 1999, 96, 12436–12441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- LUCENTIS® Solution for Intravitreal Injection 10 mg/mL Package Insert. 2019. Ver. 11. Available online: https://www.novartis.co.jp/ (accessed on 26 October 2021).
- EYLEA Solution for IVT Inj. 40 mg/mL Package Insert. 2020. Ver. 1. Available online: https://pharma-navi.bayer.jp/eylea/basic-docs (accessed on 26 October 2021).
- Mori, K.; Gehlbach, P.L.; Nishiyama, Y.; Deguchi, T.; Yoneya, S. The ultra-late phase of indocyanine green angiography for healthy subjects and patients with age-related macular degeneration. Retina 2002, 22, 309–316. [Google Scholar] [CrossRef]
- Shinojima, A.; Fujita, K.; Mori, R.; Kawamura, A.; Yuzawa, M.; Yasukawa, T. Investigation of the Etiology of Central Serous Chorioretinopathy Using En-Face Optical Coherence Tomography and Indocyanine Green Angiography. Ophthalmologica 2016, 236, 100–107. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.-M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Gorin, M.B. Von Hippel-Lindau Disease: Clinical Considerations and the Use of Fluorescein-Potentiated Argon Laser Therapy for Treatment of Retinal Angiomas. Semin. Ophthalmol. 1992, 7, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.P.; Lips, C.J.; Hsia, Y.E.; Zbar, B. Von Hippel-Lindau Syndrome. Brain Pathol. 1995, 5, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Gu, Y.-F.; Wolff, N.; Stefanius, K.; Christie, A.; Dey, A.; Hammer, R.E.; Xie, X.-J.; Rakheja, D.; Pedrosa, I.; et al. Bap1is essential for kidney function and cooperates withVhlin renal tumorigenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 16538–16543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.L.; Jiang, B.-H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; McKnight, S.L.; Russell, D. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Ema, M.; Taya, S.; Yokotani, N.; Sogawa, K.; Matsuda, Y.; Fujii-Kuriyama, Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1 regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 4273–4278. [Google Scholar] [CrossRef] [Green Version]
- Berra, E.; Benizri, E.; Ginouvès, A.; Volmat, V.; Roux, D.; Pouysségur, J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1 in normoxia. EMBO J. 2003, 22, 4082–4090. [Google Scholar] [CrossRef] [Green Version]
- Appelhoff, R.J.; Tian, Y.-M.; Raval, R.R.; Turley, H.; Harris, A.; Pugh, C.; Ratcliffe, P.; Gleadle, J. Differential Function of the Prolyl Hydroxylases PHD1, PHD2, and PHD3 in the Regulation of Hypoxia-inducible Factor. J. Biol. Chem. 2004, 279, 38458–38465. [Google Scholar] [CrossRef] [Green Version]
- Landázuri, M.O.; Vara-Vega, A.; Vitón, M.; Cuevas, Y.; del Peso, L. Analysis of HIF-prolyl hydroxylases binding to substrates. Biochem. Biophys. Res. Commun. 2006, 351, 313–320. [Google Scholar] [CrossRef]
- Koivunen, P.; Tiainen, P.; Hyvärinen, J.; Williams, K.E.; Sormunen, R.; Klaus, S.J.; Kivirikko, K.I.; Myllyharju, J. An Endoplasmic Reticulum Transmembrane Prolyl 4-Hydroxylase Is Induced by Hypoxia and Acts on Hypoxia-inducible Factor α. J. Biol. Chem. 2007, 282, 30544–30552. [Google Scholar] [CrossRef] [Green Version]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, T.; Westenskow, P.D.; Friedlander, M. Hypoxia-Inducible Factor (HIF)/Vascular Endothelial Growth Factor (VEGF) Signaling in the Retina. Adv. Exp. Med. Biol. 2014, 801, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Flamme, I.; Krieg, M.; Plate, K.H. Up-Regulation of Vascular Endothelial Growth Factor in Stromal Cells of Hemangioblastomas Is Correlated with Up-Regulation of the Transcription Factor HRF/HIF-2α. Am. J. Pathol. 1998, 153, 25–29. [Google Scholar] [CrossRef]
- Schödel, J.; Oikonomopoulos, S.; Ragoussis, J.; Pugh, C.W.; Ratcliffe, P.J.; Mole, D.R. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 2011, 117, e207–e217. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, T.; Kubota, Y.; Ozawa, Y.; Takubo, K.; Noda, K.; Simon, M.C.; Johnson, R.; Suematsu, M.; Tsubota, K.; Ishida, S.; et al. von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development 2010, 137, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Kubota, Y.; Hirashima, M.; Kishi, K.; Stewart, C.L.; Suda, T. Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J. Clin. Investig. 2008, 118, 2393–2403. