Optimization of RAASi Therapy with New Potassium Binders for Patients with Heart Failure and Hyperkalemia: Rapid Review and Meta-Analysis
Abstract
:1. Introduction
1.1. Description of the Condition
1.2. Description of the Intervention
1.3. How the Intervention Might Work
1.4. Why It Is Important to Do This Review
1.5. Objectives
2. Materials and Methods
2.1. Criteria for Considering Studies for this Review
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
- Proportion of patients on MRA therapy (guideline target dose) compared with placebo at the end of the study
- Proportion of patients on ACE/ARB therapy (guideline target dose) compared with placebo at the end of the study
- Proportion of patients on ARNi therapy (guideline target dose) compared with placebo at the end of the study
- Safety of treatments in patients with heart failure
2.2. Search Methods for Identification of Studies
2.3. Data Collection and Analysis
Selection of Studies
2.4. Data Extraction and Management
2.5. Assessment of Risk of Bias in Included Studies
2.6. Measures of Treatment Effect
2.7. Assessment of Heterogeneity
2.8. Assessment of Reporting Biases
2.9. Data Synthesis
2.10. Subgroup Analysis and Investigation of Heterogeneity
2.11. Sensitivity Analysis
- Repeating the analysis with the fixed effects model
- Repeating the analysis excluding studies with high risks of bias
3. Results
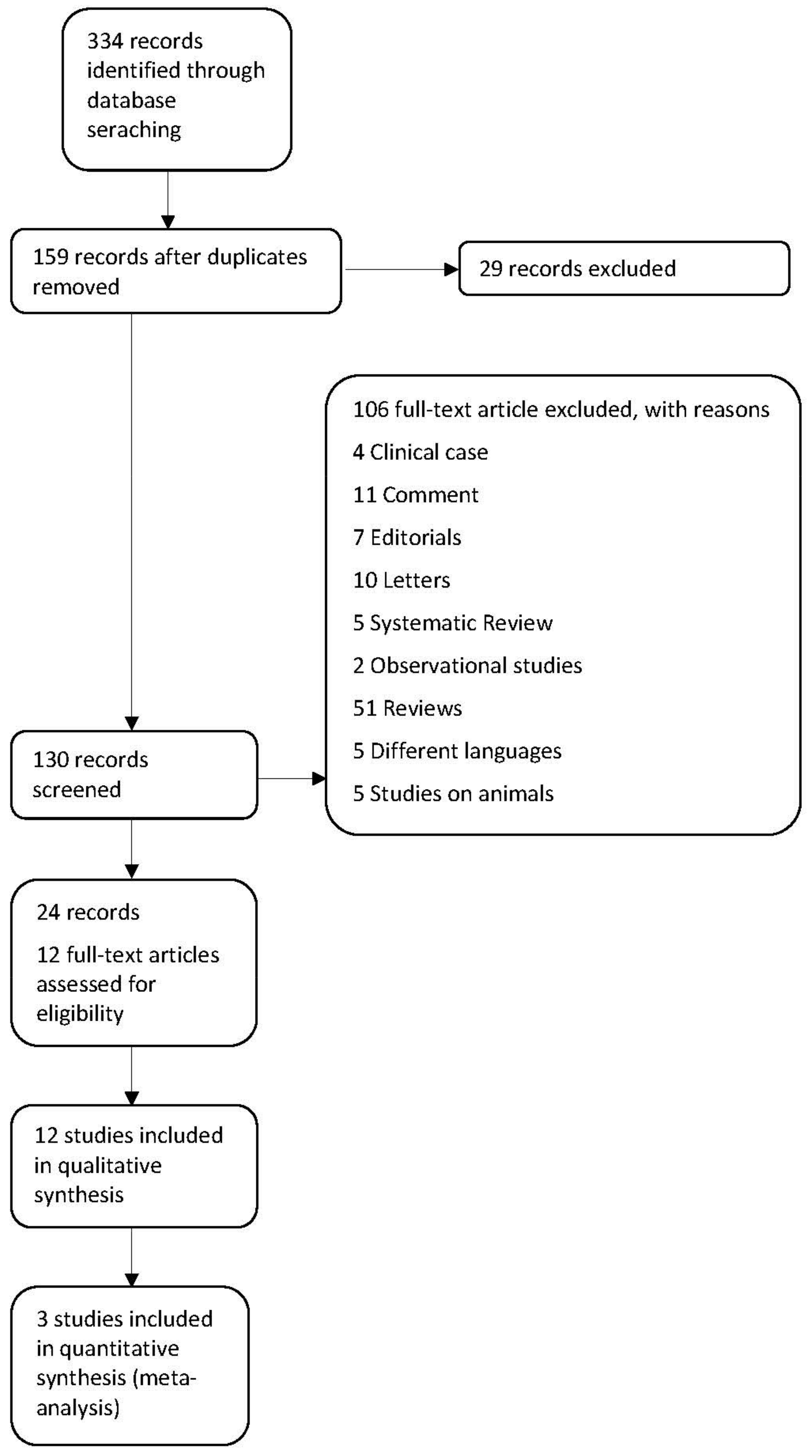
3.1. Description of Studies
3.2. Included Studies
3.3. Excluded Studies
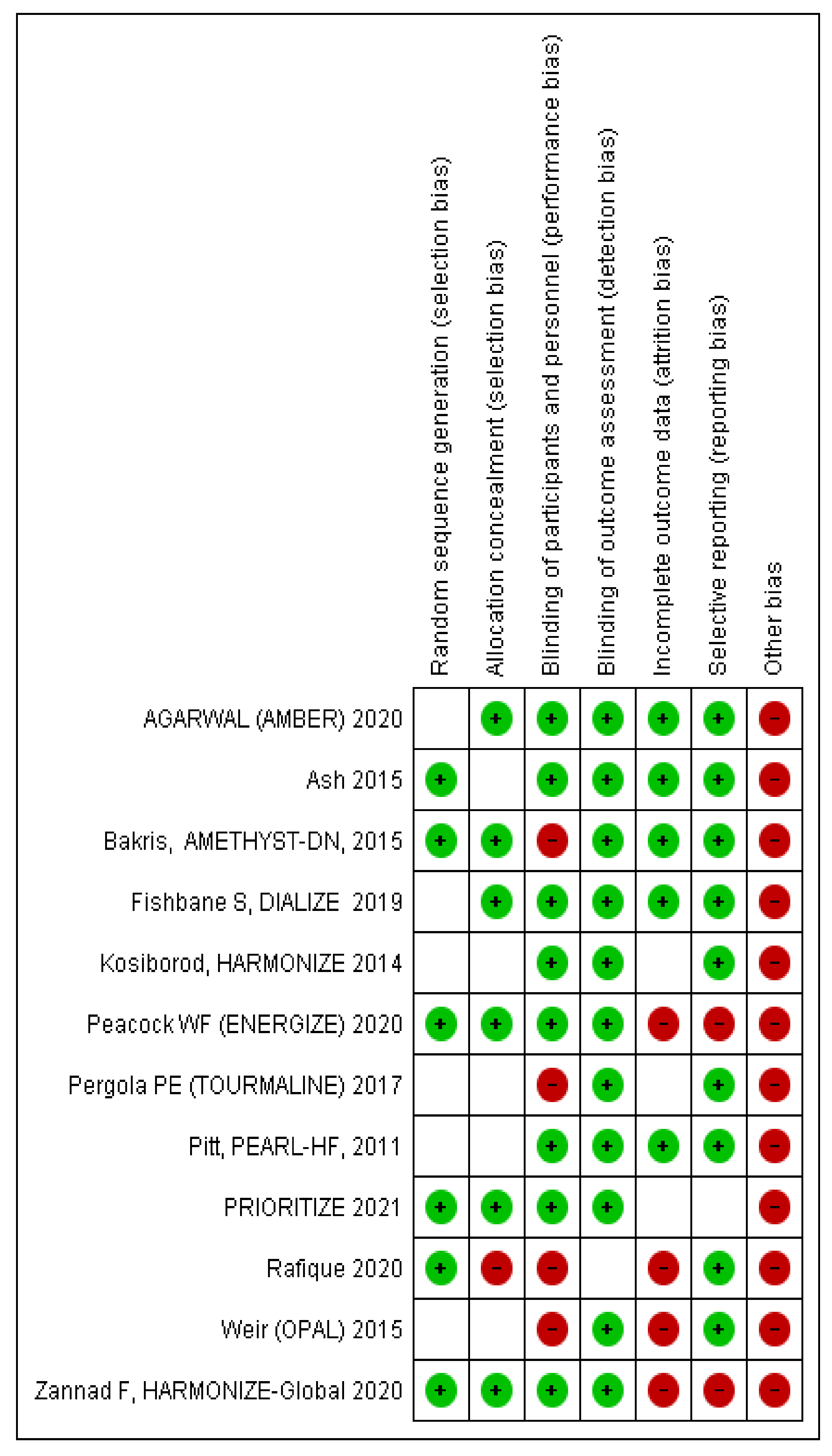
3.4. Risk of Bias in Included Studies the Review
3.5. Selective Reporting (Reporting Bias)
3.6. Other Potential Sources of Bias
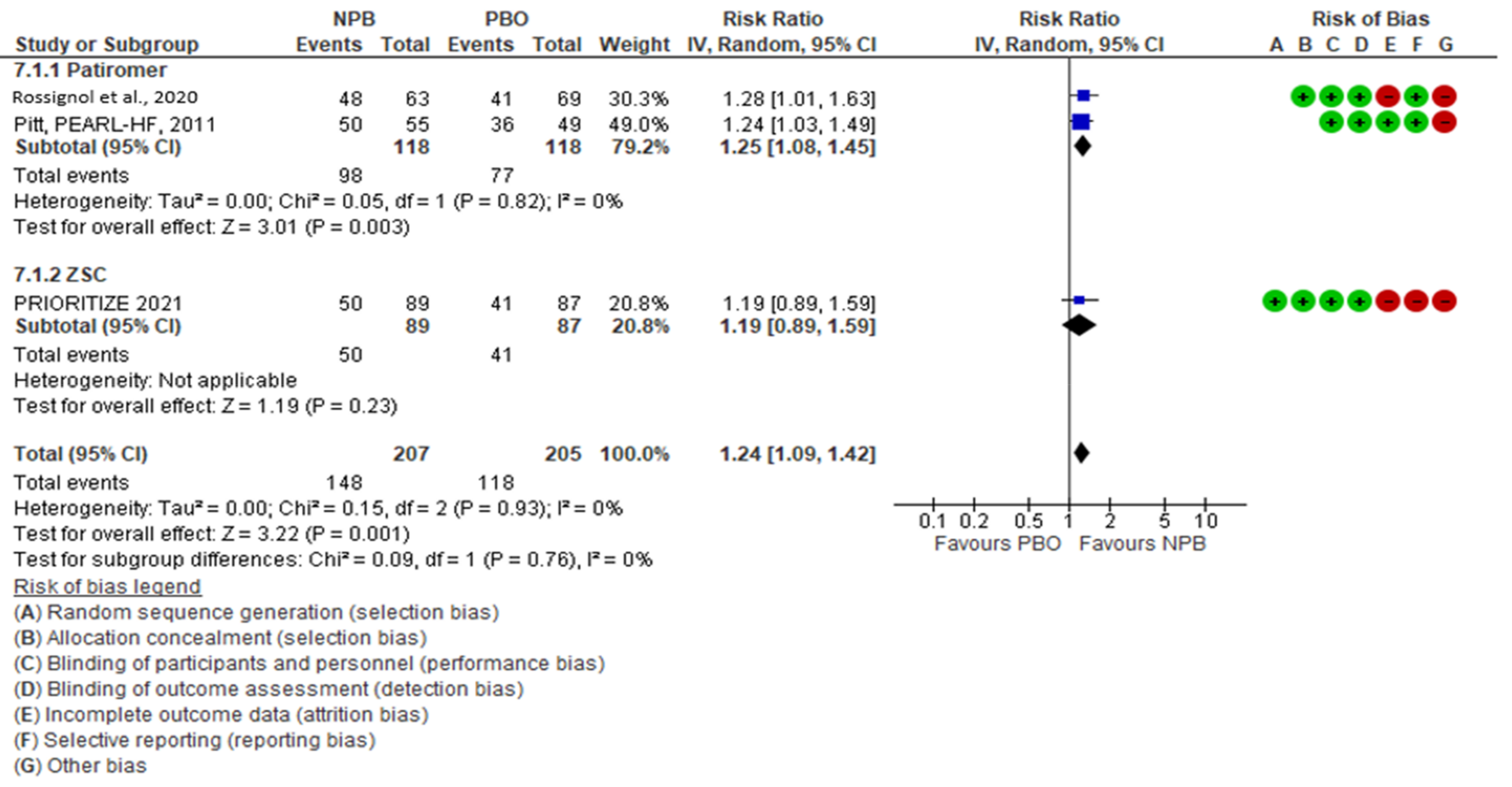
3.7. Effects of Interventions
3.8. Safety
4. Discussion
4.1. Overall Completeness and Applicability of Evidence
4.2. Quality of the Evidence
4.3. Potential Biases in the Review Process
4.4. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.; Coats, A.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Epstein, M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Oestreicher, N.; Knispel, J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am. J. Manag. Care 2015, 21, S212–S220. [Google Scholar] [PubMed]
