Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury
Abstract
1. Introduction
2. Obesity Decreases Functional Mobility
3. Electrically Evoked Training to Improve Body Composition
4. Optimizing Electrical Stimulation Training Parameters
5. Management of Spasticity
6. Clinical Applications of FES/NMES
7. Retraining Standing Postural Control with FES
8. Improved Function through Less Invasive and Invasive Electrical Stimulation
9. Neuromodulation of the Spinal Cord
10. Combined Epidural Stimulation and Exoskeletal-Assisted Walking
11. Applications of Epidural Stimulation on Cardiovascular Performance
12. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preiser, J.-C.; Ichai, C.; Orban, J.-C.; Groeneveld, A. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.N.; Plank, L.D.; Franch-Arcas, G.; Finn, P.J.; Streat, S.J.; Hill, G.L. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann. Surg. 1996, 223, 395. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.J.; Apple, D.F., Jr.; Hillegass, E.A.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. 1999, 80, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.; Dudley, G. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007, 45, 304–309. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: An open-label randomized clinical trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Holman, M.E.; Gorgey, A.S. Testosterone and Resistance Training Improve Muscle Quality in Spinal Cord Injury. Med. Sci. Sport Exerc. 2019, 51, 1591–1598. [Google Scholar] [CrossRef]
- Ghatas, M.P.; Khan, M.R.; Gorgey, A.S. Skeletal muscle stiffness as measured by magnetic resonance elastography after chronic spinal cord injury: A cross-sectional pilot study. Neural Regen. Res. 2021, 16, 2486. [Google Scholar]
- O’Brien, L.C.; Wade, R.C.; Segal, L.; Chen, Q.; Savas, J.; Lesnefsky, E.J.; Gorgey, A.S. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol. Rep. 2017, 5, e13080. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dudley, G.A. The role of pulse duration and stimulation duration in maximizing the normalized torque during neuromuscular electrical stimulation. J. Orthop. Sport. Phys. Ther. 2008, 38, 508–516. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Ennasr, A.N.; Farkas, G.J.; Gater, D.R.; Gorgey, A.S. Role of exercise on visceral adiposity after spinal cord injury: A cardiometabolic risk factor. Eur. J. Appl. Physiol. 2021, 121, 2143–2163. [Google Scholar] [CrossRef]
- Buchholz, A.C.; McGillivray, C.F.; Pencharz, P.B. Physical activity levels are low in free-living adults with chronic paraplegia. Obes. Res. 2003, 11, 563–570. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gater, D.R. Energy expenditure and nutrition in neurogenic obesity following spinal cord injury. J. Phys. Med. Rehabil. 2020, 2, 11. [Google Scholar]
- Farkas, G.J.; Pitot, M.A.; Gater, D.R. A systematic review of the accuracy of estimated and measured resting metabolic rate in chronic spinal cord injury. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 548–558. [Google Scholar] [CrossRef]
- Farkas, G.J.; Pitot, M.A.; Berg, A.S.; Gater, D.R. Nutritional status in chronic spinal cord injury: A systematic review and meta-analysis. Spinal Cord 2019, 57, 3–17. [Google Scholar] [CrossRef]
- Gater, D.R., Jr.; Farkas, G.J.; Dolbow, D.R.; Berg, A.; Gorgey, A.S. Body Composition and Metabolic Assessment after Motor Complete Spinal Cord Injury: Development of a Clinically Relevant Equation to Estimate Body Fat. Top. Spinal Cord Inj. Rehabil. 2021, 27, 11–22. [Google Scholar] [CrossRef]
- Spungen, A.M.; Wang, J.; Pierson, R.N., Jr.; Bauman, W.A. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J. Appl. Physiol. 2000, 88, 1310–1315. [Google Scholar] [CrossRef]