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Lustig, M.; Francois, F.; Sellinger, M.; Plesken, H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development 1994, 120, 3395–3403. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Kurihara, T.; Okuno, Y.; Ozawa, Y.; Kishi, K.; Goda, N.; Tsubota, K.; Okano, H.; Suda, T.; Kubota, Y. The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev. Biol. 2012, 363, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Usui, Y.; Westenskow, P.; Kurihara, T.; Aguilar, E.; Sakimoto, S.; Paris, L.; Wittgrove, C.; Feitelberg, D.; Friedlander, M.; Moreno, S.K.; et al. Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Investig. 2015, 125, 2335–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidemann, A.; Krohne, T.U.; Aguilar, E.; Kurihara, T.; Takeda, N.; Dorrell, M.I.; Simon, M.C.; Haase, V.H.; Friedlander, M.; Johnson, R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 2010, 58, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, T.; Westenskow, P.; Bravo, S.; Aguilar, E.; Friedlander, M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Investig. 2012, 122, 4213–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; SEVEN-UP Study Group. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Lactoferrin Has a Therapeutic Effect via HIF Inhibition in a Murine Model of Choroidal Neovascularization. Front. Pharmacol. 2020, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef]
- Lee, C.S.; Choi, E.Y.; Lee, S.C.; Koh, H.J.; Lee, J.H.; Chung, J.H. Resveratrol Inhibits Hypoxia-Induced Vascular Endothelial Growth Factor Expression and Pathological Neovascularization. Yonsei Med. J. 2015, 56, 1678–1685. [Google Scholar] [CrossRef] [Green Version]
- Shoda, C.; Miwa, Y.; Nimura, K.; Okamoto, K.; Yamagami, S.; Tsubota, K.; Kurihara, T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients 2020, 12, 1055. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.; Park, S.W.; Jo, D.H.; Kim, D.; Kim, J.H.; Cho, H.-Y.; Kim, J.; Kim, J.H.; Kim, J.-S. CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat. Commun. 2018, 9, 1855. [Google Scholar] [CrossRef]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones? Proc. Jpn. Acad. Ser. B 2019, 95, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchinson, A. Fairy chemicals. Nat. Cell Biol. 2014, 505, 298. [Google Scholar] [CrossRef]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A fairy chemical suppresses retinal angiogenesis as a HIF inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Kunimi, H.; Miwa, Y.; Inoue, H.; Tsubota, K.; Kurihara, T. A Novel HIF Inhibitor Halofuginone Prevents Neurodegeneration in a Murine Model of Retinal Ischemia-Reperfusion. Int. J. Mol. Sci. 2019, 20, 3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimi, H.; Miwa, Y.; Katada, Y.; Tsubota, K.; Kurihara, T. HIF inhibitor topotecan has a neuroprotective effect in a murine retinal ischemia-reperfusion model. PeerJ 2019, 7, e7849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimi, H.; Lee, D.; Ibuki, M.; Katada, Y.; Negishi, K.; Tsubota, K.; Kurihara, T. Inhibition of the HIF-1α/BNIP3 pathway has a retinal neuroprotective effect. FASEB J. 2021, 35, e21829. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.L.; Berger, J.W.; Maguire, M.G.; Ho, A.C. Age-related macular degeneration. N. Engl. J. Med. 2000, 342, 483–492. [Google Scholar] [CrossRef]
- Rim, T.H.; Cheng, C.-Y.; Kim, D.W.; Kim, S.S.; Wong, T.Y. A nationwide cohort study of cigarette smoking and risk of neovascular age-related macular degeneration in East Asian men. Br. J. Ophthalmol. 2017, 101, 1367–1373. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Jensen, S.C.; Meuer, S.M. The Five-year Incidence and Progression of Age-related Maculopathy. Ophthalmology 1997, 104, 7–21. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Cherepanoff, S.; Dolz-Marco, R.; Killingsworth, M.; Chen, F.; Mendis, R.; Mrejen, S.; Too, L.K.; Gal-Or, O.; Curcio, C.A.; et al. Cuticular Drusen. Ophthalmology 2018, 125, 100–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Stambolian, D.; Edwards, A.O.; Branham, K.; Othman, M.; Jakobsdottir, J.; Tosakulwong, N.; Pericak-Vance, M.A.; Campochiaro, P.A.; Klein, M.L.; et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7401–7406. [Google Scholar] [CrossRef] [Green Version]
