Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Assessment of Brachial Artery Flow-Mediated Dilation
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Clinical Course and In-Hospital Outcomes
3.3. Covariates of bFMD
3.4. Covariates of ICU Admission/In-Hospital Death
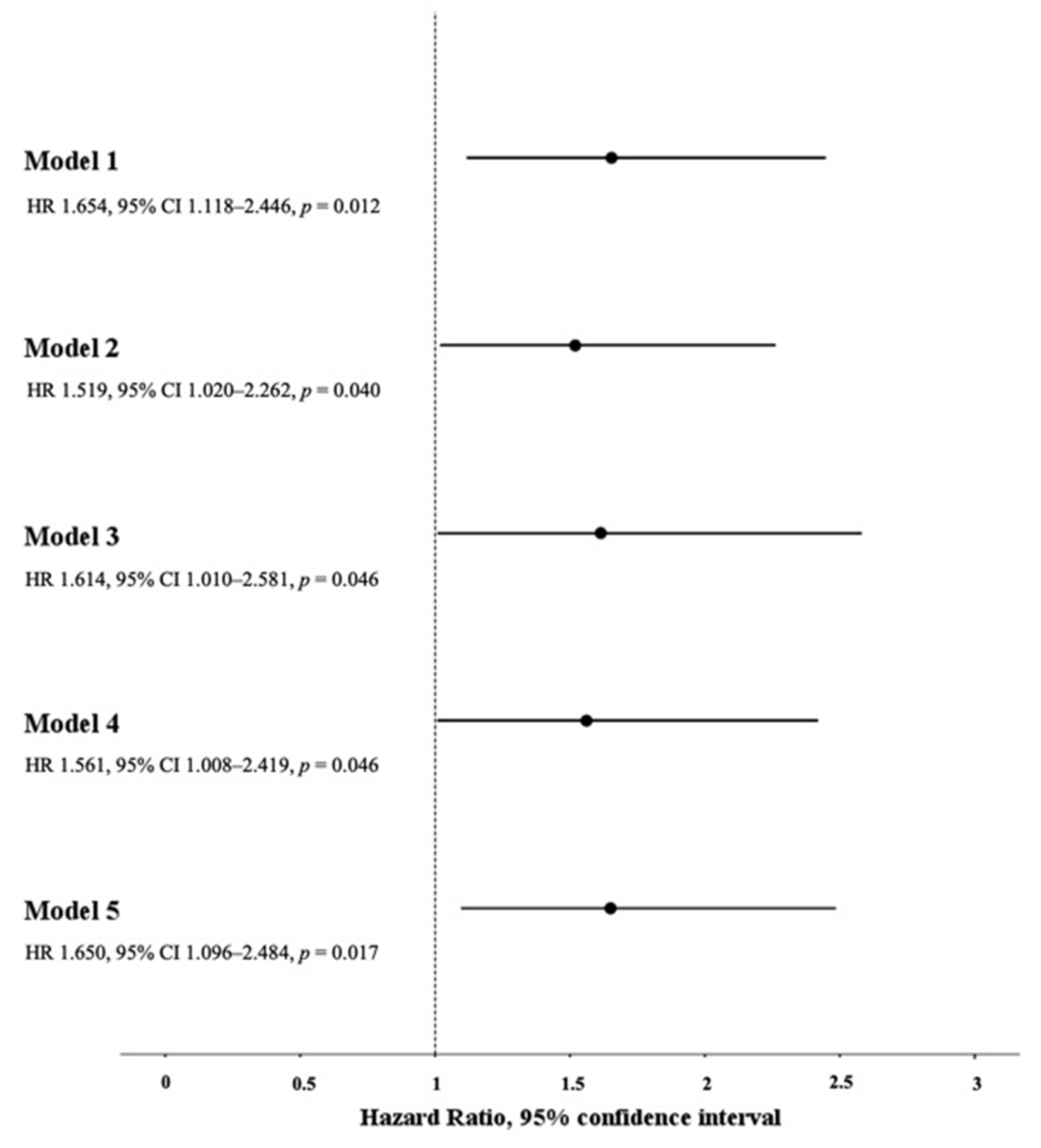
3.5. Association between Low bFMD and the Composite Endpoint of ICU Admission/In-Hospital Death
3.6. Exploratory Analyses on the Association between Low bFMD and either ICU Admission or In-Hospital Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Statement on the Sixth Meeting of the International Health Regulations (2005) Emergency Committee regarding the Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int (accessed on 27 September 2021).