- Rosano, G.; Tamargo, J.; Kjeldsen, K.P.; Lainscak, M.; Agewall, S.; Anker, S.D.; Ceconi, C.; Coats, A.; Drexel, H.; Filippatos, G.; et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: Coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 180–188. [Google Scholar] [CrossRef]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; EMPHASIS-HF Study Group EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M.; et al. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Carrero, J.J.; Pitt, B.; Anker, S.D.; Rosano, G.; Dahlström, U.; Lund, L.H. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: An analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2018, 20, 1326–1334. [Google Scholar] [CrossRef]
- Rossignol, P.; Lainscak, M.; Crespo-Leiro, M.G.; Laroche, C.; Piepoli, M.F.; Filippatos, G.; Rosano, G.; Savarese, G.; Anker, S.D.; Seferovic, P.M.; et al. Unravelling the interplay between hyperkalaemia, renin–angiotensin–aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 1378–1389. [Google Scholar] [CrossRef]
- Aldahl, M.; Jensen, A.C.; Davidsen, L.; Eriksen, M.A.; Møller Hansen, S.; Nielsen, B.J.; Krogager, M.L.; Køber, L.; Torp-Pedersen, C.; Søgaard, P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur. Heart J. 2017, 38, 2890–2896. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Harrison, S.D.; Cope, M.J.; Park, C.; Lee, L.; Salaymeh, F.; Madsen, D.; Benton, W.W.; Berman, L.; Buysse, J. Mechanism of Action and Pharmacology of Patiromer, a Nonabsorbed Cross-Linked Polymer That Lowers Serum Potassium Concentration in Patients With Hyperkalemia. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 456–465. [Google Scholar] [CrossRef]
- Stavros, F.; Yang, A.; Leon, A.; Nuttall, M.; Rasmussen, H.S. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS ONE 2014, 9, e114686. [Google Scholar] [CrossRef]
- Meaney, C.J.; Beccari, M.V.; Yang, Y.; Zhao, J. Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacotherapy 2017, 37, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, D.B.; Budhathoki, P.; Sedhai, Y.R.; Baniya, R.; Cable, C.A.; Kashiouris, M.G.; Dixon, D.L.; Kidd, J.M.; Adhikari, Y.; Marasini, A.; et al. Patiromer and Sodium Zirconium Cyclosilicate in Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis. Curr. Ther. Res. Clin. Exp. 2021, 95, 100635. [Google Scholar] [CrossRef]
- Natale, P.; Palmer, S.C.; Ruospo, M.; Saglimbene, V.M.; Strippoli, G.F. Potassium binders for chronic hype Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 6, CD013165. [Google Scholar]
