Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Outcome Measurement during Follow-Up
2.3. Statistical Analysis
3. Results
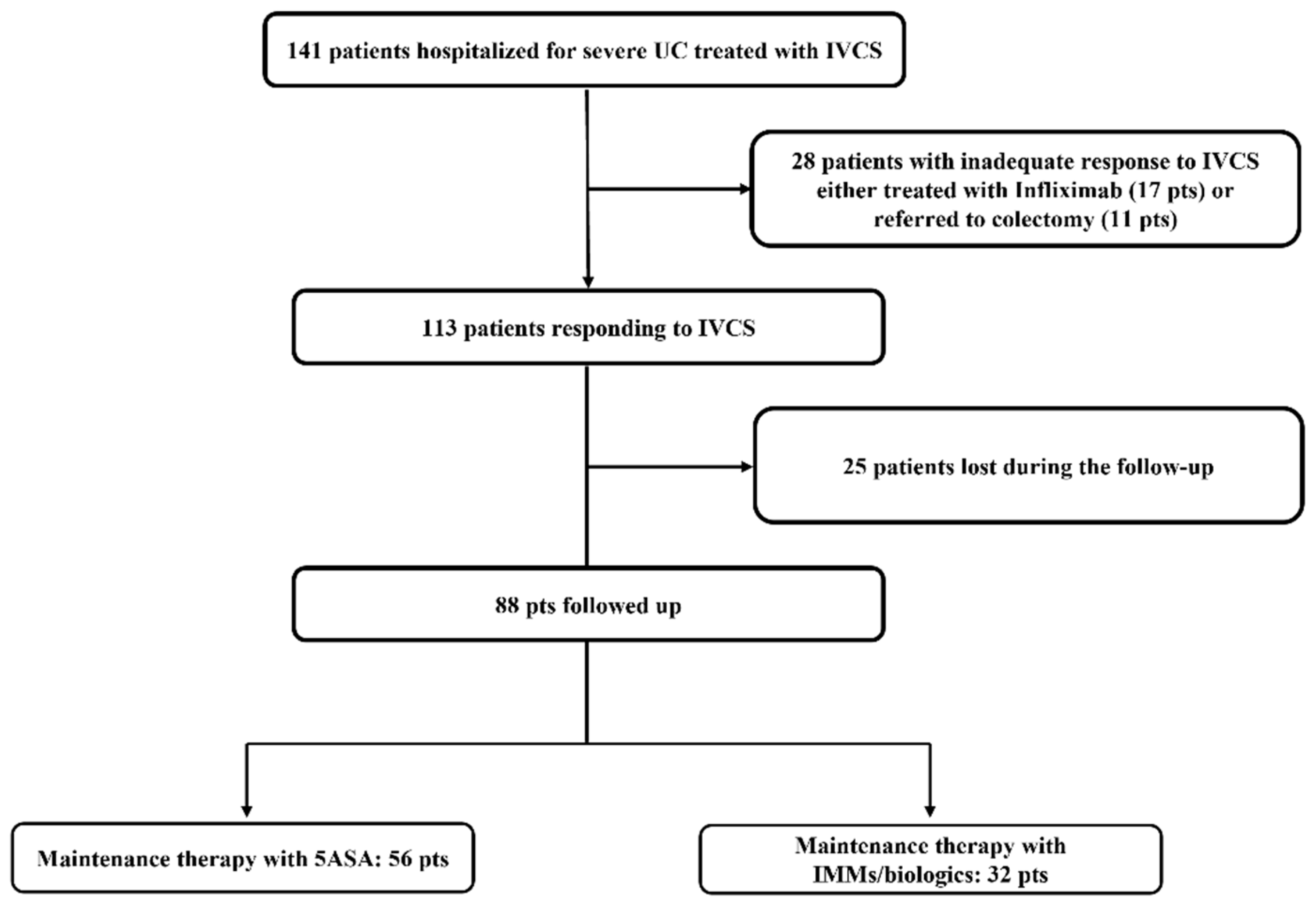
3.1. Study Population
3.2. Characteristics at Baseline of Patients Responding to IVCS and Maintenance Therapies
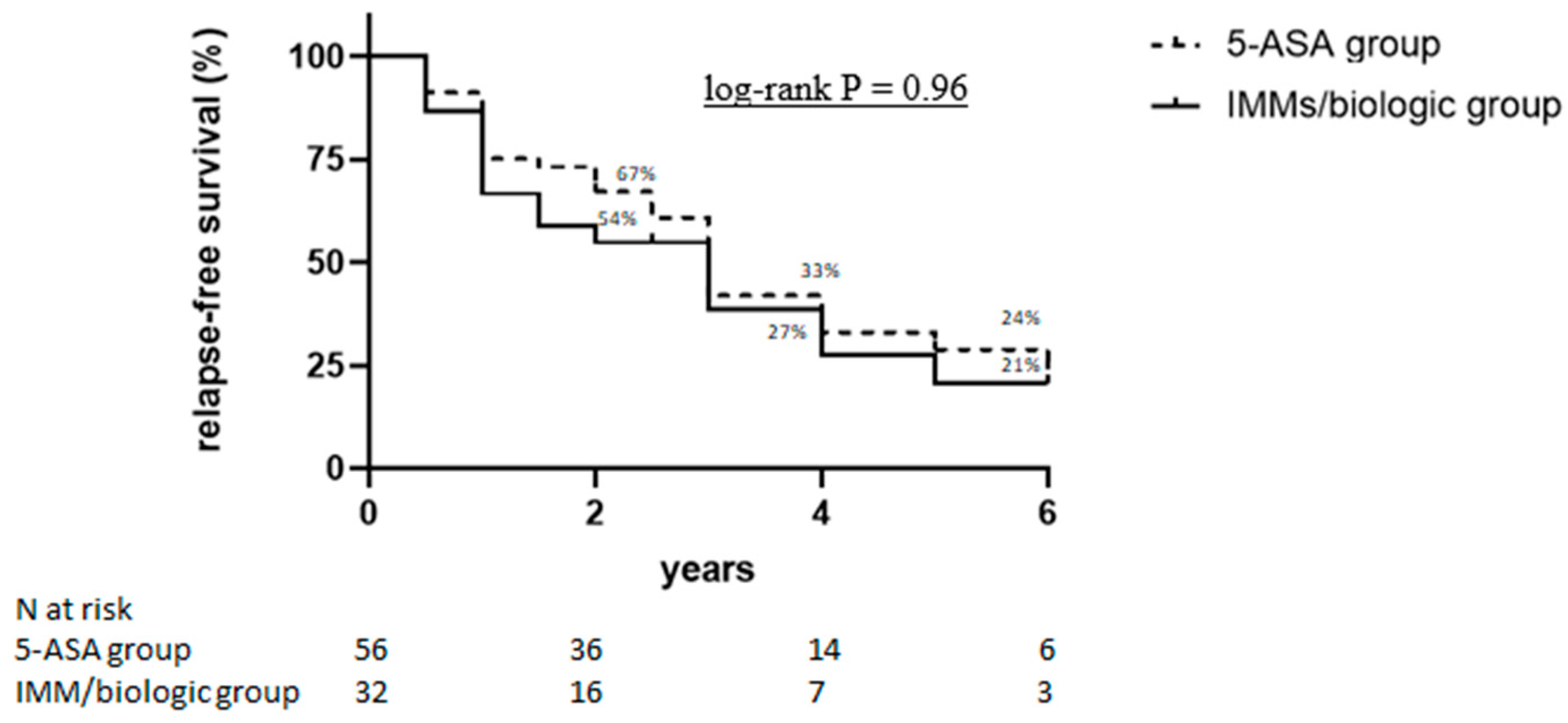
3.3. Long-Term Outcomes in Patients Responding to IVCS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Cosnes, J.; Gowerrousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; George, B.; Mortensen, N.J.; Travis, S.P. The pattern and outcome of acute severe colitis. J. Crohn’s Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Benchimol, E.; Mann, E.H.; E Thomas, K.; Chow, C.; A McLernon, R.; Walters, T.D.; Swales, J.; Steinhart, A.H.; et al. Severe paediatric ulcerative colitis: Incidence, outcomes and optimal timing for second-line therapy. Gut 2008, 57, 331–338. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to Corticosteroids in Severe Ulcerative Colitis: A Systematic Review of the Literature and a Meta-Regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef]
- Park, D. Cortisone in Ulcerative Colitis. Br. Med. J. 1955, 2, 1386. [Google Scholar] [CrossRef][Green Version]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Dias, F.J.M.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: Surgical management and special situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef]
- Lindgren, S.C.; Flood, L.M.; Kilander, A.F.; Löfberg, R.; Persson, T.B.; Sjödahl, R.I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 831–835. [Google Scholar] [CrossRef]
- Ho, G.T.; Mowat, C.; Goddard, C.J.R.; Fennell, J.M.; Shah, N.B.; Prescott, R.J.; Satsangi, J. Predicting the outcome of severe ulcerative colitis: Development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment. Pharmacol. Ther. 2004, 19, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.L. Predicting outcome in severe ulcerative colitis. Dig. Liver Dis. 2004, 36, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, F.; Gargouri, D.; Lémann, M.; Beaugerie, L.; Cattan, S.; Cosnes, J.; Gendre, J.P. Predictive factors of outcome of intensive intravenous treatment for attacks of ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 273–279. [Google Scholar]