- Edwards, L.A.; Bugaresti, J.M.; Buchholz, A.C. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am. J. Clin. Nutr. 2008, 87, 600–607. [Google Scholar] [CrossRef]
- Van den Berg-Emons, R.J.; Bussmann, J.B.; Stam, H.J. Accelerometry-based activity spectrum in persons with chronic physical conditions. Arch. Phys. Med. Rehabil. 2010, 91, 1856–1861. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Mather, K.J.; Gater, D.R. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. Metabolism 2011, 60, 843–851. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Gater, D.R. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl. Physiol. Nutr. Metab. 2011, 36, 107–114. [Google Scholar] [CrossRef]
- Galea, M. Spinal cord injury and physical activity: Preservation of the body. Spinal Cord 2012, 50, 344–351. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Allen, V.; Richards, J.S. Weight matters: Physical and psychosocial well being of persons with spinal cord injury in relation to body mass index. Arch. Phys. Med. Rehabil. 2011, 92, 391–398. [Google Scholar] [CrossRef]
- Tian, W.; Hsieh, C.-H.; DeJong, G.; Backus, D.; Groah, S.; Ballard, P.H. Role of body weight in therapy participation and rehabilitation outcomes among individuals with traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, S125–S136. [Google Scholar] [CrossRef]
- Kimura, T. The items and level contributing to improve in less than 50 motor functional independence measure upon admission in the stroke recovery rehabilitation ward. J. Phys. Ther. Sci. 2019, 31, 418–423. [Google Scholar] [CrossRef]
- Stenson, K.W.; Deutsch, A.; Heinemann, A.W.; Chen, D. Obesity and inpatient rehabilitation outcomes for patients with a traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2011, 92, 384–390. [Google Scholar] [CrossRef]
- Blackmer, J.; Marshall, S. Obesity and spinal cord injury: An observational study. Spinal Cord 1997, 35, 245–247. [Google Scholar] [CrossRef]
- Beck, L. Morbid obesity and spinal cord injury: A case study. SCI Nurs. Publ. Am. Assoc. Spinal Cord Inj. Nurses 1998, 15, 3–5. [Google Scholar]
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Khalil, R.K.; Gater, D.R. The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury—Part II. J. Spinal Cord Med. 2015, 38, 23–37. [Google Scholar] [CrossRef]
- Mahoney, E.T.; Bickel, C.S.; Elder, C.; Black, C.; Slade, J.M.; Apple, D., Jr.; Dudley, G.A. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 1502–1504. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Mather, K.J.; Cupp, H.R.; Gater, D.R. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med. Sci. Sport. Exerc. 2012, 44, 165–174. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brizendine, J.T.; Backus, D.; McCully, K.K. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.M.; Bickel, C.S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys. Ther. 2005, 85, 358–364. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Gill, S.; Holman, M.E.; Davis, J.C.; Atri, R.; Bai, O.; Goetz, L.; Lester, D.L.; Trainer, R.; Lavis, T.D. The feasibility of using exoskeletal-assisted walking with epidural stimulation: A case report study. Ann. Clin. Transl. Neurol. 2020, 7, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.E.; Gorgey, A.S. Low-dose testosterone replacement therapy and electrically evoked resistance training enhance muscle quality after spinal cord injury. Neural Regen. Res. 2021, 16, 1544–1545. [Google Scholar] [PubMed]
- Demchak, T.J.; Linderman, J.K.; Mysiw, W.J.; Jackson, R.; Sunn, J.; Devor, S.T. Functional Electric Stimulation Cycle Ergometry Training Effect on Lower Limb Muscles in Acute SCI Individuals. J. Sport. Sci. Med. 2005, 4, 263–271. [Google Scholar]
