Multi-Parametric Diagnostic Approach and Potential Markers of Early Onset Subclinical Cardiovascular Disease in a Cohort of Children, Adolescents and Young Adults Vertically Infected with HIV on cART
Abstract
:1. Introduction
2. Method
- Left ventricle and left atrium-aorta M-mode examination [11];
- Tricuspid Annular Plane Systolic Excursion (TAPSE) M-mode examination [12];
- Left ventricle volume and ejection fraction measured through biplane Simpson’s method [13];
- Left atrium volume through biplane area/length method [13];
- Right ventricle fractional area charge [14];
- Trans-mitral and trans-tricuspid Doppler to assess E, A, E/A and deceleration time (DT) [15];
- Tissue Doppler to assess E′ values taken from lateral mitral annulus, right ventricle septum and free wall [15];
- Epicardial adipose tissue (EAT) was measured during end-systole from the parasternal long axis view, slightly off axis [16];
- 3D full volumes through a four beats acquisition, obtained online [17];
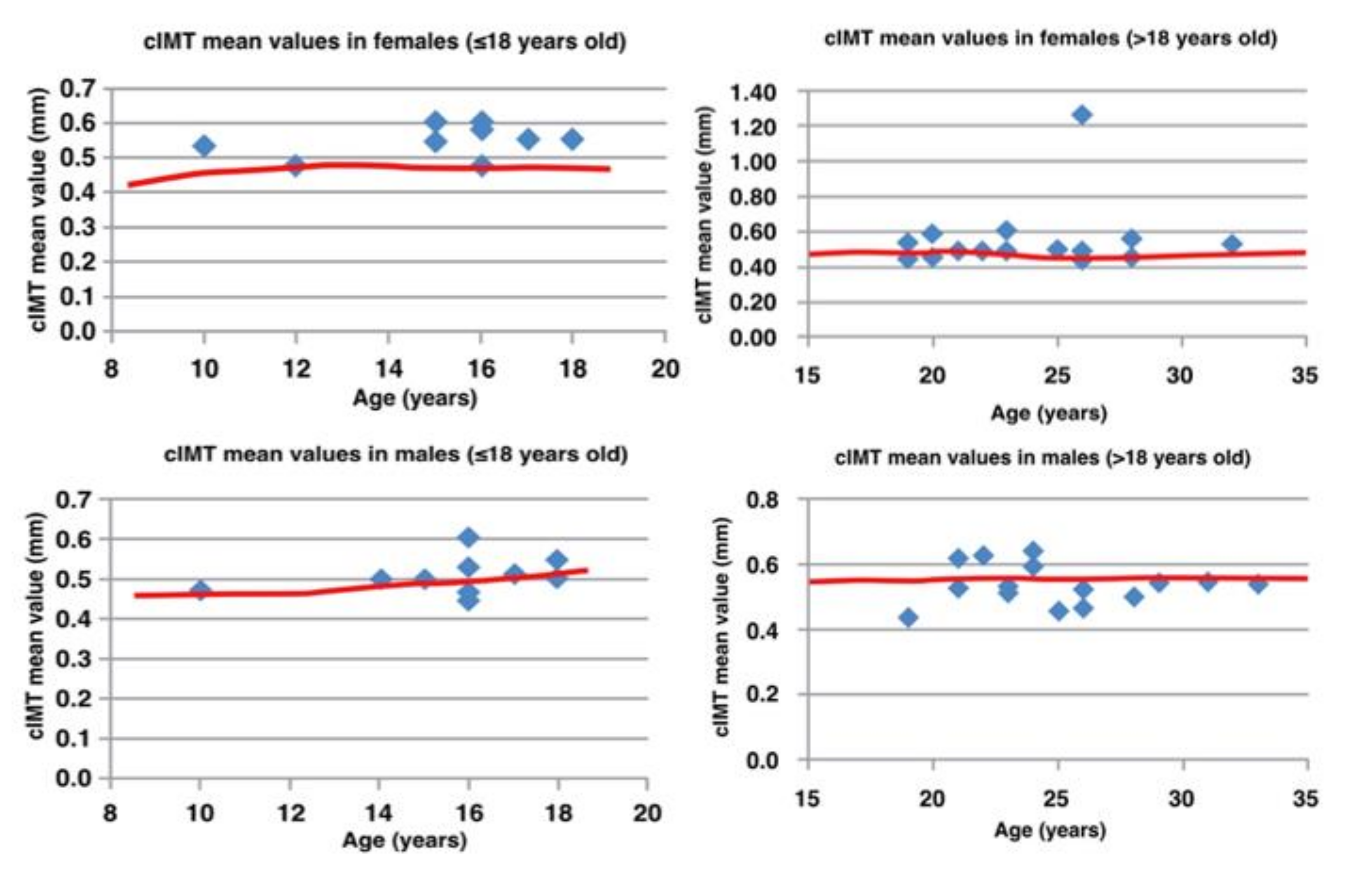
- Bilateral carotid IMT measurement, which is traditionally used as an early marker of cardiovascular pathology, was measured from the posterior right common carotid wall, once the machine recorded the mean value on a 15 mm long segment [18]. Based on the age of our population, cut-off values (<95th centile) were between 0.47 and 0.51 mm in males and 0.44 and 0.48 mm in females [ref];
- Flow-mediated dilatation, as a marker of endothelial dysfunction, was calculated after a 4 min ischemic period from brachial artery [19]. The cut-off used for peak diameter change was 9%;
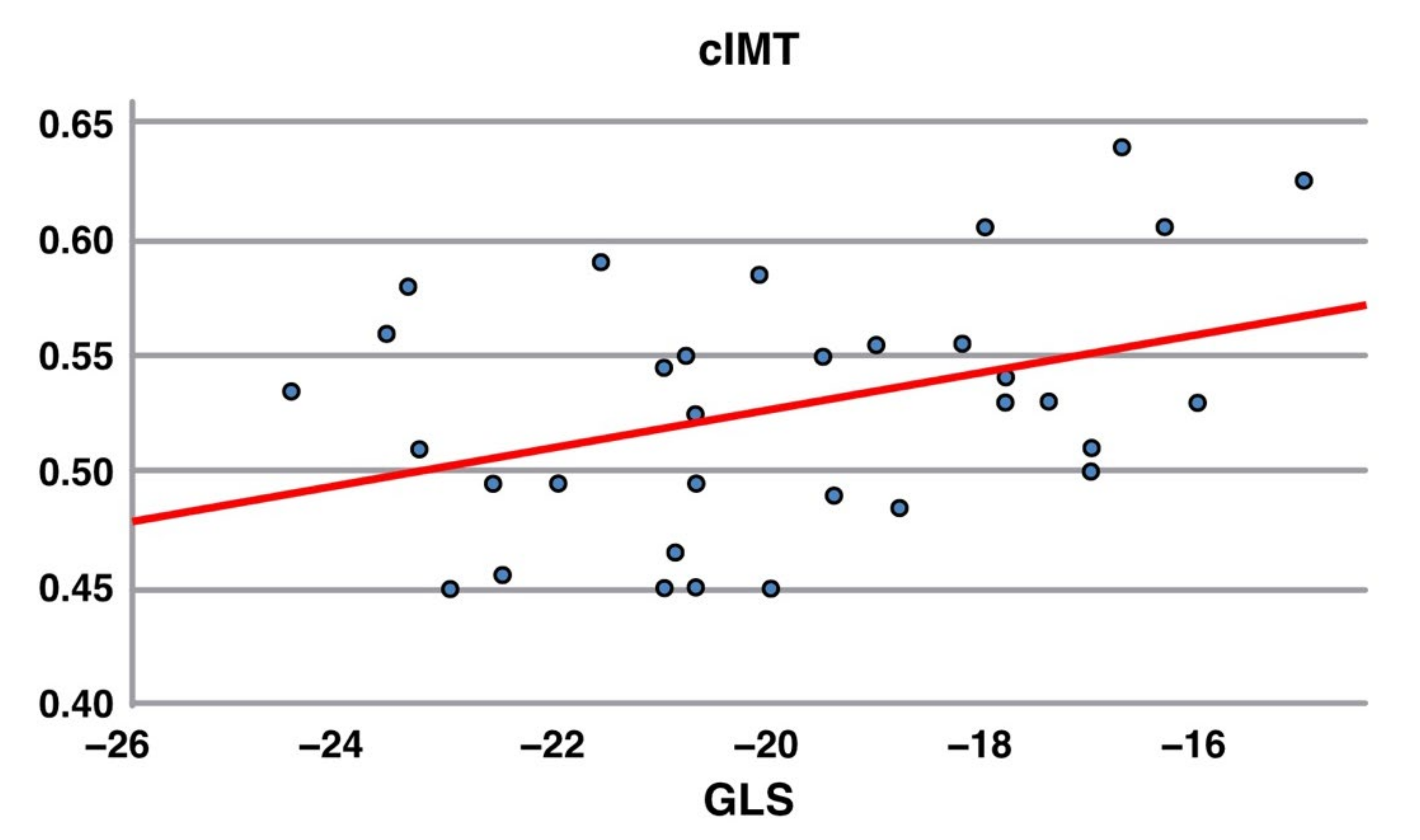
- Global longitudinal strain is a very sensible and specific marker of subclinical myocardial dysfunction and was calculated after speckle tracking echocardiography with AFI software [12]. Based on the published age-related lower GLS normal limit, the cut-off used was −19.0% [ref x2].