- Zhang, Y.; Xu, R.; Wang, F.; Liu, Y.; Xu, J.; Zhao, N.; Cheng, F.; Long, L.; Jia, J.; Lin, S. Effects and Safety of a Novel Oral Potassium-Lowering Drug-Sodium Zirconium Cyclosilicate for the Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis. Cardiovasc. Drugs Ther. 2021, 35, 1057–1066. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021). Available online: https://training.cochrane.org/handbook/current (accessed on 8 September 2021).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, B.; Bakris, G.L.; Bushinsky, D.A.; Garza, D.; Mayo, M.R.; Stasiv, Y.; Christ-Schmidt, H.; Berman, L.; Weir, M.R. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur. J. Heart Fail. 2015, 17, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, P.; Williams, B.; Mayo, M.R.; Warren, S.; Arthur, S.; Ackourey, G.; White, W.B.; Agarwal, R. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): Results in the pre-specified subgroup with heart failure. Eur. J. Heart Fail. 2020, 22, 1462–1471. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef]
- Ash, S.R.; Singh, B.; Lavin, P.T.; Stavros, F.; Rasmussen, H.S. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015, 88, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Bakris, G.L.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Zawadzki, R.; Berman, L.; Bushinsky, D.A.; et al. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 2015, 314, 151–161. [Google Scholar] [CrossRef]
- Fishbane, S.; Ford, M.; Fukagawa, M.; McCafferty, K.; Rastogi, A.; Spinowitz, B.; Staroselskiy, K.; Vishnevskiy, K.; Lisovskaja, V.; Al-Shurbaji, A.; et al. A phase 3b, randomized, double-Blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J. Am. Soc. Nephrol. 2019, 30, 1723–1733. [Google Scholar] [CrossRef]
- Anker, S.D.; Kosiborod, M.; Zannad, F.; Piña, I.L.; McCullough, P.A.; Filippatos, G.; van der Meer, P.; Ponikowski, P.; Rasmussen, H.S.; Lavin, P.T.; et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: Results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur. J. Heart Fail. 2015, 17, 1050–1056. [Google Scholar] [CrossRef]
- Peacock, W.F.; Rafique, Z.; Vishnevskiy, K.; Michelson, E.; Vishneva, E.; Zvereva, T.; Nahra, R.; Li, D.; Miller, J. Emergency Potassium Normalization Treatment Including Sodium Zirconium Cyclosilicate: A Phase II, Randomized, Double-blind, Placebo-controlled Study (ENERGIZE). Acad. Emerg. Med. 2020, 27, 475–486. [Google Scholar] [CrossRef]