- Dolbow, D.R.; Credeur, D.P.; Lemacks, J.L.; Stokic, D.S.; Pattanaik, S.; Corbin, G.N.; Courtner, A.S. Electrically induced cycling and nutritional counseling for counteracting obesity after spinal cord injury: A pilot study. J. Spinal Cord Med. 2021, 44, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fornusek, C.; Davis, G.M.; Russold, M.F. Pilot study of the effect of low-cadence functional electrical stimulation cycling after spinal cord injury on thigh girth and strength. Arch. Phys. Med. Rehabil. 2013, 94, 990–993. [Google Scholar] [CrossRef]
- Johnston, T.E.; Marino, R.J.; Oleson, C.V.; Schmidt-Read, M.; Leiby, B.E.; Sendecki, J.; Singh, H.; Modlesky, C.M. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2016, 97, 1413–1422. [Google Scholar] [CrossRef]
- Deley, G.; Denuziller, J.; Babault, N.; Taylor, J.A. Effects of electrical stimulation pattern on quadriceps isometric force and fatigue in individuals with spinal cord injury. Muscle Nerve 2015, 52, 260–264. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Poarch, H.J.; Dolbow, D.R.; Castillo, T.; Gater, D.R. Effect of adjusting pulse durations of functional electrical stimulation cycling on energy expenditure and fatigue after spinal cord injury. J. Rehabil. Res. Dev. 2014, 51, 1455–1468. [Google Scholar] [CrossRef]
- Mahoney, E.; Puetz, T.W.; Dudley, G.A.; McCully, K.K. Low-frequency fatigue in individuals with spinal cord injury. J. Spinal Cord Med. 2007, 30, 458–466. [Google Scholar] [CrossRef]
- Bochkezanian, V.; Newton, R.U.; Trajano, G.S.; Blazevich, A.J. Effects of neuromuscular electrical stimulation in people with spinal cord injury. Med. Sci. Sport. Exerc. 2018, 50, 1733–1739. [Google Scholar] [CrossRef]
- Bajd, T.; Gregoric, M.; Vodovnik, L.; Benko, H. Electrical stimulation in treating spasticity resulting from spinal cord injury. Arch. Phys. Med. Rehabil. 1985, 66, 515–517. [Google Scholar]
- Ragnarsson, K.T. Functional electrical stimulation and suppression of spasticity following spinal cord injury. Bull. N. Y. Acad. Med. 1992, 68, 351. [Google Scholar]
- Bekhet, A.H.; Bochkezanian, V.; Saab, I.M.; Gorgey, A.S. The effects of electrical stimulation parameters in managing spasticity after spinal cord injury: A systematic review. Am. J. Phys. Med. Rehabil. 2019, 98, 484–499. [Google Scholar] [CrossRef]
- Bochkezanian, V. Electrotherapeutic techniques. In Rehabilitation in Spinal Cord Injuries; Elsevier: Amsterdam, The Netherlands, 2020; p. 146. [Google Scholar]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef]
- Ploutz, L.L.; Tesch, P.A.; Biro, R.L.; Dudley, G.A. Effect of resistance training on muscle use during exercise. J. Appl. Physiol. 1994, 76, 1675–1681. [Google Scholar] [CrossRef]
- Van der Scheer, J.W.; Goosey-Tolfrey, V.L.; Valentino, S.E.; Davis, G.M.; Ho, C.H. Functional electrical stimulation cycling exercise after spinal cord injury: A systematic review of health and fitness-related outcomes. J. Neuroeng. Rehabil. 2021, 18, 99. [Google Scholar] [CrossRef]
- Shields, R.K.; Dudley-Javoroski, S. Musculoskeletal plasticity after acute spinal cord injury: Effects of long-term neuromuscular electrical stimulation training. J. Neurophysiol. 2006, 95, 2380–2390. [Google Scholar] [CrossRef]
- Black, C.D.; McCully, K.K. Force per active area and muscle injury during electrically stimulated contractions. Med. Sci. Sport. Exerc. 2008, 40, 1596. [Google Scholar] [CrossRef]
- Auchstaetter, N.; Luc, J.; Lukye, S.; Lynd, K.; Schemenauer, S.; Whittaker, M.; Musselman, K.E. Physical therapists’ use of functional electrical stimulation for clients with stroke: Frequency, barriers, and facilitators. Phys. Ther. 2016, 96, 995–1005. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef]