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiappini, E.; Bianconi, M.; Dalzini, A.; Petrara, M.R.; Galli, L.; Giaquinto, C.; De Rossi, A. Accelerated aging in perinatally HIV-infected children: Clinical manifestations and pathogenetic mechanisms. Aging 2018, 10, 3610–3625. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Williams, P.L.; Wilkinson, J.D.; Leister, M.E.C.; Van Dyke, R.B.; Shearer, W.T.; Rich, K.C.; Hazra, R.; Kaltman, J.R.; Jacobson, D.L.; et al. Cardiac status of HIV-infected children treated with long-term combination antiretroviral therapy: Results from the adolescent master protocol of the NIH multicenter pediatric HIV/AIDS cohort study. JAMA Pediatr. 2013, 6, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keesler, M.J.; Fisher, S.D.; Lipshultz, S.E. Cardiac manifestations of HIV infection in infants and children. Ann. N. Y. Acad. Sci. 2001, 946, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Easley, K.A.; Orav, E.J.; Kaplan, S.; Starc, T.J.; Bricker, J.T.; Lai, W.W.; Moodie, D.S.; McIntosh, K.; Schluchter, M.D.; et al. Left ventricular structure and function in children infected with human immunodeficiency virus: The prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV). Circulation 1998, 97, 1246–1256. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, S.E.; Easley, K.A.; Orav, E.J.; Kaplan, S.; Starc, T.J.; Bricker, J.T.; Lai, W.W.; Moodie, D.S.; Sopko, G.; Colan, S.D.; et al. Cardiac dysfunction and mortality in HIV-infected children: The Prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV). Circulation 2000, 102, 1542–1548. [Google Scholar] [CrossRef] [Green Version]
- Sims, A.; Frank, L.; Cross, R.; Clauss, S.; Dimock, D.; Purdy, J.; Mikhail, I.; Hazra, R.; Hadigan, C.; Sable, C. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J. Am. Soc. Echocardiogr. 2012, 25, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Sims, A.; Hadigan, C. Cardiovascular complications in children with HIV infection. Curr. HIV/AIDS Rep. 2011, 8, 209–214. [Google Scholar] [CrossRef]
- Sainz, T.; Álvarez-Fuente, M.; Fernández-Jiménez, R.; González-Tomé, M.I.; de José, M.I.; Ramos, J.T.; Navarro, M.L.; Martínez, J.; García-Hortelano, M.; Medrano, C.; et al. Cardiac function in vertically HIV-infected children and adolescents in the era of highly active antiretroviral therapy. Pediatr. Infect. Dis. J. 2015, 34, e125–e131. [Google Scholar] [CrossRef]
