Correlation between Preoperative Serum Levels of Calcium, Phosphate, and Intact Parathyroid Hormone and Radiological Outcomes in Spinal Interbody Fusion among End-Stage Renal Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Methods
2.3. Laboratory Profiles and Radiographic Assessment
2.4. Statistical Analyses
3. Results
3.1. Patient Population Demographics
3.2. The Relationship between Radiographic Outcomes and Laboratory Data
3.2.1. Interbody Fusion Grade
3.2.2. Cage Subsidence
3.2.3. Implants Loosening
3.2.4. ASD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-C.; Hsu, C.-Y.; Kao, C.; Chen, T.; Chen, H.-H.; Hsu, C.-C.; Wu, M.-S. Incidence and Prevalence of ESRD in Taiwan Renal Registry Data System (TWRDS): 2005–2012. Acta Nephrol. 2014, 28, 65–68. [Google Scholar]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Eric Nyam, T.T.; Lim, S.W.; Ho, C.H.; Liao, J.C.; Wang, J.J.; Chio, C.C.; Kuo, J.R.; Wang, C.C. In-Hospital Mortality after Spinal Surgery in Hemodialysis Patients: An 11-Year Population-Based Study. World Neurosurg. 2019, 122, e667–e675. [Google Scholar] [CrossRef]
- Chikawa, T.; Sakai, T.; Bhatia, N.N.; Miyagi, R.; Sairyo, K.; Goda, Y.; Nakamura, M.; Nakano, S.; Shimakawa, T.; Minato, A. Clinical outcomes of spinal surgery in patients treated with hemodialysis. J. Spinal. Disord. Tech. 2013, 26, 321–324. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef] [Green Version]
- Maruo, K.; Moriyama, T.; Tachibana, T.; Inoue, S.; Arizumi, F.; Kusuyama, K.; Yoshiya, S. Prognosis and adjacent segment disease after lumbar spinal fusion surgery for destructive spondyloarthropathy in long-term hemodialysis patients. J. Orthop. Sci. 2017, 22, 248–253. [Google Scholar] [CrossRef] [PubMed]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [CrossRef]
- Garg, B.; Mehta, N. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): A review of indications, technique, results and complications. J. Clin. Orthop. Trauma 2019, 10, S156–S162. [Google Scholar] [CrossRef] [PubMed]
- Ito, Z.; Matsuyama, Y.; Sakai, Y.; Imagama, S.; Wakao, N.; Ando, K.; Hirano, K.; Tauchi, R.; Muramoto, A.; Matsui, H.; et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine 2010, 35, E1101–E1105. [Google Scholar] [CrossRef]