- Pergola, P.E.; Spiegel, D.M.; Warren, S.; Yuan, J.; Weir, M.R. Patiromer Lowers Serum Potassium When Taken without Food: Comparison to Dosing with Food from an Open-Label, Randomized, Parallel Group Hyperkalemia Study. Am. J. Nephrol. 2017, 46, 323–332. [Google Scholar] [CrossRef]
- Pitt, B.; Anker, S.D.; Bushinsky, D.A.; Kitzman, D.W.; Zannad, F.; Huang, I.Z.; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur. Heart J. 2011, 32, 820–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT03532009, Potassium Reduction Initiative to Optimize RAAS Inhibition Therapy with Sodium Zirconium Cyclosilicate in Heart Failure (PRIORITIZE HF), 22 May 2018. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03532009?term=prioritize+hf&draw=2&rank=1 (accessed on 27 October 2021).
- Rafique, Z.; Liu, M.; Staggers, K.A.; Minard, C.G.; Peacock, W.F. Patiromer for Treatment of Hyperkalemia in the Emergency Department: A Pilot Study. Acad. Emerg. Med. 2020, 27, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B.; et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015, 372, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannad, F.; Hsu, B.G.; Maeda, Y.; Shin, S.K.; Vishneva, E.M.; Rensfeldt, M.; Eklund, S.; Zhao, J. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: The randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail. 2020, 7, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Rasmussen, H.S.; Lavin, P.; Qunibi, W.Y.; Spinowitz, B.; Packham, D.; Roger, S.D.; Yang, A.; Lerma, E.; Singh, B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA 2014, 312, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M. Renin-Angiotensin-Aldosterone System Inhibition and Mineralocorticoid Receptor Antagonists: The Overriding Importance of Enablers and Dampers. Kidney Int. Rep. 2021, 6, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Ster, I.C.; Kaski, J.C.; Anderson, L.; Banerjee, D. The LIFT trial: Study protocol for a double-blind, randomised, placebo-controlled trial of K+-binder Lokelma for maximisation of RAAS inhibition in CKD patients with heart failure. BMC Nephrol. 2021, 22, 254. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]

| Study ID | Population | Intervention | Comparator | Outcome |
|---|---|---|---|---|
