Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Biomarkers Determination in Serum
2.3. Statistical Analysis
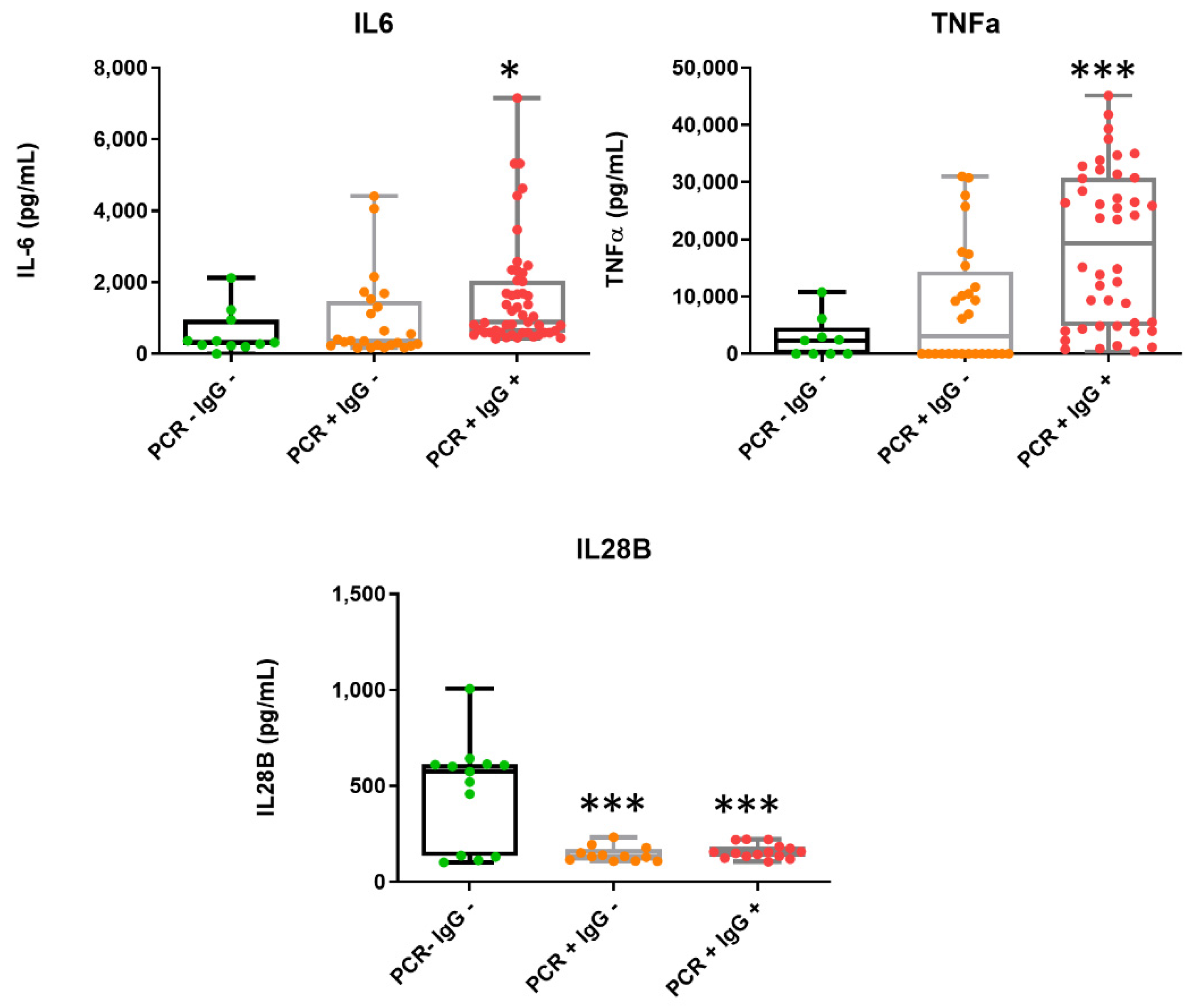