- Narula, N.; Marshall, J.K.; Colombel, J.-F.; Leontiadis, G.I.; Williams, J.G.; Muqtadir, Z.; Reinisch, W. Systematic review and meta-analysis: Infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am. J. Gastroenterol. 2016, 111, 477–491. [Google Scholar] [CrossRef]
- Salameh, R.; Kirchgesner, J.; Allez, M.; Carbonnel, F.; Meyer, A.; Gornet, J.-M.; Beaugerie, L.; Amiot, A. Long-term outcome of patients with acute severe ulcerative colitis responding to intravenous steroids. Aliment. Pharmacol. Ther. 2020, 51, 1096–1104. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis: Final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Asa for Mildely to Moderately Active Ulcerative Colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- D’Haens, G.; Sandborn, W.J.; Feagan, B.G.; Geboes, K.; Hanauer, S.B.; Irvine, E.J.; Lémann, M.; Marteau, P.; Rutgeerts, P.; Schölmerich, J.; et al. A Review of Activity Indices and Efficacy End Points for Clinical Trials of Medical Therapy in Adults with Ulcerative Colitis. Gastroenterology 2007, 132, 763–786. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Vedamurthy, A.; Xu, L.; Luther, J.; Colizzo, F.; Garber, J.J.; Khalili, H.; Ananthakrishnan, A.N. Long-term outcomes of immunosuppression-naïve steroid responders following hospitalization for ulcerative colitis. Dig. Dis. Sci. 2018, 63, 2740–2746. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Ballester, M.P.; Tosca, J.; Bosca-Watts, M.M.; Navarro, P.; Anton, R.; Pascual, I.; Mora, F.; Minguez, M. Long-term follow-up of patients treated with aminosalicylates for ulcerative colitis: Predictive factors of response: An observational case-control study. United Eur. Gastroenterol. J. 2019, 7, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Belaiche, J.; Louis, E.; Reenaers, C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J. Crohn’s Colitis 2011, 5, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. Simple score to identify colectomy risk in ulcerative colitis hospitalizations. Inflamm. Bowel. Dis. 2010, 16, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, D.N.; Van Assche, G.; Maenhout, B.; Arts, J.; Ferrante, M.; Vermeire, S.; Rutgeerts, P. Incidence of Colectomy During Long-term Follow-up After