| Agarwal, (AMBER) 2020 [22]. | 295 patients with CKD (eGFR 25 to 45 mL/min/1.73 m2) and resistant hypertension, baseline K+ levels (4.3 to 5.1 mmol/L) | Patiromer (8.4 g o.d.) | Placebo | Proportion of patients remaining on spironolactone |
| Ash, 2015 [23]. | 90 outpatients, CKD stage 3 and hyperkalemia (5.0 to 6.0 mEq/L) | 0.3 g, 3 g, 10 g of ZSC | Placebo | Rate of serum K+ decline in the first 48 h |
| Bakis, AMETHYST-DN, 2015 [24] | 306 patients with T2DM (eGFR, 15 to <60 mL/min/1.73 m2 and serum K+ level >5.0 mEq/L). | Mild hyperkalemia: 4.2 g, twice daily; moderate hyperkalemia: 8.4 g, twice daily | Mild hyperkalemia: 8.4 g, or 12.6 g twice daily; moderate hyperkalemia: 12.6 g, or 16.8 g twice daily | Mean change in serum K+ from baseline to week 4 and adverse events through 52 weeks |
| Fishbane, DIALIZE 2019 [25]. | 97 patients with ESRD and HD three times weekly, predialysis sK+ > 5.4 mmol/L after the long interdialytic interval as well as predialysis sK+ > 5.0 mmol/L after at least one short interdialytic interval | ZSC 5 g, 10 g, 15 g | Placebo | Patients with predialysis serum K+ of 4.0–5.0 mmol/L |
| Kosiborod, HARMONIZE 2014 [26]. | 258 patients with hyperkalemia (serum potassium ≥ 5.1 mEq/L) | ZSC 10 g three times a day for 48 h (correction phase), after which normokalemic patients (3.5–5.0 mEq/L) were randomized to ZSC, 5 g, 10 g, or 15 g daily (Maintenance phase) | Placebo | Mean serum potassium level in each zirconium cyclosilicate group vs. placebo in MP |
| Peacock WF, ENERGIZE 2020 [27]. | 70 ED patients with blood potassium ≥ 5.8 mmol/L | SZC 10 g | Placebo | Mean change in level of serum K+ from baseline until 4 h after the starting dose |
| Pergola, TOURMALINE, 2017 [28]. | 112 patients with serum K+ ≥ 5.0 mEq/L | Patiromer (by 8.4 g/day–25.2 g/day) with food | Patiromer (8.4 g/day–25.2 g/day) without food | Proportion of patients with level of serum K+ in the range of 3.8–5.0 mEq/L at week 3 or 4 |
| Pitt, PEARL-HF, 2011 [29]. | 105 HF patients with CKD (eGFR <60 mL/min) or a history of HK | Patiromer 25.2 g | Placebo | Change from baseline in level of serum K+ at the end of study |
| PRIORITIZE HF 2021 [30] | 182 HF patients with serum potassium > 5.0 mmol/L or at high risk of hyperkalemia | ZSC 5 g or 10 g | Placebo | Percentage of patients receiving ACEi, ARB, MRA, or ARNi treatments at month 3 |