- WHO. Situation by Country, Territory & Area. Available online: https://covid19.who.int (accessed on 27 September 2021).
- Asch, D.A.; Sheils, N.E.; Islam, M.N.; Chen, Y.; Werner, R.M.; Buresh, J.; Doshi, J.A. Variation in US Hospital Mortality Rates for Patients Admitted With COVID-19 During the First 6 Months of the Pandemic. JAMA Intern. Med. 2021, 181, 471. [Google Scholar] [CrossRef]
- Ciceri, F.; Ruggeri, A.; Lembo, R.; Puglisi, R.; Landoni, G.; Zangrillo, A.; COVID-BioB Study Group. Decreased in-hospital mortality in patients with COVID-19 pneumonia. Pathog. Glob. Health 2020, 114, 281–282. [Google Scholar] [CrossRef]
- Bose, S.; Adapa, S.; Aeddula, N.R.; Roy, S.; Nandikanti, D.; Vupadhyayula, P.M.; Naramala, S.; Gayam, V.; Muppidi, V.; Konala, V.M. Medical Management of COVID-19: Evidence and Experience. J. Clin. Med. Res. 2020, 12, 329–343. [Google Scholar] [CrossRef] [PubMed]
- CDC. Information for Clinicians on Investigational Therapeutics for Patients with COVID-19. Available online: https://www.cdc.gov (accessed on 27 September 2021).
- Bianconi, V.; Violi, F.; Fallarino, F.; Pignatelli, P.; Sahebkar, A.; Pirro, M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19? Drugs 2020, 80, 1383–1396. [Google Scholar] [CrossRef]
- Shojaei, A.; Salari, P. COVID-19 and off label use of drugs: An ethical viewpoint. Daru 2020, 28, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Wynants, L.; Sotgiu, G. Improving clinical management of COVID-19: The role of prediction models. Lancet Respir. Med. 2021, 9, 320–321. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef] [PubMed]
- Yoganandamoorthy, S.; Munasinghe, M.A.D.S.N.; Wanigasuriya, L.V.U.; Priyankara, M.K.K.; Jayasinghe, S. Non-invasive assessment of endothelial dysfunction: A novel method to predict severe COVID-19? Med. Hypotheses 2020, 144, 110229. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, C.; Burtey, S.; Sabatier, F.; Cauchois, R.; Lano, G.; Abdili, E.; Daviet, F.; Arnaud, L.; Brunet, P.; Hraiech, S.; et al. Circulating Endothelial Cells as a Marker of Endothelial Injury in Severe COVID-19. J. Infect. Dis. 2020, 222, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Petito, E.; Becattini, C.; De Robertis, E.; Paliani, U.; Sebastiano, M.; Vaudo, G.; Guglielmini, G.; Paciullo, F.; Cerotto, V.; et al. COVIR study investigators. Role of endothelial dysfunction in the thrombotic complications of COVID-19 patients. J. Infect. 2021, 82, 186–230. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Philippe, A.; Chocron, R.; Gendron, N.; Bory, O.; Beauvais, A.; Peron, N.; Khider, L.; Guerin, C.L.; Goudot, G.; Levasseur, F.; et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021, 24, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Mannarino, M.R.; Pucci, G.; Pirro, M.; Helou, J.; Savarese, G.; Vaudo, G.; Mannarino, E. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension 2007, 49, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 371, m4334, Erratum for: BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Guo, L.; Wei, D.; Zhang, X.; Wu, Y.; Li, Q.; Zhou, M.; Qu, J. Clinical Features Predicting Mortality Risk in Patients With Viral Pneumonia: The MuLBSTA Score. Front. Microbiol. 2019, 10, 2752. [Google Scholar] [CrossRef] [Green Version]
- Pirro, M.; Mannarino, M.R.; Francisci, D.; Schiaroli, E.; Bianconi, V.; Bagaglia, F.; Sahebkar, A.; Mannarino, E.; Baldelli, F. Urinary albumin-to-creatinine ratio is associated with endothelial dysfunction in HIV-infected patients receiving antiretroviral therapy. Sci. Rep. 2016, 6, 28741. [Google Scholar] [CrossRef]
- Pasqualini, L.; Cortese, C.; Marchesi, S.; Siepi, D.; Pirro, M.; Vaudo, G.; Liberatoscioli, L.; Gnasso, A.; Schillaci, G.; Mannarino, E. Paraoxonase-1 activity modulates endothelial function in patients with peripheral arterial disease. Atherosclerosis 2005, 183, 349–354. [Google Scholar] [CrossRef]
- NIH. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 27 September 2021).