Cyclosporine-Induced Remission of Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2006, 4, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Bojic, D.; Radojicic, Z.; Nedeljkovic-Protic, M.; Al-Ali, M.; Jewell, D.P.; Travis, S.P. Long-term outcome after admission for acute severe ulcerative colitis in Oxford: The 1992–1993 cohort. Inflamm. Bowel Dis. 2009, 15, 823–828. [Google Scholar] [CrossRef]

| Characteristics | Maintenance Therapy with 5-ASA (n = 56) | Maintenance Therapy with IMM/Biologic (n = 32) | p |
|---|---|---|---|
| Male gender, n (%) | 33 (59%) | 14 (44%) | 0.19 |
| Age, y (mean ± SD) | 52 ± 19.0 | 44 ± 14.2 | 0.08 |
| Appendectomy | |||
| Yes, n (%) | 3 (5%) | 3 (9%) | 0.056 |
| Age at diagnosis: | |||
| A2: 17–40 y | 31 (55%) | 16 (50%) | 0.66 |
| A3: >40 y | 25 (45%) | 16 (50%) | 0.66 |
| Ulcerative colitis | |||
| E2: left-sided colitis | 20 (36%) | 8 (25%) | 0.35 |
| E3: extensive colitis | 36 (64%) | 24 (75%) | 0.35 |
| Duration of disease, months—median (IQR) | 132 (108) | 108 (138) | 0.46 |
| Previous anti-TNF therapies | 6 (11%) | 8 (25%) | 0.044 |
| Smoking habits | |||
| Former | 18 (32%) | 14 (44%) | 0.74 |
| Current | 7 (13%) | 6 (19%) | 0.34 |
| Steroid dependence, | |||
| Yes, n (%) | 13 (23%) | 15 (47%) | 0.032 |
| Partial Mayo Clinic score, (mean ± SD) | 6.7 ± 1.1 | 6.6 ± 0.8 | 0.79 |
| Endoscopic Mayo Clinic subscore, | |||
| (mean ± SD) | 2.6 ± 0.6 | 2.6 ± 0.5 | 0.79 |
| C-reactive-protein (CRP) | |||
| Positive (>5 mg/L), n (%) | 55 (98%) | 28 (87%) | 0.056 |
| Hemoglobin g/dL | |||
| median (IQR) | 11. 9 (2.8) | 11. 7 (2.3) | 0.909 |
| Albumin g/dL | |||
| median (IQR) | 3 (0.7) | 3.5 (0.8) | 0.0048 |
| Long-Term Outcomes, n pts (%) | Maintenance Therapy with 5-ASA (n = 56) | Maintenance Therapy with IMM/Biologic (n = 32) | p |
|---|---|---|---|
| Relapse, n (%) | |||
| (partial Mayo score >4), | 39 (70%) | 21 (66%) | 0.81 |
| Steroid-free clinical remission | 24 (43%) | 14 (44%) | 0.83 |
| Re-hospitalization, n (%) | 17 (30%) | 11 (34%) | 0.81 |
| Colectomy, n (%) | 11 (20%) | 4 (13%) | 0.56 |
| Corticosteroids, n (%) | 32 (57%) | 17 (53%) | 0.82 |
| IMMs, n (%) | 9 (16%) | 3 (9%) | 0.52 |
| Biologic therapy, n (%) | 15 (27%) | 16 (50%) | 0.037 |
| Month 6 (73 pts) | Month 12 (62 pts) | Month 24 (52 pts) | |