- Sibley, K.M.; Beauchamp, M.K.; Van Ooteghem, K.; Straus, S.E.; Jaglal, S.B. Using the systems framework for postural control to analyze the components of balance evaluated in standardized balance measures: A scoping review. Arch. Phys. Med. Rehabil. 2015, 96, 122–132. [Google Scholar] [CrossRef]
- Lemay, J.-F.; Gagnon, D.H.; Nadeau, S.; Grangeon, M.; Gauthier, C.; Duclos, C. Center-of-pressure total trajectory length is a complementary measure to maximum excursion to better differentiate multidirectional standing limits of stability between individuals with incomplete spinal cord injury and able-bodied individuals. J. Neuroeng. Rehabil. 2014, 11, 8. [Google Scholar] [CrossRef]
- Houston, D.J.; Lee, J.W.; Unger, J.; Masani, K.; Musselman, K.E. Functional electrical stimulation plus visual feedback balance training for standing balance performance among individuals with incomplete spinal cord injury: A case series. Front. Neurol. 2020, 11, 680. [Google Scholar] [CrossRef]
- Houston, D.J.; Unger, J.; Lee, J.W.; Masani, K.; Musselman, K.E. Perspectives of individuals with chronic spinal cord injury following novel balance training involving functional electrical stimulation with visual feedback: A qualitative exploratory study. J. Neuroeng. Rehabil. 2021, 18, 57. [Google Scholar] [CrossRef]
- Chan, K.; Lee, J.W.; Unger, J.; Yoo, J.; Masani, K.; Musselman, K.E. Reactive stepping after a forward fall in people living with incomplete spinal cord injury or disease. Spinal Cord 2020, 58, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Musselman, K.E.; Lanovaz, J.L.; Linassi, G.; Arnold, C.; Milosavljevic, S.; Oates, A. Reactive balance responses to an unexpected slip perturbation in individuals with incomplete spinal cord injury. Clin. Biomech. 2020, 78, 105099. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pujol, C.; Laylor, M.; Unic, N.; Pakosh, M.; Dawe, J.; Musselman, K.E. Falls after spinal cord injury: A systematic review and meta-analysis of incidence proportion and contributing factors. Spinal Cord 2019, 57, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Scovil, C.Y.; Yoshida, K.; Oosman, S.; Kaiser, A.; Jaglal, S.B.; Musselman, K.E. Capturing the psychosocial impacts of falls from the perspectives of wheelchair users with spinal cord injury through photo-elicitation. Disabil. Rehabil. 2020, 43, 2680–2689. [Google Scholar] [CrossRef]
- Vette, A.H.; Masani, K.; Nakazawa, K.; Popovic, M.R. Neural-mechanical feedback control scheme generates physiological ankle torque fluctuation during quiet stance. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 86–95. [Google Scholar] [CrossRef]
- Gauthier, C.; Chan, K.; Masani, K.; Musselman, K.E. Perturbation-Based Training in Combination with Functional Electrical Stimulation: A Promising Mixed-methods Case Study. Arch. Phys. Med. Rehabil. 2020, 101, e26. [Google Scholar] [CrossRef]
- Bekhet, A.H.; Jahan, A.M.; Bochkezanian, V.; Musselman, K.E.; Elsareih, A.A.; Gorgey, A.S. Effects of electrical stimulation training on body composition parameters after spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 2021, 16, 1544–1545. [Google Scholar] [CrossRef]
- Unger, J.; Chan, K.; Lee, J.W.; Craven, B.C.; Mansfield, A.; Alavinia, M.; Masani, K.; Musselman, K.E. The Effect of Perturbation-Based Balance Training and Conventional Intensive Balance Training on Reactive Stepping Ability in Individuals with Incomplete Spinal Cord Injury or Disease: A Randomized Clinical Trial. Front. Neurol. 2021, 12, 49. [Google Scholar] [CrossRef]
- Gater, D.R., Jr.; Dolbow, D.; Tsui, B.; Gorgey, A.S. Functional electrical stimulation therapies after spinal cord injury. NeuroRehabilitation 2011, 28, 231–248. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef]