- Neale, B.M.; Fagerness, J.; Reynolds, R.; Sobrin, L.; Parker, M.; Raychaudhuri, S.; Tan, P.L.; Oh, E.C.; Merriam, J.E.; Souied, E.; et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. USA 2010, 107, 7395–7400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorés-Motta, L.; van Beek, A.E.; Willems, E.; Zandstra, J.; van Mierlo, G.; Einhaus, A.; Mary, J.-L.; Stucki, C.; Bakker, B.; Hoyng, C.B.; et al. Common haplotypes at the CFH locus and low-frequency variants in CFHR2 and CFHR5 associate with systemic FHR concentrations and age-related macular degeneration. Am. J. Hum. Genet. 2021, 108, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Bressler, N.M.; Boyer, D.S.; Williams, D.F.; Butler, S.; Francom, S.F.; Brown, B.; Di Nucci, F.; Cramm, T.; Tuomi, L.L.; Ianchulev, T.; et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012, 32, 1821–1828. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins c and e, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Vives-Bauza, C.; Anand, M.; Shiraz, A.K.; Magrane, J.; Gao, J.; Vollmer-Snarr, H.R.; Manfredi, G.; Finnemann, S.C. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J. Biol. Chem. 2008, 36, 24770–24780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bai, Y.; Huang, L.; Qi, Y.; Zhang, Q.; Li, S.; Wu, Y.; Li, X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: Implications for age-related macular degeneration. Cell Death Dis. 2015, 11, e1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, J.D. Pathogenesis of disciform detachment of the neuroepithelium. Am. J. Ophthalmol. 1967, 63, 6019308. [Google Scholar]
- Ross, A.; Ross, A.H.; Mohamed, Q. Review and update of central serous chorioretinopathy. Curr. Opin. Ophthalmol. 2011, 22, 166–173. [Google Scholar] [CrossRef]
- Fong, A.H.; Lai, T.Y. Central serous chorioretinopathy. In Spectral Domain Optical Coherence Tomography in Macular Diseases; Meyer, C.H., Sandeep, S., Sadda, S.R., Eds.; Springer: New Delhi, India, 2017; Chapter 18. [Google Scholar]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, C.M.; Owens, S.L.; Smith, P.D.; Fine, S.L. Long-term follow-up of central serous chorioretinopathy. Br. J. Ophthalmol. 1984, 68, 815–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujarborua, D.; Chatterjee, S.; Choudhury, A.; Bori, G.; Sarma, A.K. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina 2005, 25, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Fujiwara, T.; Margolis, R.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009, 29, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Hanumunthadu, D.; Van Dijk, E.H.C.; Dumpala, S.; Rajesh, B.; Jabeen, A.; Jabeen, A.; Ansari, M.; Mehta, P.; Shah, S.; et al. Evaluation of choroidal layer thickness in central serous chorioretinopathy. J. Ophthalmic Vis. Res. 2019, 14, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, N.; Terao, N.; Nakamine, S.; Tamashiro, T.; Wakugawa, S.; Sawaguchi, K.; Koizumi, H. Scleral Thickness in Central Serous Chorioretinopathy. Ophthalmol. Retin. 2021, 5, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Matsumoto, H.; Sonoda, S.; Hiroe, T.; Sakamoto, T.; Akiyama, H. Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS ONE 2018, 13, e0206646. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin Eye Res. 2021, 100973. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, A.; Mehanna, C.; Lavia, C.A.; Gaudric, A.; Tadayoni, R.; Bousquet, E. Central serous chorioretinopathy: Risk factors for serous retinal detachment in fellow eyes. Br. J. Ophthalmol. 2020, 6, 852–856. [Google Scholar] [CrossRef]
- Shinojima, A.; Kawamura, A.; Mori, R.; Fujita, K.; Yuzawa, M. Findings of Optical Coherence Tomographic Angiography at the Choriocapillaris Level in Central Serous Chorioretinopathy. Ophthalmologica 2016, 236, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, Y.; Shinojima, A.; Kawamura, A.; Yuzawa, M. Correlation of Aging and Segmental Choroidal Thickness Measurement using Swept Source Optical Coherence Tomography in Healthy Eyes. PLoS ONE 2015, 12, e0144156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia Parodi, M.; Arrigo, A.; Iacono, P.; Falcomatà, B.; Bandello, F. Central Serous Chorioretinopathy: Treatment with Laser. Pharmaceuticals 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Imamura, Y.; Shinoda, K.; Matsumoto, C.S.; Mizutani, Y.; Hashizume, K.; Mizota, A.; Yuzawa, M. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2015, 3, 555–561. [Google Scholar] [CrossRef]