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Kalinskaya, A.; Dukhin, O.; Molodtsov, I.; Maltseva, A.; Sokorev, D.; Elizarova, A.; Sapozhnikova, O.; Glebova, K.; Stonogina, D.; Shakhidzhanov, S.; et al. Dynamics of coagulopathy in patients with different COVID-19 severity. medRxiv 2020. [Preprint]. [Google Scholar] [CrossRef]
- Heubel, A.D.; Viana, A.A.; Linares, S.N.; do Amaral, V.T.; Schafauser, N.S.; de Oliveira, G.Y.O.; Ramírez, P.C.; Martinelli, B.; da Silva Alexandre, T.; Borghi Silva, A.; et al. Determinants of endothelial dysfunction in non-critically ill hospitalized COVID-19 patients: A cross-sectional study. Obesity 2021. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Back, G.D.; da Luz Goulart, C.; Domingos, B.C.; Arena, R.; Borghi-Silva, A. Endothelial function provides early prognostic information in patients with COVID-19: A cohort study. Respir. Med. 2021, 185, 106469. [Google Scholar] [CrossRef] [PubMed]
- Sabioni, L.; De Lorenzo, A.; Lamas, C.; Muccillo, F.; Castro-Faria-Neto, H.C.; Estato, V.; Tibirica, E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc. Res. 2021, 134, 104119. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. The cytokine storm of COVID-19: A spotlight on prevention and protection. Expert. Opin. Ther. Targets 2020, 24, 723–730. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, Y.; Tao, C. An enlightening role for cytokine storm in coronavirus infection. Clin. Immunol. 2021, 222, 108615. [Google Scholar] [CrossRef]
- Solages, A.; Vita, J.A.; Thornton, D.J.; Murray, J.; Heeren, T.; Craven, D.E.; Horsburgh, C.R., Jr. Endothelial function in HIV-infected persons. Clin. Infect. Dis. 2006, 42, 1325–1332. [Google Scholar] [CrossRef]
- Marchesi, S.; Lupattelli, G.; Lombardini, R.; Sensini, A.; Siepi, D.; Mannarino, M.; Vaudo, G.; Mannarino, E. Acute inflammatory state during influenza infection and endothelial function. Atherosclerosis 2005, 178, 345–350. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Mencarelli, A.; Distrutti, E.; Baldoni, M.; del Soldato, P.; Morelli, A. Nitric oxide regulates immune cell bioenergetic: A mechanism to understand immunomodulatory functions of nitric oxide-releasing anti-inflammatory drugs. J. Immunol. 2004, 173, 874–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Ha, E.K.; Yeniova, A.Ö.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Shie, C.B.; Yang, C.C.; Lee, T.M. Blockade of Cardiac Proton Pump Impairs Ventricular Remodeling Through a Superoxide-DDAH-Dependent Pathway in Infarcted Rats. Acta Cardiol. Sin. 2019, 35, 165–178. [Google Scholar] [CrossRef]
- Ancion, A.; Tridetti, J.; Trung, M.L.N.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.R.; Adhikari, S.; Iturrate, E. RAAS Inhibitors and Risk of Covid-19. Reply. N. Engl. J. Med. 2020, 383, 1993–1994. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Almansa, R.; Torres, A.; González-Rivera, M.; Kelvin, D.J. COVID-19 as a cardiovascular disease: The potential role of chronic endothelial dysfunction. Cardiovasc. Res. 2020, 116, e132–e133. [Google Scholar] [CrossRef] [PubMed]