- Dakhil-Jerew, F.; Jadeja, H.; Cohen, A.; Shepperd, J.A. Inter-observer reliability of detecting Dynesys pedicle screw using plain X-rays: A study on 50 post-operative patients. Eur. Spine J. 2009, 18, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Uei, H. Clinical course and significance of the clear zone around the pedicle screws in the lumbar degenerative disease. Spine 2008, 33, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Choi, B.W.; Ham, D.H.; Kim, H.J. Prognosis of Hardware-Related Problems in Anterior Cervical Discectomy and Fusion with Cage and Plate Constructs. World Neurosurg. 2020, 134, e249–e255. [Google Scholar] [CrossRef]
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. Sci. World J. 2012, 2012, 456346. [Google Scholar] [CrossRef]
- Fukushima, M.; Oshima, Y.; Yuzawa, Y.; Tanaka, S.; Inanami, H. Clinical and radiographic analysis of unilateral versus bilateral instrumented one-level lateral lumbar interbody fusion. Sci. Rep. 2020, 10, 3105. [Google Scholar] [CrossRef] [Green Version]
- Cheh, G.; Bridwell, K.H.; Lenke, L.G.; Buchowski, J.M.; Daubs, M.D.; Kim, Y.; Baldus, C. Adjacent Segment Disease FollowingLumbar/Thoracolumbar Fusion With Pedicle Screw Instrumentation: A Minimum 5-Year Follow-up. Spine 2007, 32, 2253–2257. [Google Scholar] [CrossRef]
- Basques, B.A.; Louie, P.K.; Mormol, J.; Khan, J.M.; Movassaghi, K.; Paul, J.C.; Varthi, A.; Goldberg, E.J.; An, H.S. Multi- versus single-level anterior cervical discectomy and fusion: Comparing sagittal alignment, early adjacent segment degeneration, and clinical outcomes. Eur. Spine J. 2018, 27, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Paik, H.K.; Chin, D.K.; Kim, S.H.; Kim, D.W.; Ku, M.G. The Fate of Adjacent Segments After Anterior Cervical Discectomy and Fusion: The Influence of an Anterior Plate System. World Neurosurg. 2016, 89, 42–50. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.N.; Liao, J.C.; Chen, W.J. Instrumented Posterolateral fusion versus instrumented Interbody fusion for degenerative lumbar diseases in uremic patients under hemodialysis. BMC Musculoskelet. Disord. 2020, 21, 815. [Google Scholar] [CrossRef]