- Williams, P.L.; Correia, K.; Karalius, B.; Van Dyke, R.B.; Wilkinson, J.D.; Shearer, W.T.; Colan, S.D.; Lipshultz, S.E.; Study, A.C. Cardiac status of perinatally HIV-infected children: Assessing combination antiretroviral regimens in observational studies. AIDS 2018, 32, 2337–2346. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Gilman, G.; Nelson, T.A.; Hansen, W.H.; Khandheria, B.K.; Ommen, S.R. Diastolic function: A sonographer’s approach to the essential echocardiographic measurements of left ventricular diastolic function. J. Am. Soc. Echocardiogr. 2007, 20, 199–209. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1–46. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Urbina, E.M.; Williams, R.V.; Alpert, B.S.; Collins, R.T.; Daniels, S.R.; Hayman, L.; Jacobson, M.; Mahoney, L.; Mietus-Snyder, M.; Rocchini, A.; et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research: A scientific statement from the American Heart Association. Hypertension 2009, 54, 919–950. [Google Scholar] [CrossRef]
- Marsico, F.; Vecchio, A.L.; Paolillo, S.; D’Andrea, C.; De Lucia, V.; Bruzzese, E.; Vallone, G.; Dellegrottaglie, S.; Marciano, C.; Trimarco, B. Left Ventricular Function, Epicardial Adipose Tissue, and Carotid Intima-Media Thickness in Children and Adolescents With Vertical HIV Infection. J. Acquir. Immune Defic. Syndr. 2019, 82, 462–467. [Google Scholar] [CrossRef]
- Giaquinto, C.; Penazzato, M.; Rosso, R.; Bernardi, S.; Rampon, O.; Nasta, P.; Ammassari, A.; Antinori, A.; Badolato, R.; Gattinara, G.C.; et al. Italian consensus statement on paediatric HIV infection. Infection 2010, 38, 301–319. [Google Scholar] [CrossRef]
- Penazzato, M.; Donà, D.; Wool, P.S.; Rampon, O.; Giaquinto, C. Update on antiretroviral therapy in paediatrics. Antiviral Res. 2010, 85, 266–275. [Google Scholar] [CrossRef]
- Nelson, M.R.; Mookadam, F.; Thota, V.; Emani, U.; Al Harthi, M.; Lester, S.J.; Cha, S.; Stepanek, J.; Hurst, R.T. Epicardial fat: An additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J. Am. Soc. Echocardiogr. 2011, 24, 339–345. [Google Scholar] [CrossRef]
- McComsey, G.A.; O’Riordan, M.; Hazen, S.L.; El-Bejjani, D.; Bhatt, S.; Brennan, M.L.; Storer, N.; Adell, J.; Nakamoto, D.A.; Dogra, V. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS 2007, 21, 921–927. [Google Scholar] [CrossRef]