|---|---|---|---|
| Relapse (partial Mayo score >4), n (%) | 24 (33%) | 18 (29%) | 19 (36%) |
| Steroid-free clinical remission, n (%) | 44 (60%) | 41(66%) | 33 (63%) |
| Re-hospitalization, n (%) | 4 (5%) | 6 (10%) | 4 (8%) |
| Colectomy, n (%) | 2 (3%) | 0 | 0 |
| Corticosteroids, n (%) | 17 (23%) | 14 (23%) | 12 (23%) |
| IMMs, n (%) | 1 (1.4%) | 11 (18%) | 7 (13%) |
| Biologic therapy, n (%) | 6 (8%) | 5 (8%) | 5 (10%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Risk Factors | HR (95% CI) | p Value | HR (95% CI) | p Value |
| 5-ASA maintenance therapy | 1.01 (0.39 to 2.57) | 0.99 | - | |
| IMM/biologic maintenance therapy | 0.85 (0.34 to 2.16) | 0.74 | - | |
| Previous anti-TNF exposure | 8.71 (1.85 to 69.93) | 0.008 | 6.51 (0.67 to 62.86) | 0.89 |
| Steroid dependence | 3.04 (1.13 to 8.19) | 0.02 | 1.09 (0.33 to 3.53) | 0.11 |
| Partial Mayo Clinic score <2 at 6 months after discharge | 0.20 (0.05 to 0.76) | 0.02 | 0.22 (0.05 to 0.88) | 0.03 |
| Disease duration (months) | 0.99 (0.99 to 1.01) | 0.78 | - | |
| Smoking habits | 0.77 (0.23 to 2.59) | 0.6 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cristofaro, E.; Salvatori, S.; Marafini, I.; Zorzi, F.; Alfieri, N.; Musumeci, M.; Biancone, L.; Calabrese, E.; Monteleone, G. Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids. J. Clin. Med. 2021, 10, 5413. https://doi.org/10.3390/jcm10225413
De Cristofaro E, Salvatori S, Marafini I, Zorzi F, Alfieri N, Musumeci M, Biancone L, Calabrese E, Monteleone G. Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids. Journal of Clinical Medicine. 2021; 10(22):5413. https://doi.org/10.3390/jcm10225413
Chicago/Turabian StyleDe Cristofaro, Elena, Silvia Salvatori, Irene Marafini, Francesca Zorzi, Norma Alfieri, Martina Musumeci, Livia Biancone, Emma Calabrese, and Giovanni Monteleone. 2021. "Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids" Journal of Clinical Medicine 10, no. 22: 5413. https://doi.org/10.3390/jcm10225413
APA StyleDe Cristofaro, E., Salvatori, S., Marafini, I., Zorzi, F., Alfieri, N., Musumeci, M., Biancone, L., Calabrese, E., & Monteleone, G. (2021). Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids. Journal of Clinical Medicine, 10(22), 5413. https://doi.org/10.3390/jcm10225413