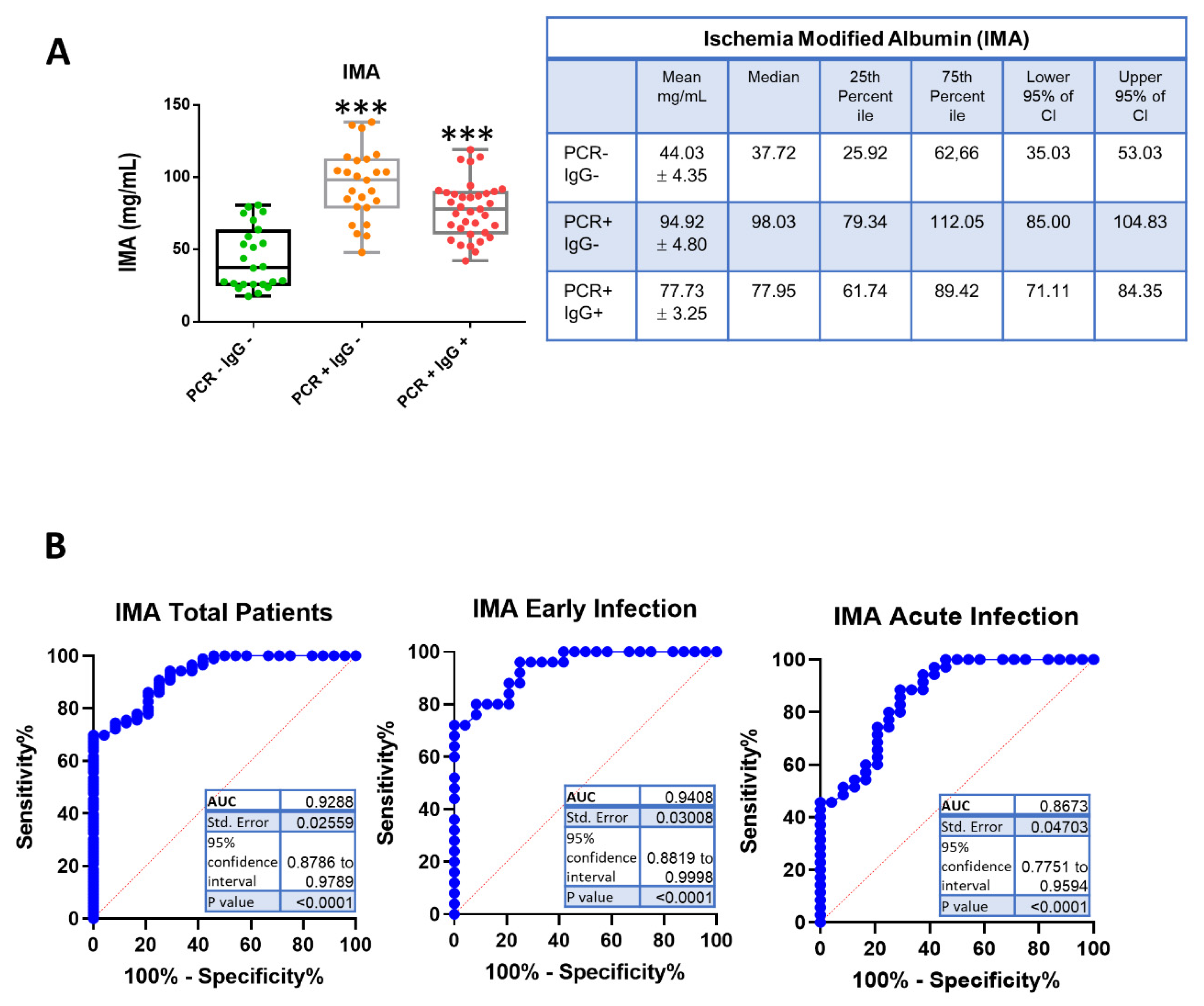
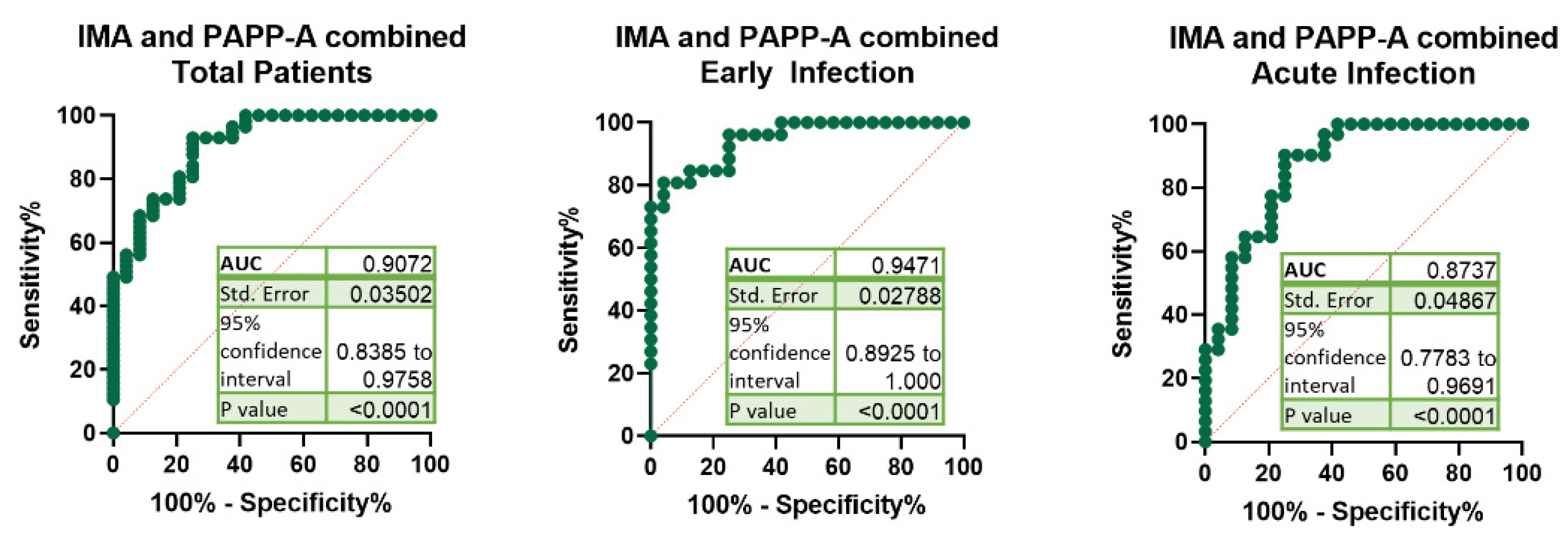
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pettersson, H.; Manley, B.; Hernandez, S.; McPhillips, D. Tracking COVID-19 Vaccinations Worldwide. Available online: https://edition.cnn.com/interactive/2021/health/global-covid-vaccinations/ (accessed on 6 September 2021).