- Sainz, T.; Álvarez-Fuente, M.; Navarro, M.L.; Díaz, L.; Rojo, P.; Blázquez, D.; de José, M.I.; Ramos, J.T.; Serrano-Villar, S.; Martínez, J.; et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: The CaroVIH Study. J. Acquir. Immune Defic. Syndr. 2014, 65, 4249. [Google Scholar] [CrossRef]
- Cincin, A.; Ozben, B.; Tigen, E.T.; Sunbul, M.; Sayar, N.; Gurel, E.; Tigen, K.; Korten, V. Ventricular and atrial functions assessed by speckle-tracking echocardiography in patients with human immunodeficiency virus. J. Clin. Ultrasound 2020, 49, 341–350. [Google Scholar] [CrossRef]
- Rose, K.A.M.; Vera, J.H.; Drivas, P.; Banya, W.; Keenan, N.; Pennell, D.; Winston, A. Atherosclerosis is Evident in Treated HIV-Infected Subjects With Low Cardiovascular Risk by Carotid Cardiovascular Magnetic Resonance. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 71, 514–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetkens, J.A.; Doerner, J.; Schwarze-Zander, C.; Wasmuth, J.-C.; Boesecke, C.; Sprinkart, A.M.; Schmeel, F.C.; Homsi, R.; Gieseke, J.; Schild, H.H.; et al. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ. Cardiovasc. Imaging 2016, 9, e004091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echecopar-Sabogal, J.; D’Angelo-Piaggio, L.; Chanamé-Baca, D.M.; Ugarte-Gil, C. Association between the use of protease inhibitors in highly active antiretroviral therapy and incidence of diabetes mellitus and/or metabolic syndrome in HIV-infected patients: A systematic review and meta-analysis. Int. J. STD AIDS 2017, 29, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Grant, Y.T.; Almeida, D.N.; Sharma, T.; Lipshultz, S.E. Cardiometabolic disease in human immunodeficiency virus-infected children. J. CardioMetab. Syndr. 2008, 3, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.-L.; Lee, Y.-T.; Sheu, G.-T. Metabolic Syndrome Prevalence and Cardiovascular Risk Assessment in HIV-Positive Men with and without Antiretroviral Therapy. Medicina 2021, 57, 578. [Google Scholar] [CrossRef]
- Karavidas, A.; Xylomenos, G.; Matzaraki, V.; Papoutsidakis, N.; Leventopoulos, G.; Farmakis, D.; Lazaros, G.; Perpinia, A.; Arapi, S.; Paisios, N.; et al. Myocardial deformation imaging unmasks subtle left ventricular systolic dysfunction in asymptomatic and treatment-naïve HIV patients. Clin. Res. Cardiol. 2015, 104, 975–981. [Google Scholar] [CrossRef]