- Alley, H.; Owens, C.D.; Gasper, W.J.; Grenon, S.M. Ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery in clinical research. J. Vis. Exp. 2014, 92, e52070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganjali, S.; Bianconi, V.; Penson, P.E.; Pirro, M.; Banach, M.; Watts, G.F.; Sahebkar, A. Commentary: Statins, COVID-19, and coronary artery disease: Killing two birds with one stone. Metabolism 2020, 113, 154375. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Banach, M.; Reiner, Ž.; Pirro, M.; Bianconi, V.; Al-Rasadi, K.; Sahebkar, A. Interaction Between Coronavirus S-Protein and Human ACE2: Hints for Exploring Efficient Therapeutic Targets to Treat COVID-19. Angiology 2021, 72, 122–130. [Google Scholar] [CrossRef] [PubMed]

| Total Study Population n = 408 | |
|---|---|
| Age, years | 72 (16) |
| Male gender, % | 52 |
| BMI, kg/m2 | 26.5 (4.3) |
| Current smoking, % | 16 |
| Hypertension, % | 61 |
| Type 2 diabetes, % | 19 |
| CKD, % | 11 |
| Previous CV event, % | 16 |
| Active cancer, % | 9 |
| Previous VTE, % | 3 |
| AF, % | 15 |
| COPD, % | 12 |
| ACE inhibitors, % | 27 |
| ARBs, % | 17 |
| Statins, % | 19 |
| DOACs, % | 10 |
| VKAs, % | 2 |
| LMWH, % | 19 |
| Anti-platelets, % | 23 |
| BBs, % | 25 |
| CCBs, % | 24 |
| Diuretics, % | 32 |
| Insulin, % | 13 |
| Oral hypoglycemic agents, % | 9 |
| SBP, mmHg | 131 (21) |
| DBP, mmHg | 80 (11) |
| Leukocytes, X 103/μL | 7.2 (5.1–10.3) |
| Platelets, X 103/μL | 203 (154–265) |
| D-dimer, ng/mL | 839 (531–1732) |
| hs-cTn, ng/L | 13.5 (6.9–29.5) |
| CRP, mg/dL | 6.5 (3.1–11.6) |
| eGFR, mL/min | 71 (27) |
| LDH, UI/L | 292 (224–407) |
| PaO2/FiO2 | 250 (171–304) |
| CURB-65 score | 2 (1–3) |
| 4C mortality score | 12 (9–15) |
| MuLBSTA score | 8 (4–11) |
| bFMD, % | 4.4 (2.7–6.8) |
| bFMD < 4.4% n = 201 | bFMD ≥ 4.4% n = 207 | p | |
|---|---|---|---|
| Age, years | 73 (14) | 72 (18) | 0.323 |
| Male gender, % | 54 | 51 | 0.479 |
| BMI, kg/m2 | 27.1 (4.6) | 26.0 (4.1) | 0.022 |
| Current smoking, % | 19 | 12 | 0.198 |
| Hypertension, % | 65 | 57 | 0.097 |
| Type 2 diabetes, % | 24 | 14 | 0.011 |
| CKD, % | 13 | 10 | 0.226 |
| Previous CV event, % | 18 | 14 | 0.276 |
| Active cancer, % | 9 | 10 | 0.956 |
| Previous VTE, % | 2 | 4 | 0.307 |
| AF, % | 16 | 13 | 0.401 |
| COPD, % | 12 | 11 | 0.899 |
| ACE inhibitors, % | 28 | 27 | 0.769 |
| ARBs, % | 19 | 11 | 0.027 |
| Statins, % | 21 | 16 | 0.215 |
| DOACs, % | 11 | 9 | 0.435 |
| VKAs, % | 2 | 2 | 0.776 |
| LMWH, % | 17 | 21 | 0.338 |
| Anti-platelets, % | 26 | 21 | 0.174 |
| BBs, % | 34 | 24 | 0.022 |
| CCBs, % | 24 | 18 | 0.163 |
| Diuretics, % | 36 | 32 | 0.380 |
| Insulin, % | 15 | 9 | 0.071 |
| Oral hypoglycemic agents, % | 12 | 9 | 0.273 |
| SBP, mmHg | 131 (20) | 131 (21) | 0.682 |
| DBP, mmHg | 76 (11) | 77 (11) | 0.492 |
| Leukocytes, X 103/μL | 9.4 (5.3–11.0) | 8.1 (4.9–10.0) | 0.155 |
| Platelets, X 103/μL | 216 (157–257) | 223 (146–269) | 0.740 |
| D-dimer, ng/mL | 871 (539–1682) | 824 (531–1765) | 0.921 |
| hs-cTn, ng/L | 14.3 (7.1–40.5) | 12.7 (6.2–26.3) | 0.084 |
| CRP, mg/dL | 6.5 (3.2–12.4) | 6.5 (3.2–12.4) | 0.484 |
| eGFR, mL/min | 68 (26) | 74 (28) | 0.079 |
| LDH, UI/L | 305 (234–416) | 278 (214–396) | 0.101 |