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Y.; Ullah, W.; Rauf, H.; Virk, H.U.H.; Yadav, S.; Chowdhury, M.; Connerney, M.; Mamtani, S.; Pahuja, M.; Patel, R.D.; et al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int. J. Cardiol. Heart Vasc. 2020, 29, 100589. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V., 3rd; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.S.; Drazner, M.H. Biomarkers of Cardiac Stress and Cytokine Release Syndrome in COVID-19: A Review. Curr. Heart Fail. Rep. 2021, 18, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Coverdale, J.P.C.; Katundu, K.G.H.; Sobczak, A.I.S.; Arya, S.; Blindauer, C.A.; Stewart, A.J. Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding. Prostaglandins Leukot Essent Fatty Acids 2018, 135, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Role of “Ischemia modified albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg. Med. J. 2004, 21, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Bolatkale, M.; Duger, M.; Ulfer, G.; Can, C.; Acara, A.C.; Yigitbasi, T.; Seyhan, E.C.; Bulut, M. A novel biochemical marker for community-acquired pneumonia: Ischemia-modified albumin. Am. J. Emerg. Med. 2017, 35, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Duger, M.; Seyhan, E.C.; Gunluoglu, M.Z.; Bolatkale, M.; Ozgul, M.A.; Turan, D.; Ugur, E.; Ulfer, G. Does ischemia-modified albumin level predict severity of obstructive sleep apnea? Sleep Breath. 2021, 25, 65–73. [Google Scholar] [CrossRef]
- Wang, J.; Tan, G.J.; Han, L.N.; Bai, Y.Y.; He, M.; Liu, H.B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. 2017, 14, 135–150. [Google Scholar] [CrossRef]
- Conover, C.A.; Bale, L.K.; Harrington, S.C.; Resch, Z.T.; Overgaard, M.T.; Oxvig, C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: Inhibition by resveratrol. Am. J. Physiol. Cell Physiol. 2006, 290, C183–C188. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Flack, J.M.; Ito, S.; Muntner, P.; Webb, R.C. Hypertension and COVID-19. Am. J. Hypertens. 2020, 33, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The RBD of The Spike Protein of SARS-Group Coronaviruses Is A Highly Specific Target of SARS-CoV-2 Antibodies but not Other Pathogenic Human and Animal Coronavirus Antibodies. MedRxiv 2020. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Zhou, X.X.; Lui, I.; Elledge, S.K.; Glasgow, J.E.; Lim, S.A.; Loudermilk, R.P.; Chiu, C.Y.; Wang, T.T.; Wilson, M.R.; et al. Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding. mSphere 2020, 5, e00802-20. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, D.; Wen, X.S.; Cheng, X.C.; Sun, M.; He, B.; You, L.N.; Lei, P.; Tan, X.W.; Qin, S.; et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir. Res. 2020, 21, 83. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, F.B.Q.; Aguilar, A.M.J.; Montero-Perez, F.J. Acute arterial ischemia due to SARS-CoV-2 infection. Med. Clin. 2021, 157, 266. [Google Scholar] [CrossRef]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Wang, Y.N.; Chang, Q.Y.; Ma, P.; Hu, Y.; Cao, X. Type III Interferons in Viral Infection and Antiviral Immunity. Cell. Physiol. Biochem. 2018, 51, 173–185. [Google Scholar] [CrossRef]
- Felgenhauer, U.; Schoen, A.; Gad, H.H.; Hartmann, R.; Schaubmar, A.R.; Failing, K.; Drosten, C.; Weber, F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020, 295, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Vanderheiden, A.; Ralfs, P.; Chirkova, T.; Upadhyay, A.A.; Zimmerman, M.G.; Bedoya, S.; Aoued, H.; Tharp, G.M.; Pellegrini, K.L.; Manfredi, C.; et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J. Virol. 2020, 94, e00985-20. [Google Scholar] [CrossRef]