- Kumru, H.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Flores, Á.; Soriano, I.; Opisso, E.; Gerasimenko, Y.; Navarro, X.; García-Alías, G. Transcutaneous Electrical Neuromodulation of the Cervical Spinal Cord Depends Both on the Stimulation Intensity and the Degree of Voluntary Activity for Training. A Pilot Study. J. Clin. Med. 2021, 10, 3278. [Google Scholar] [CrossRef]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and subcortical effects of transcutaneous spinal cord stimulation in humans with tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Hofstoetter, U.; Hofer, C.; Kern, H.; Danner, S.; Mayr, W.; Dimitrijevic, M.; Minassian, K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Eng. Biomed. Tech. 2013, 58, 4014. [Google Scholar] [CrossRef]
- Freyvert, Y.; Yong, N.A.; Morikawa, E.; Zdunowski, S.; Sarino, M.E.; Gerasimenko, Y.; Edgerton, V.R.; Lu, D.C. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci. Rep. 2018, 8, 15546. [Google Scholar] [CrossRef]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-invasive activation of cervical spinal networks after severe paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing Over-ground stepping in an exoskeleton with non-invasive spinal Cord neuromodulation after motor complete paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Hackman, J.; Yousak, A.; Wallner, J.J.; Gad, P.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, in press. [Google Scholar] [CrossRef]
- Benavides, F.D.; Santamaria, A.J.; Bodoukhin, N.; Guada, L.G.; Solano, J.P.; Guest, J.D. Characterization of motor and somatosensory evoked potentials in the Yucatan micropig using transcranial and epidural stimulation. J. Neurotrauma 2017, 34, 2595–2608. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Minassian, K.; McKay, W.B.; Binder, H.; Hofstoetter, U.S. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics 2016, 13, 284–294. [Google Scholar] [CrossRef]
- Jervis Rademeyer, H.; Gauthier, C.; Masani, K.; Pakosh, M.; Musselman, K.E. The effects of epidural stimulation on individuals living with spinal cord injury or disease: A scoping review. Phys. Ther. Rev. 2021, 26, 344–369. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A.; Bryant, N.; Harkema, S.J. Effects of Stand and Step Training with epidural stimulation on motor function for standing in chronic complete paraplegics. J. Neurotrauma 2017, 34, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin. Neurophysiol. 2010, 121, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Linde, M.; Fautsch, K.; Hale, R.; Lopez, C.; Veith, D.; Calvert, J.; Beck, L.; Garlanger, K.; Edgerton, R. Epidural electrical stimulation of the lumbosacral spinal cord improves trunk stability during seated reaching in two humans with severe thoracic spinal cord injury. Front. Syst. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.S.; Grahn, P.J.; Strommen, J.A.; Lavrov, I.A.; Beck, L.A.; Gill, M.L.; Linde, M.B.; Brown, D.A.; Van Straaten, M.G.; Veith, D.D. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J. Neurotrauma 2019, 36, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Galley, D.; Rettori, R.; Boccalon, H.; Medvedowsky, A.; Lefebvre, J.; Sellier, F.; Chauvreau, C.; Serisé, J.; Pieronne, A. Electric stimulation of the spinal cord in arterial diseases of the legs. A multicenter study of 244 patients. J. Mal. Vasc. 1992, 17, 208–213. [Google Scholar] [PubMed]
- Claeys, L.G.Y. Improvement of Microcirculatory Blood Flow underEpidural Spinal Cord Stimulation in Patients withNonreconstructible Peripheral Arterial Occlusive Disease. Artif. Organs 1997, 21, 201–206. [Google Scholar] [CrossRef]
- Yamasaki, F.; Ushida, T.; Yokoyama, T.; Ando, M.; Yamashita, K.; Sato, T. Artificial baroreflex: Clinical application of a bionic baroreflex system. Circulation 2006, 113, 634–639. [Google Scholar] [CrossRef]