| PaO2/FiO2 | 242 (159–291) | 266 (185–319) | 0.010 |
| CURB-65 score | 2 (1–3) | 2 (1–3) | 0.253 |
| 4C mortality score | 12 (9–15) | 11 (7–14) | 0.092 |
| MuLBSTA score | 12 (9–15) | 7 (4–9) | 0.014 |
| Non-ICU Admitted/ Discharged Alive n = 290 | ICU Admitted/ Non-Survivors n = 118 | p | |
|---|---|---|---|
| Age, years | 70 (17) | 78 (13) | <0.001 |
| Male gender, % | 50 | 57 | 0.182 |
| BMI, kg/m2 | 26.8 (4.5) | 25.7 (4.1) | 0.038 |
| Current smoking, % | 17 | 15 | 0.719 |
| Hypertension, % | 60 | 66 | 0.248 |
| Type 2 diabetes, % | 16 | 28 | 0.004 |
| CKD, % | 9 | 19 | 0.004 |
| Previous CV event, % | 14 | 23 | 0.022 |
| Active cancer, % | 9 | 11 | 0.506 |
| Previous VTE, % | 3 | 3 | 0.988 |
| AF, % | 13 | 20 | 0.094 |
| COPD, % | 12 | 11 | 0.786 |
| ACE inhibitors, % | 28 | 26 | 0.808 |
| ARBs, % | 14 | 18 | 0.382 |
| Statins, % | 18 | 19 | 0.809 |
| DOACs, % | 9 | 11 | 0.581 |
| VKAs, % | 2 | 3 | 0.293 |
| LMWH, % | 17 | 24 | 0.082 |
| Anti-platelets, % | 21 | 30 | 0.015 |
| BBs, % | 28 | 32 | 0.361 |
| CCBs, % | 18 | 28 | 0.026 |
| Diuretics, % | 32 | 40 | 0.119 |
| Insulin, % | 9 | 18 | 0.007 |
| Oral hypoglycemic agents, % | 9 | 14 | 0.157 |
| SBP, mmHg | 132 (20) | 128 (22) | 0.053 |
| DBP, mmHg | 78 (11) | 74 (12) | 0.004 |
| Leukocytes, X 103/μL | 7.0 (4.9–10.2) | 7.7 (5.8–11.1) | 0.030 |
| Platelets, X 103/μL | 208 (159–268) | 192 (144–254) | 0.135 |
| D-dimer, ng/mL | 757 (532–1538) | 1211 (529–2807) | 0.015 |
| hs-cTn, ng/L | 10.2 (5.6–23) | 24.1 (11.4–44.6) | <0.001 |
| CRP, mg/dL | 5.7 (2.4–10.0) | 9.1 (4.7–15.0) | <0.001 |
| eGFR, mL/min | 76 (26) | 58 (27) | <0.001 |
| LDH, UI/L | 268 (216–368) | 361 (263–466) | <0.001 |
| PaO2/FiO2 | 271 (213–319) | 159 (114–257) | <0.001 |
| CURB-65 score | 2 (1–2) | 2 (1–3) | <0.001 |
| 4C mortality score | 11 (7–13) | 14 (12–16) | <0.001 |
| MuLBSTA score | 7 (4–9) | 9 (6–13) | <0.001 |
| bFMD, % | 4.7 (3.0–7.4) | 3.6 (2.0–5.2) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Schiaroli, E.; Cosentini, E.; Batori, G.; Marini, E.; Sahebkar, A.; Grignani, F.; Gidari, A.; et al. Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19. J. Clin. Med. 2021, 10, 5456. https://doi.org/10.3390/jcm10225456
Bianconi V, Mannarino MR, Figorilli F, Schiaroli E, Cosentini E, Batori G, Marini E, Sahebkar A, Grignani F, Gidari A, et al. Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19. Journal of Clinical Medicine. 2021; 10(22):5456. https://doi.org/10.3390/jcm10225456
Chicago/Turabian StyleBianconi, Vanessa, Massimo Raffaele Mannarino, Filippo Figorilli, Elisabetta Schiaroli, Elena Cosentini, Giuseppe Batori, Ettore Marini, Amirhossein Sahebkar, Francesco Grignani, Anna Gidari, and et al. 2021. "Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19" Journal of Clinical Medicine 10, no. 22: 5456. https://doi.org/10.3390/jcm10225456
APA StyleBianconi, V., Mannarino, M. R., Figorilli, F., Schiaroli, E., Cosentini, E., Batori, G., Marini, E., Sahebkar, A., Grignani, F., Gidari, A., Francisci, D., & Pirro, M. (2021). Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19. Journal of Clinical Medicine, 10(22), 5456. https://doi.org/10.3390/jcm10225456