- Holder, S.M.; Bruno, R.M.; Shkredova, D.A.; Dawson, E.A.; Jones, H.; Hopkins, N.D.; Hopman, M.T.; Bailey, T.G.; Coombes, J.S.; Askew, C.D.; et al. Reference Intervals for Brachial Artery Flow-Mediated Dilation and the Relation With Cardiovascular Risk Factors. Hypertension 2021, 77, 1469–1480. [Google Scholar] [CrossRef]
- Rodriguez, D.; Coll, M.; Guerrero, R.; Henao, L. Flow mediated vasodilation in overweight children. Rev. Chil. Pediatr. 2015, 86, 410–414. [Google Scholar]
- Kaplan, R.C.; Kingsley, L.; Gange, S.; Benning, L.; Jacobson, L.P.; Lazar, J.; Anastos, K.; Tien, P.C.; Sharrett, A.R.; Hodis, H.N. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008, 22, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Bernal, E.; Serrano, J.; Perez, A.; Valero, S.; Garcia, E.; Marín, I.; Muñoz, A.; Gómez-Verdú, J.M.; Vera, C.; Cano, A. The CD4:CD8 ratio is associated with IMT progression in HIV-infected patients on antiretroviral treatment. J. Int. AIDS Soc. 2014, 17, 19723. [Google Scholar] [CrossRef]

| n (%) | Mean (SD) | |
|---|---|---|
| Male | 26 (50%) | - |
| Age at examination (years) | - | 20.96 (5.69) |
| Ethnicity # | - | |
| E. Europe | 4 (7.69%) | |
| W. Europe | 33 (63.46%) | |
| Africa | 15 (28.85%) | |
| Other | 0 (0%) | |
| Current smoker | 21 (40.38%) | - |
| Age ART initiated # | - | |
| <5 years old | 16 (37.2%) | |
| ≥5 years old | 27 (62.8%) | |
| Therapy duration (years) | - | 13.89 (5.16) |
| PI therapy # | 37 (71.15%) | - |
| Abacavir therapy # | 21 (40.38%) | - |
| Kaletra therapy # | 7 (13.46%) | - |
| NNRTI therapy # | 14 (26.92%) | - |
| Integrase therapy # | 8 (15.38%) | - |
| HIV RNA #/mL | - | 0.29 (0.46) |
| CD4 nadir #/mm3 | - | 223.11 (229.51) |
| CD4 count #/mm3 | - | 654.33 (273.52) |
| CD4 % (%) | - | 31.21 (10.35) |
| CD8 count #/mm3 | - | 774.88 (332.46) |
| CD8 % (%) | - | 37.08 (12.52) |
| CD4/CD8 ratio | - | 0.95 (0.47) |
| HDL cholesterol mmol/L | - | 1.43 (0.40) |
| LDL cholesterol mmol/L | - | 3.07 (0.95) |
| Total cholesterol mmol/L | - | 4.38 (0.96) |
| Triglycerides mmol/L | - | 1.25 (0.84) |
| Glycemia mmol/L | - | 4.71 (1.09) |
| Mean blood pressure mmHg | - | 90.51 (9.34) |
| BMI (kg/m2) | - | 21.94 (3.97) |
| Lipodystrophy # | 12 (23.08%) | - |