- Han, I.H.; Kim, K.S.; Park, H.C.; Chin, D.K.; Jin, B.H.; Yoon, Y.S.; Ahn, J.Y.; Cho, Y.E.; Kuh, S.U. Spinal surgery in patients with end-stage renal disease undergoing hemodialysis therapy. Spine 2009, 34, 1990–1994. [Google Scholar] [CrossRef]
- Kanaya, K.; Kato, Y.; Murata, Y.; Wada, H.; Wada, K.; Shimamoto, S.; Shiba, M.; Hatta, S. Low parathyroid hormone levels in patients who underwent/would undergo hemodialysis result in bone graft failure after posterolateral fusion. Spine 2014, 39, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.T.; Shinde, A.; Tan, K.G. Hip hemiarthroplasty for femoral neck fractures in end-stage renal disease patients on dialysis compared to patients with late-stage chronic kidney disease. Singap. Med. J. 2019, 60, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Covic, A.; Rastogi, A. Hyperphosphatemia in patients with ESRD: Assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol. 2013, 14, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131. [Google Scholar] [CrossRef] [Green Version]
- Nitta, K.; Yajima, A.; Tsuchiya, K. Management of Osteoporosis in Chronic Kidney Disease. Intern. Med. 2017, 56, 3271–3276. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Shi, J.; Wu, J.; Cheng, Y.; Peng, K.; Chen, J.; Jiang, H. Pedicle screw loosening: The value of radiological imagings and the identification of risk factors assessed by extraction torque during screw removal surgery. J. Orthop. Surg. Res. 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Marie-Hardy, L.; Pascal-Moussellard, H.; Barnaba, A.; Bonaccorsi, R.; Scemama, C. Screw Loosening in Posterior Spine Fusion: Prevalence and Risk Factors. Glob. Spine J. 2020, 10, 598–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opsenak, R.; Hanko, M.; Snopko, P.; Varga, K.; Kolarovszki, B. Subsidence of anchored cage after anterior cervical discectomy. Bratisl. Med. J. 2019, 120, 356–361. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, J.H.; Lee, J.H.; Lee, D.Y.; Shim, H.J. The Correlation Between Cage Subsidence, Bone Mineral Density, and Clinical Results in Posterior Lumbar Interbody Fusion. Clin. Spine Surg. 2017, 30, E683–E689. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular Calcification: The Killer of Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, M.; Dusso, A.S.; Slatopolsky, E. Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J. Am. Soc. Nephrol. 2001, 12, 2511–2516. [Google Scholar] [CrossRef]
- Block, G.A.; Port, F.K. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am. J. Kidney Dis. 2000, 35, 1226–1237. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, F.M.; Gou, Y.; Wang, P.; Zhang, H.; Song, H.P.; Shen, Y.; Zhang, Y.Z.; Zhang, L. Enhancement of Lumbar Fusion and Alleviation of Adjacent Segment Disc Degeneration by Intermittent PTH(1-34) in Ovariectomized Rats. J. Bone Miner. Res. 2016, 31, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Madiraju, P.; Gawri, R.; Wang, H.; Antoniou, J.; Mwale, F. Mechanism of parathyroid hormone-mediated suppression of calcification markers in human intervertebral disc cells. Eur. Cell Mater. 2013, 25, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Goss, B.G.; Thorpe, P.J.; Williams, R.P. CT-based classification of long spinal allograft fusion. Eur. Spine J. 2007, 16, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Suzuki, A.; Takahashi, S.; Yasuda, H.; Tada, M.; Sugioka, Y.; Okano, T.; Koike, T.; Nakamura, H. MRI Evaluation of Lumbar Endplate and Facet Erosion in Rheumatoid Arthritis. Clin. Spine Surg. 2014, 27, E128–E135. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Huang, W.C.; Tsai, H.W.; Ko, C.C.; Wu, C.L.; Tu, T.H.; Cheng, H. Pedicle screw loosening in dynamic stabilization: Incidence, risk, and outcome in 126 patients. Neurosurg. Focus 2011, 31, E9. [Google Scholar] [CrossRef] [Green Version]
- Son, H.J.; Choi, S.H.; Heo, D.R.; Kook, I.; Lee, M.K.; Ahn, H.S.; Kang, C.N. Outcomes of the use of cement-augmented cannulated pedicle screws in lumbar spinal fusion. Spine J. 2021, 21, 1857–1865. [Google Scholar] [CrossRef]
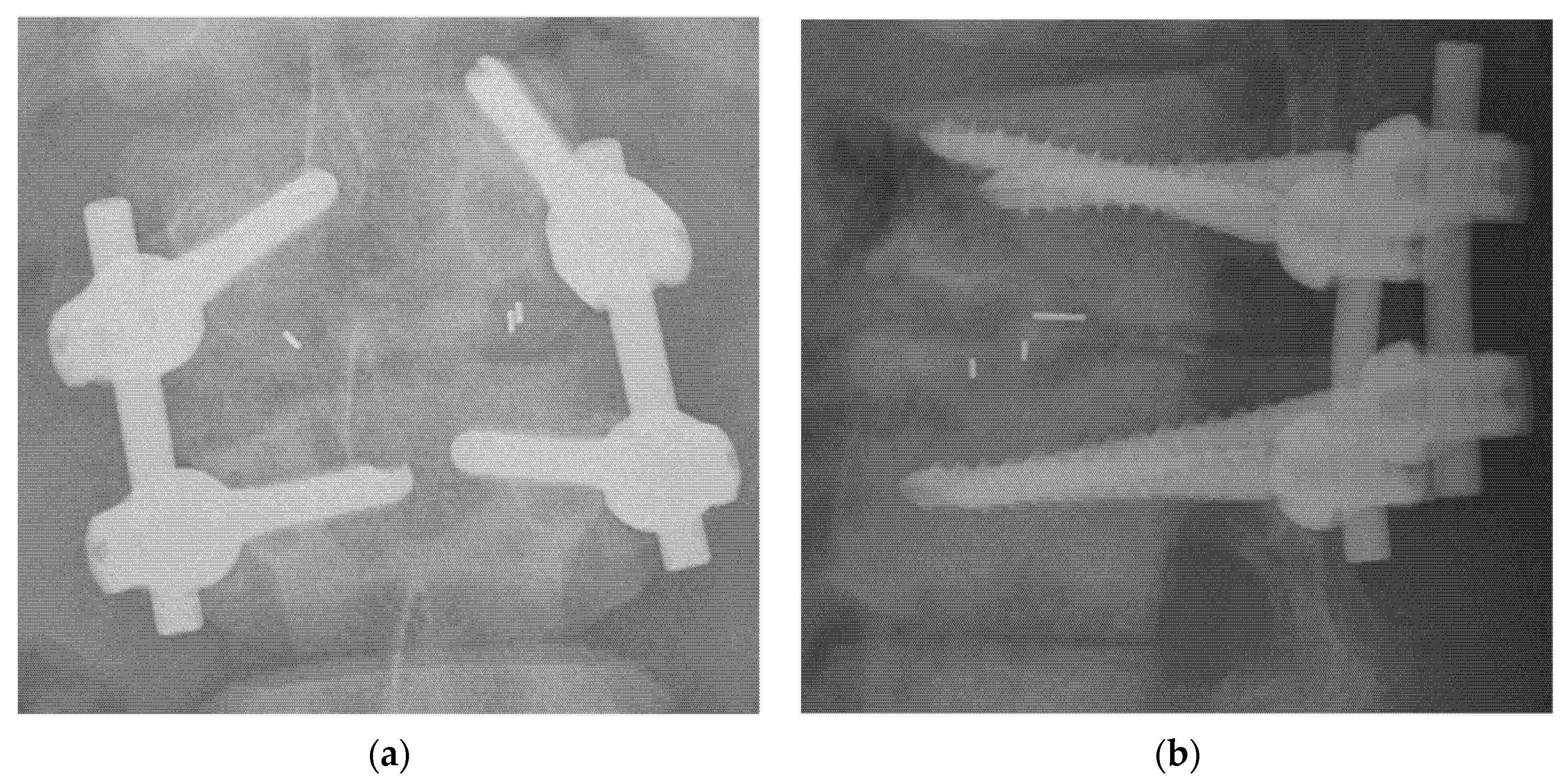
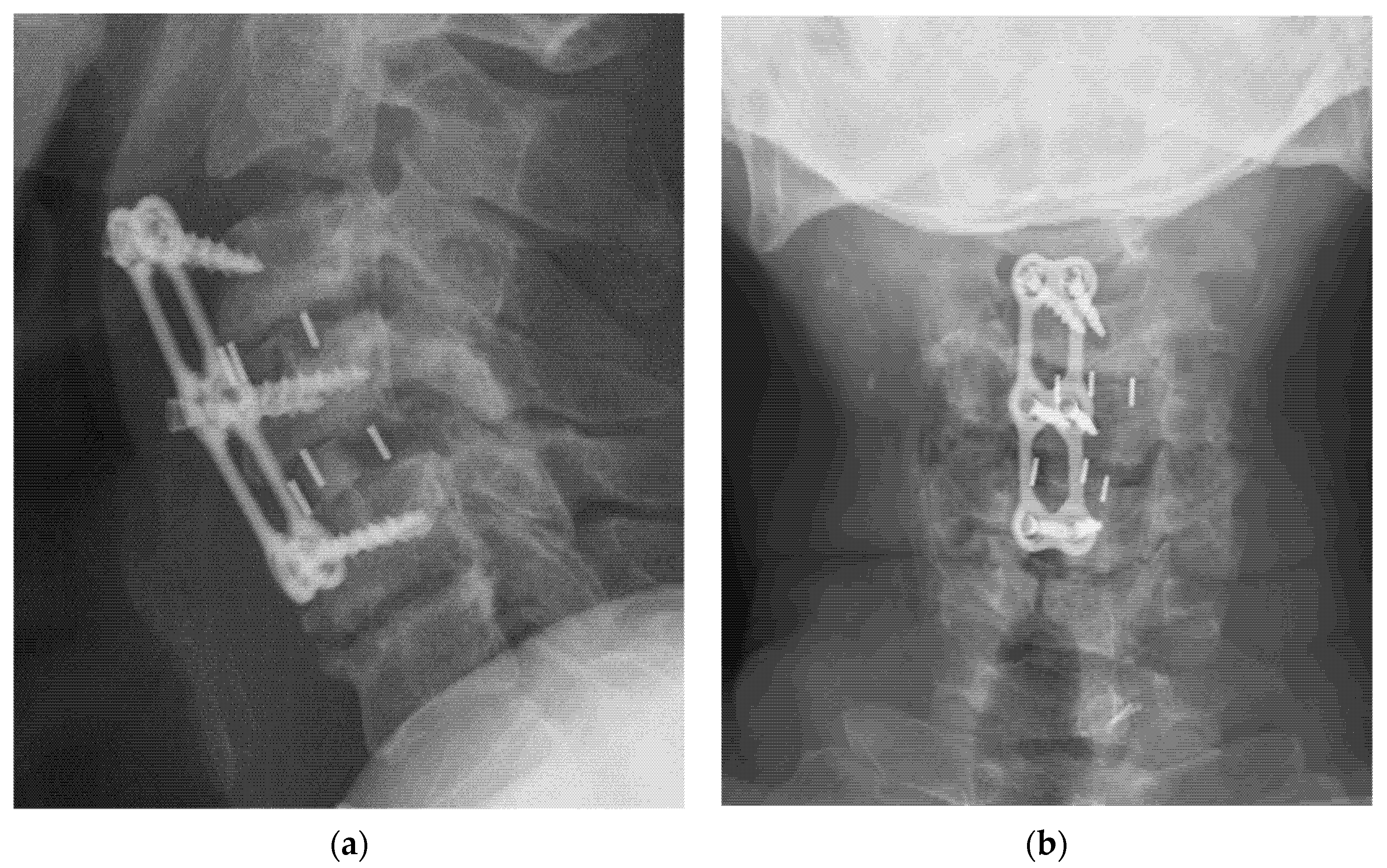