| Rafique 2020 [31]. | 30 ESRD patients, serum K+ ≥ 6.0 mEq/L | Patiromer 25.2 g + SOC | SOC | Difference in serum K+ between groups at 6 h |
| Weir, OPAL, 2015 [32] | Initial phase: 243 CKD patients in RAASi and with serum K+ levels between 5.1 and 6.5 mmol/L. Withdrawal phase: 105 patients in normokalemic range | Patiromer 4.2 g or 8.4 g twice/day | Placebo in withdrawal phase | Initial phase: mean change in the serum K+ level from baseline to week 4. Withdrawl phase: between-group difference in the median change in the serum K+ level |
| Zannad, HARMONIZE GLOBAL, 2020 [33]. | 267 patients with serum K+ ≥ 5.1 mmol/L | CP: ZSC 10 g 3 times a day for 48 h. MP: ZSC 5 g to 10 g | Placebo in MP | Mean serum K+ level during days 8–29 of the MP |
| Study ID | Design of Study | Outcome | Duration | MRA NPB (End of Study) | MRA PBO (End of Study) |
|---|---|---|---|---|---|
| Rossignol et al., 2020 [21] | RCT | Patients taking spironolactone at week 12 | 3 months | 48/63 | 41/69 |
| PEARL HF, Pitt B et al., 2011 [29] | RCT | Change in serum K+ from baseline to day 28 | 28 days | 50/55 | 36/49 |
| PRIORITIZE-HF, 2021 [30] | RCT | Percentage of patients receiving different categories of RAASi | 3 months | 50/89 | 41/87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagnani, A.; Frasson, S.; Gussoni, G.; Manfellotto, D. Optimization of RAASi Therapy with New Potassium Binders for Patients with Heart Failure and Hyperkalemia: Rapid Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5483. https://doi.org/10.3390/jcm10235483
Montagnani A, Frasson S, Gussoni G, Manfellotto D. Optimization of RAASi Therapy with New Potassium Binders for Patients with Heart Failure and Hyperkalemia: Rapid Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(23):5483. https://doi.org/10.3390/jcm10235483
Chicago/Turabian StyleMontagnani, Andrea, Stefania Frasson, Gualberto Gussoni, and Dario Manfellotto. 2021. "Optimization of RAASi Therapy with New Potassium Binders for Patients with Heart Failure and Hyperkalemia: Rapid Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 23: 5483. https://doi.org/10.3390/jcm10235483
APA StyleMontagnani, A., Frasson, S., Gussoni, G., & Manfellotto, D. (2021). Optimization of RAASi Therapy with New Potassium Binders for Patients with Heart Failure and Hyperkalemia: Rapid Review and Meta-Analysis. Journal of Clinical Medicine, 10(23), 5483. https://doi.org/10.3390/jcm10235483