| Measurements | Reference Values | Mean Value (SD) | Prevalence of Alteration (%) |
|---|---|---|---|
| c IMT |
| 0.54 (0.12) | 38 (73%) |
| GLS | <−19.0% | −20.04 (2.52) | 15 (29%) |
| EF (2D) | ≥55% | 66.04 (7.37) | 3 (5%) |
| EF (3D) | ≥53% | 65.59 (4.88) | 0% |
| E/E′ ratio | <8% | 7.15 (1.25) | 4 (8%) |
| FMD | >9% | 9.4 (4.7) | 22 (45%) |
| Total | Normal cIMT (n = 30) | Elevated cIMT (n = 22) | p Value | |
|---|---|---|---|---|
| Male, n (%) | 26 (50%) | 14 (46.67%) | 12 (54.55%) | 0.575 |
| Age at examination (y) | 20.96 (5.69) | 24.1 (4.63) | 16.68 (3.98) | <0.001 |
| Ethnicity, # | <0.05 | |||
| E. Europe | 4 (7.69%) | 4 (13.33%) | 0 (0.00%) | |
| W. Europe | 33 (63.46%) | 25 (83.33%) | 8 (36.36%) | |
| Africa | 15 (28.85%) | 1 (3.33%) | 14 (63.64%) | |
| other | 0 (0%) | - | - | |
| Current smoker, | 21 (40.38%) | 15 (50.00%) | 6 (27.27%) | 0.146 |
| Age ART initiated, # | 0.04 | |||
| <5 years old | 19 (36.54%) | 7 (23.33%) | 12 (54.55%) | |
| ≥5 years old | 33 (63.46%) | 23 (76.67%) | 10 (45.45%) | |
| Therapy duration (y) | 13.89 (5.23) | 16.44 (3.60) | 10.67 (5.26) | <0.001 |
| PI therapy # | 37 (71.15%) | 22 (73.33%) | 15 (68.18%) | 0.685 |
| Abacavir therapy # | 21 (40.38%) | 9 (30.00%) | 12 (54.55%) | 0.075 |
| Kaletra therapy # | 7 (13.46%) | 3 (10.00%) | 4 (18.18%) | 0.393 |
| NNRTI therapy # | 14 (26.92%) | 8 (26.67%) | 6 (27.27%) | 0.961 |
| Integrase therapy # | 8 (15.38%) | 5 (16.67%) | 3 (13.64%) | 0.765 |
| HIV RNA copies/mL | 0.29 (0.46) | 0.34 (0.48) | 0.23 (0.43) | 0.366 |
| CD4 nadir #/mm3 | 223.11 (229.51) | 141.00 (150.16) | 409.72 (274.02) | <0.05 |
| CD4 count #/mm3 | 654.33 (273.52) | 635.70 (296.13) | 679.73 (243.77) | 0.345 |
| CD4 % | 31.21 (10.35) | 31.27 (11.66) | 31.14 (8.51) | 0.585 |
| CD8 #/mm3 | 774.88 (332.46) | 804.00 (398.65) | 735.16 (214.72) | 0.875 |
| CD8 % | 37.08 (12.52) | 38.79 (14.68) | 34.75 (8.57) | 0.560 |
| CD4/CD8 ratio | 0.95 (0.47) | 0.96 (0.54) | 0.941 (0.358) | 0.802 |
| HDL cholesterol mmol/L | 1.43 (0.40) | 1.22 (0.34) | 1.63 (0.35) | 0.022 |
| LDL cholesterol mmol/L | 3.07 (0.95) | 3.44 (1.07) | 2.73 (0.72) | 0.105 |
| Total cholesterol mmol/L | 4.38 (0.96) | 4.37 (1.01) | 4.40 (0.91) | 0.875 |
| Triglicerides mmol/L | 1.25 (0.84) | 1.28 (0.77) | 1.20 (0.94) | 0.225 |