- Shinojima, A.; Bousquet, E.; Lavia, C.; Gaudric, A.; Tadayoni, R. Natural course of late-phase hypofluorescent areas on in-docyanine green angiography in patients with central serous chorioretinopathy. In Proceedings of the 18th EURETINA, Vienna, Austria, 20–23 September 2018. [Google Scholar]
- Shinojima, A.; Bousquet, E.; Lavia, C.; Gaudric, A.; Tadayoni, R. The course of ultra-late-phase hypofluorescent foci on indo-cyanine green angiography in patients with central serous chorioretinopathy before and after photodynamic therapy. In Proceedings of the 19th EURETINA, Paris, France, 5–8 September 2019. [Google Scholar]
- Shinojima, A.; Ozawa, Y.; Uchida, A.; Nagai, N.; Shinoda, H.; Kurihara, T.; Suzuki, M.; Minami, S.; Negishi, K.; Tsubota, K. Assessment of Hypofluorescent Foci on Late-Phase Indocyanine Green Angiography in Central Serous Chorioretinopathy. J. Clin. Med. 2021, 10, 2178. [Google Scholar] [CrossRef]
- Castro-Navarro, V.; Behar-Cohen, F.; Chang, W.; Joussen, A.M.; Lai, T.Y.Y.; Navarro, R.; Pearce, I.; Yanagi, Y.; Okada, A.A. Pachychoroid: Current concepts on clinical features and pathogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Mrejen, S.; Krivosic, V.; Quentel, G.; Tadayoni, R.; Gaudric, A. Flat Irregular Retinal Pigment Epithelium Detachments in Chronic Central Serous Chorioretinopathy and Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 159, 890–903. [Google Scholar] [CrossRef]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef]
- Blaauwgeers, H.G.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.; Kijlstra, A.; van Hinsbergh, V.W.; et al. Polarized Vascular Endothelial Growth Factor Secretion by Human Retinal Pigment Epithelium and Localization of Vascular Endothelial Growth Factor Receptors on the Inner Choriocapillaris: Evidence for a Trophic Paracrine Relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular Endothelial Growth Factor Expression in the Retinal Pigment Epithelium Is Essential for Choriocapillaris Development and Visual Function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Schlingemann, R.O. Role of growth factors and the wound healing response in age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 242, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.C. Towards an understanding of age-related macular disease. Eye 2003, 17, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Ramrattan, R.S.; van der Schaft, T.L.; Mooy, C.M.; de Bruijn, W.C.; Mulder, P.G.; de Jong, P.T. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2857–2864. [Google Scholar]
- Kurihara, T.; Westenskow, P.; Gantner, M.L.; Usui, Y.; Schultz, A.; Bravo, S.; Aguilar, E.; Wittgrove, C.; Friedlander, M.; Paris, L.; et al. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. eLife 2016, 5, e14319. [Google Scholar] [CrossRef] [PubMed]


| Representative HIF Inhibitors | References |
|---|---|
| Garcinia cambogia extract | Ibuki, et al. [38] |
| Hydroxycitric acid | Ibuki, et al. [38] |
| Lactoferrin | Ibuki, et al. [39] |
| Rice bran | Ibuki, et al. [40] |
| Vitamin B6 (pyridoxine hydrochloride) | Ibuki, et al. [40] |
| Resveratrol | Lee, et al. [41] |
| Selar crumenophthalmus | Shoda, et al. [42] |
| Seriola dumerili | Shoda, et al. [42] |
| Spratelloides gracilis | Shoda, et al. [42] |
| Decapterus macarellus | Shoda, et al. [42] |
| Decapterus tabl | Shoda, et al. [42] |
| Decapterus muroadsi | Shoda, et al. [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinojima, A.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Retinal Diseases Regulated by Hypoxia—Basic and Clinical Perspectives: A Comprehensive Review. J. Clin. Med. 2021, 10, 5496. https://doi.org/10.3390/jcm10235496
Shinojima A, Lee D, Tsubota K, Negishi K, Kurihara T. Retinal Diseases Regulated by Hypoxia—Basic and Clinical Perspectives: A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(23):5496. https://doi.org/10.3390/jcm10235496
Chicago/Turabian StyleShinojima, Ari, Deokho Lee, Kazuo Tsubota, Kazuno Negishi, and Toshihide Kurihara. 2021. "Retinal Diseases Regulated by Hypoxia—Basic and Clinical Perspectives: A Comprehensive Review" Journal of Clinical Medicine 10, no. 23: 5496. https://doi.org/10.3390/jcm10235496
APA StyleShinojima, A., Lee, D., Tsubota, K., Negishi, K., & Kurihara, T. (2021). Retinal Diseases Regulated by Hypoxia—Basic and Clinical Perspectives: A Comprehensive Review. Journal of Clinical Medicine, 10(23), 5496. https://doi.org/10.3390/jcm10235496