- Galani, I.E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021, 22, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhuang, M.W.; Han, L.; Zhang, J.; Nan, M.L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Tabassum, T.; Araf, Y.; Al Nahid, A.; Ullah, M.A.; Hosen, M.J. Silent hypoxia in COVID-19: Pathomechanism and possible management strategy. Mol. Biol. Rep. 2021, 48, 3863–3869. [Google Scholar] [CrossRef]
- Yildiz, H.; Alp, H.H.; Ekin, S.; Arisoy, A.; Gunbatar, H.; Asker, S.; Cilingir, B.M.; Sunnetcioglu, A.; Celikel, M.; Esen, N.; et al. Analysis of endogenous oxidative damage markers and association with pulmonary involvement severity in patients with SARS-CoV-2 pneumonia. Infect. Dis. Now 2021, 51, 429–434. [Google Scholar] [CrossRef]
- Nilsson, E.; Kastrup, J.; Sajadieh, A.; Jensen, G.B.; Kjoller, E.; Kolmos, H.J.; Wuopio, J.; Nowak, C.; Larsson, A.; Jakobsen, J.C.; et al. Pregnancy Associated Plasma Protein-A as a Cardiovascular Risk Marker in Patients with Stable Coronary Heart Disease During 10 Years Follow-Up-A CLARICOR Trial Sub-Study. J. Clin. Med. 2020, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.H.; He, L.H.; Gao, J.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. Pregnancy-associated plasma protein-A in atherosclerosis: Molecular marker, mechanistic insight, and therapeutic target. Atherosclerosis 2018, 278, 250–258. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Gu, F. CRP and TNF-alpha induce PAPP-A expression in human peripheral blood mononuclear cells. Mediat. Inflamm. 2012, 2012, 697832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, H.; Zhou, L.; Wang, Z.; Hua, B. Pregnancy-Associated Plasma Protein A Induces Inflammatory Cytokine Expression by Activating IGF-I/PI3K/Akt Pathways. Mediat. Inflamm. 2019, 2019, 8436985. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Yang, M.; Qu, A.J.; Mintz, G.S.; Yang, Y.; Shang, Y.P.; Gao, H.; Zhang, Y.C.; Ge, C.J.; Wang, L.Y.; et al. Level of Pregnancy-associated Plasma Protein-A Correlates with Coronary Thin-cap Fibroatheroma Burden in Patients With Coronary Artery Disease: Novel Findings From 3-Vessel Virtual Histology Intravascular Ultrasound Assessment. Medicine 2016, 95, e2563. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Conover, C.A.; Overgaard, M.T.; Bailey, K.R.; Christiansen, M.; Holmes, D.R., Jr.; Virmani, R.; Oxvig, C.; Schwartz, R.S. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N. Engl. J. Med. 2001, 345, 1022–1029. [Google Scholar] [CrossRef]
- Cediel, G.; Rueda, F.; Oxvig, C.; Oliveras, T.; Labata, C.; de Diego, O.; Ferrer, M.; Aranda-Nevado, M.C.; Serra-Gregori, J.; Nunez, J.; et al. Prognostic value of the Stanniocalcin-2/PAPP-A/IGFBP-4 axis in ST-segment elevation myocardial infarction. Cardiovasc. Diabetol. 2018, 17, 63. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Cervio, E.; Lionetti, V.; Milano, G.; Ciullo, A.; Biemmi, V.; Bolis, S.; Altomare, C.; Matteucci, M.; Di Silvestre, D.; et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: Role of pregnancy-associated plasma protein-A. Cardiovasc. Res. 2018, 114, 992–1005. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, H.; Gao, W.H.; Zou, T.; Yao, S.; Du, M.F.; Zhang, X.Y.; Chu, C.; Liao, Y.Y.; Chen, C.; et al. Associations of plasma PAPP-A2 and genetic variations with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. J. Hypertens. 2021, 39, 1817–1825. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, B.; Weng, W.; Yang, L.; Shi, G.; Xue, S.; Fu, X. Associations of pregnancy-associated plasma protein-A level with essential hypertension and hypertensive disorders in pregnancy in Chinese population: A meta-analysis of 20 research studies involving 3332 individuals. BMJ Open 2015, 5, e008210. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, H.; Liu, X.; Li, P.; Du, W.; Han, Q. Association of pregnancy-associated plasma protein A and vascular endothelial growth factor with pregnancy-induced hypertension. Exp. Ther. Med. 2019, 18, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Petla, L.T.; Chikkala, R.; Ratnakar, K.S.; Kodati, V.; Sritharan, V. Biomarkers for the management of pre-eclampsia in pregnant women. Indian J. Med. Res. 2013, 138, 60–67. [Google Scholar]
- Ferrer-Oliveras, R.; Mendoza, M.; Capote, S.; Pratcorona, L.; Esteve-Valverde, E.; Cabero-Roura, L.; Alijotas-Reig, J. Immunological and physiopathological approach of COVID-19 in pregnancy. Arch. Gynecol. Obstet. 2021, 304, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]

| Total | Female | Male | |
|---|---|---|---|
| Participants (n) | 84 (100%) | 33 (40%) | 51 (60%) |
| Age (median, IQR, years) | 65 (35−90) | 61 (44−89) | 67 (35−90) |
| SARS-CoV-2 infection | |||
| PCR+ | 60 | 28 | 32 |
| PCR− | 24 | 5 | 19 |
| SARS-CoV-2 serology | |||
| IgG+ | 36 | 15 | 21 |
| IgG− | 48 | 18 | 30 |
| Biomarker | COVID-19 Patients | Cut-Off mg/mL | Sensitivity% | 95% CI | Specificity% | 95% CI | Likelihood Ratio | Positive Predictive Value % | Negative Predictive Value % |
|---|---|---|---|---|---|---|---|---|---|
| IMA | Total | >59.26 | 90.70 | 82.70% to 95.21% | 75.00 | 55.10% to 88.00% | 3.628 | 39 | 97 |
| Early Infection | >59.26 | 96.00 | 80.46% to 99.79% | 75.00 | 55.10% to 88.00% | 3.840 | 40 | 99 | |
| Acute Infection | >54.83 | 88.57 | 74.05% to 95.46% | 70.83 | 50.83% to 85.09% | 3.037 | 34 | 97 | |
| PAPP-A | Total | >248.9 | 58.49 | 45.09% to 70.74% | 59.09 | 38.73% to 76.74% | 1.430 | 20 | 88 |
| Early Infection | >237.3 | 73.91 | 53.53% to 87.45% | 59.09 | 38.73% to 76.74% | 1.807 | 24 | 92 | |
| Acute Infection | >181.2 | 56.67 | 39.20% to 72.62% | 45.45 | 26.92% to 65.34% | 1.039 | 15 | 85 | |
| IMA + PAPP-A | Total | >0.5195 | 92.98 | 83.30% to 97.24% | 75.00 | 55.10% to 88.00% | 3.719 | 39 | 98 |
| Early Infection | >0.5689 | 92.31 | 75.86% to 98.63% | 75.00 | 55.10% to 88.00% | 3.692 | 39 | 98 | |
| Acute Infection | >0.4606 | 93.55 | 79.28% to 98.85% | 62.50 | 42.71% to 78.84% | 2.495 | 30 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, B.G.; Gasalla, J.M.; Sánchez-Chapado, M.; Bort, A.; Diaz-Laviada, I. Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients. J. Clin. Med. 2021, 10, 5474. https://doi.org/10.3390/jcm10235474
Sanchez BG, Gasalla JM, Sánchez-Chapado M, Bort A, Diaz-Laviada I. Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients. Journal of Clinical Medicine. 2021; 10(23):5474. https://doi.org/10.3390/jcm10235474
Chicago/Turabian StyleSanchez, Belén G., Jose M. Gasalla, Manuel Sánchez-Chapado, Alicia Bort, and Inés Diaz-Laviada. 2021. "Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients" Journal of Clinical Medicine 10, no. 23: 5474. https://doi.org/10.3390/jcm10235474
APA StyleSanchez, B. G., Gasalla, J. M., Sánchez-Chapado, M., Bort, A., & Diaz-Laviada, I. (2021). Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients. Journal of Clinical Medicine, 10(23), 5474. https://doi.org/10.3390/jcm10235474