| Glycemia mmol/L | 4.71 (1.09) | 4.60 (0.47) | 4.85 (1.59) | 0.402 |
| Mean blood pressure mmHg | 90.51 (9.34) | 92.59 (9.03) | 87.27 (9.12) | 0.079 |
| BMI kg/m2 | 21.94 (3.97) | 22.40 (4.09) | 21.30 (3.81) | 0.313 |
| Lipodystrophy # | 12 (23.08%) | 10 (33.33%) | 2 (9.09%) | 0.040 |
| cIMT mm | 0.54 (0.12) | 0.51 (0.05) | 0.58 (0.16) | 0.014 |
| GLS % | −20.04 (2.52) | −20.33 (2.23) | −19.68 (2.85) | 0.558 |
| EF (2d) % | 66.04 (7.37) | 66.74 (6.43) | 65.11 (8.53) | 0.955 |
| EF (3d) % | 65.59 (4.88) | 65.38 (4.67) | 65.84 (5.29) | 0.594 |
| E/E′ ratio | 7.15 (11.25) | 5.52 (1.09) | 9.30 (17.07) | 0.403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldi, B.; Lanzoni, G.; Rampon, O.; Donà, D.; Di Candia, A.; Milanesi, O.; Giaquinto, C.; Di Salvo, G. Multi-Parametric Diagnostic Approach and Potential Markers of Early Onset Subclinical Cardiovascular Disease in a Cohort of Children, Adolescents and Young Adults Vertically Infected with HIV on cART. J. Clin. Med. 2021, 10, 5455. https://doi.org/10.3390/jcm10225455
Castaldi B, Lanzoni G, Rampon O, Donà D, Di Candia A, Milanesi O, Giaquinto C, Di Salvo G. Multi-Parametric Diagnostic Approach and Potential Markers of Early Onset Subclinical Cardiovascular Disease in a Cohort of Children, Adolescents and Young Adults Vertically Infected with HIV on cART. Journal of Clinical Medicine. 2021; 10(22):5455. https://doi.org/10.3390/jcm10225455
Chicago/Turabian StyleCastaldi, Biagio, Gloria Lanzoni, Osvalda Rampon, Daniele Donà, Angela Di Candia, Ornella Milanesi, Carlo Giaquinto, and Giovanni Di Salvo. 2021. "Multi-Parametric Diagnostic Approach and Potential Markers of Early Onset Subclinical Cardiovascular Disease in a Cohort of Children, Adolescents and Young Adults Vertically Infected with HIV on cART" Journal of Clinical Medicine 10, no. 22: 5455. https://doi.org/10.3390/jcm10225455
APA StyleCastaldi, B., Lanzoni, G., Rampon, O., Donà, D., Di Candia, A., Milanesi, O., Giaquinto, C., & Di Salvo, G. (2021). Multi-Parametric Diagnostic Approach and Potential Markers of Early Onset Subclinical Cardiovascular Disease in a Cohort of Children, Adolescents and Young Adults Vertically Infected with HIV on cART. Journal of Clinical Medicine, 10(22), 5455. https://doi.org/10.3390/jcm10225455