| TLIF (n = 36) | ACDF (n = 26) | p Value | |||
|---|---|---|---|---|---|
| Age (years) | 65.5 | ±9.2 | 63.5 | ±4.3 | 0.932 |
| Sex | 0.391 | ||||
| Female | 19 | (52.8%) | 10 | (38.5%) | |
| Male | 17 | (47.2%) | 16 | (61.5%) | |
| BMI (kg/m2) | 23.6 | ±2.2 | 22.9 | ±3.5 | 0.843 |
| Mortality | 8 | (22.2%) | 2 | (7.7%) | 0.171 |
| Comorbidity | |||||
| Hyperparathyroidism | 2 | 3 | 0.641 | ||
| Peptic ulcer | 2 | 4 | 0.227 | ||
| Hypertension | 17 | 16 | 0.391 | ||
| CVA | 1 | 0 | 1.000 | ||
| CAD | 2 | 4 | 0.194 | ||
| DM | 12 | 8 | 1.000 | ||
| Atrial fibrillation | 2 | 3 | 0.641 | ||
| Segment(s) | |||||
| 1 | 21 | (58.3%) | 9 | (34.6%) | |
| 2 | 13 | (36.1%) | 6 | (23.1%) | |
| 3 | 2 | (5.6%) | 9 | (34.6%) | |
| 5 | 0 | (0%) | 1 | (3.8%) | |
| Serum level | |||||
| Ca (mg/dL) | 9.3 | (8.3–10.1) | 9.8 | (8.7–10.4) | 0.169 |
| P (mg/dL) | 5.3 | (3.5–7.2) | 4.2 | (3.5–5.5) | 0.103 |
| Ca × P (mg2/dL2) | 44.6 | (29.9–65.5) | 42.63 | (31.7–49.4) | 0.391 |
| iPTH (pg/mL) | 206.8 | (64.6–714.6) | 333 | (51.6–760.0) | 0.944 |
| Ca | P | Ca × P | iPTH | |
|---|---|---|---|---|
| 6 months after operation | ||||
| TLIF | 0.018 | 0.103 | 0.099 | −0.225 |
| ACDF | −0.220 | −0.100 | −0.153 | 0.011 |
| 12 months after operation | ||||
| TLIF | 0.080 | 0.130 | 0.140 | 0.310 |
| ACDF | −0.014 | −0.158 | −0.106 | −0.321 |
| 24 months after operation | ||||
| TLIF | 0.091 | 0.375 | 0.375 | 0.384 |
| ACDF | 0.092 | 0.126 | −0.359 | −0.370 |
| Cage Subsidence (Grade) | ||
|---|---|---|
| TLIF (p-Value) | ACDF (p-Value) | |
| Ca | 0.191 (0.184) | 0.123 (0.385) |
| P | 0.360 * (0.021) | −0.196 (0.207) |
| Ca × P | 0.390 * (0.012) | −0.227 (0.154) |
| iPTH | 0.532 ** (0.001) | 0.071 (0.680) |
| Implant Loosening (+) | Implant Loosening (−) | p Value | |||
|---|---|---|---|---|---|
| TLIF | |||||
| Ca | 9.1 | (8.1–10.0) | 9.3 | (8.3–10.3) | 0.483 |
| P | 7.2 | (3.5–9.0) | 4.6 | (2.8–5.6) | 0.008 ** |
| Ca × P | 65.57 | (35.0–85.5) | 38.7 | (27.2–54.2) | 0.013 * |
| iPTH | 389.7 | (82.0–1279.0) | 121.0 | (64.0–274.0) | 0.157 |
| ACDF | |||||
| Ca | 10.4 | (7.9–10.8) | 9.2 | (8.6–10.0) | 0.296 |
| P | 4.0 | (3.3–5.1) | 4.9 | (3.8–6.0) | 0.146 |
| Ca × P | 42.6 | (32.2–46.4) | 42.6 | (35.0–61.4) | 0.484 |
| iPTH | 708.5 | (65.8–855.0) | 255.0 | (47.1–755.5) | 0.054 |
| ASD (+) | ASD (−) | pValue | |||
| TLIF | |||||
| Ca | 10.2 | (8.9–11.1) | 9.2 | (8.1–10.0) | 0.109 |
| P | 2.7 | (2.5–5.7) | 5.6 | (3.5–7.2) | 0.076 |
| Ca × P | 30.24 | (26.4–50.5) | 49.1 | (35.0–65.6) | 0.163 |
| iPTH | 68 | (44.6–468.0) | 208.0 | (86.9–916.4) | 0.199 |
| ACDF | |||||
| Ca | 10.4 | (8.7–10.6) | 9.5 | (8.5–10.4) | 0.300 |
| P | 4.0 | (2.9–5.2) | 4.3 | (3.6–5.7) | 0.129 |
| Ca × P | 32.2 | (30.7–50.5) | 45.8 | (35.0–49.4) | 0.124 |
| iPTH | 60.6 | (49.5–318.0) | 731.3 | (255.0–815.3) | 0.002 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, T.-Y.; Wu, Y.-C.; Tseng, S.-C.; Chen, K.-H.; Pan, C.-C.; Lee, C.-H. Correlation between Preoperative Serum Levels of Calcium, Phosphate, and Intact Parathyroid Hormone and Radiological Outcomes in Spinal Interbody Fusion among End-Stage Renal Disease Patients. J. Clin. Med. 2021, 10, 5447. https://doi.org/10.3390/jcm10225447
Shih T-Y, Wu Y-C, Tseng S-C, Chen K-H, Pan C-C, Lee C-H. Correlation between Preoperative Serum Levels of Calcium, Phosphate, and Intact Parathyroid Hormone and Radiological Outcomes in Spinal Interbody Fusion among End-Stage Renal Disease Patients. Journal of Clinical Medicine. 2021; 10(22):5447. https://doi.org/10.3390/jcm10225447
Chicago/Turabian StyleShih, Ting-Yu, Yun-Che Wu, Sheng-Chieh Tseng, Kun-Hui Chen, Chien-Chou Pan, and Cheng-Hung Lee. 2021. "Correlation between Preoperative Serum Levels of Calcium, Phosphate, and Intact Parathyroid Hormone and Radiological Outcomes in Spinal Interbody Fusion among End-Stage Renal Disease Patients" Journal of Clinical Medicine 10, no. 22: 5447. https://doi.org/10.3390/jcm10225447
APA StyleShih, T.-Y., Wu, Y.-C., Tseng, S.-C., Chen, K.-H., Pan, C.-C., & Lee, C.-H. (2021). Correlation between Preoperative Serum Levels of Calcium, Phosphate, and Intact Parathyroid Hormone and Radiological Outcomes in Spinal Interbody Fusion among End-Stage Renal Disease Patients. Journal of Clinical Medicine, 10(22), 5447. https://doi.org/10.3390/jcm10225447