- Schultz, D.M.; Musley, S.; Beltrand, P.; Christensen, J.; Euler, D.; Warman, E. Acute cardiovascular effects of epidural spinal cord stimulation. Pain Physician 2007, 10, 677–685. [Google Scholar] [CrossRef]
- Foreman, R.D.; Linderoth, B.; Ardell, J.L.; Barron, K.W.; Chandler, M.; Hull, S.S., Jr.; TerHorst, G.J.; DeJongste, M.J.; Armour, J.A. Modulation of intrinsic cardiac neurons by spinal cord stimulation: Implications for its therapeutic use in angina pectoris. Cardiovasc. Res. 2000, 47, 367–375. [Google Scholar] [CrossRef]
- Eliasson, T.; Mannheimer, C.; Waagstein, F.; Andersson, B.; Bergh, C.-H.; Augustinsson, L.-E.; Hedner, T.; Larson, G. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology 1998, 89, 170–177. [Google Scholar] [CrossRef]
- Croom, J.E.; Foreman, R.D.; Chandler, M.J.; Barron, K.W. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am. J. Physiol.-Heart Circ. Physiol. 1997, 272, H950–H957. [Google Scholar] [CrossRef]
- Yanagiya, Y.; Sato, T.; Kawada, T.; Inagaki, M.; Tatewaki, T.; Zheng, C.; Kamiya, A.; Takaki, H.; Sugimachi, M.; Sunagawa, K. Bionic epidural stimulation restores arterial pressure regulation during orthostasis. J. Appl. Physiol. 2004, 97, 984–990. [Google Scholar] [CrossRef]
- West, C.R.; Phillips, A.A.; Squair, J.W.; Williams, A.M.; Walter, M.; Lam, T.; Krassioukov, A.V. Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol. 2018, 75, 630–632. [Google Scholar] [CrossRef]
- Harkema, S.J.; Wang, S.; Angeli, C.A.; Chen, Y.; Boakye, M.; Ugiliweneza, B.; Hirsch, G.A. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front. Hum. Neurosci. 2018, 12, 83. [Google Scholar] [CrossRef]
- Aslan, S.C.; Legg Ditterline, B.E.; Park, M.C.; Angeli, C.A.; Rejc, E.; Chen, Y.; Ovechkin, A.V.; Krassioukov, A.; Harkema, S.J. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front. Physiol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Legg Ditterline, B.E.; Aslan, S.C.; Wang, S.; Ugiliweneza, B.; Hirsch, G.A.; Wecht, J.M.; Harkema, S. Restoration of autonomic cardiovascular regulation in spinal cord injury with epidural stimulation: A case series. Clin. Auton. Res. 2021, 31, 317–320. [Google Scholar] [CrossRef]
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J. Neurotrauma 2019, 36, 2325–2336. [Google Scholar] [CrossRef]
- Ditterline, B.E.L.; Wade, S.; Ugiliweneza, B.; Singam, N.S.; Harkema, S.J.; Stoddard, M.F.; Hirsch, G.A. Beneficial cardiac structural and functional adaptations after lumbosacral spinal cord epidural stimulation and task-specific interventions: A pilot study. Front. Neurosci. 2020, 14, 554018. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 2021, 590, 308–314. [Google Scholar] [CrossRef]
- Wahlgren, C.; Levi, R.; Amezcua, S.; Thorell, O.; Thordstein, M. Prevalence of discomplete sensorimotor spinal cord injury as evidenced by neurophysiological methods: A cross-sectional study. J. Rehabil. Med. 2021, 53, jrm00156. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Tesini, S.; Bersch, U.; Frotzler, A. Functional electrical stimulation in spinal cord injury: Clinical evidence versus daily practice. Artif. Organs 2015, 39, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Stucki, G.; Reinhardt, J.D.; Cieza, A.; Brach, M.; Celio, M.; Joggi, D.; Villiger, B.; Zäch, G.A.; Krieg, W. Developing Swiss paraplegic research: Building a research institution from the comprehensive perspective. Disabil. Rehabil. 2008, 30, 1063–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gainforth, H.L.; Hoekstra, F.; McKay, R.; McBride, C.B.; Sweet, S.N.; Ginis, K.A.M.; Anderson, K.; Chernesky, J.; Clarke, T.; Forwell, S. Integrated knowledge translation guiding principles for conducting and disseminating spinal cord injury research in partnership. Arch. Phys. Med. Rehabil. 2021, 102, 656–663. [Google Scholar] [CrossRef]
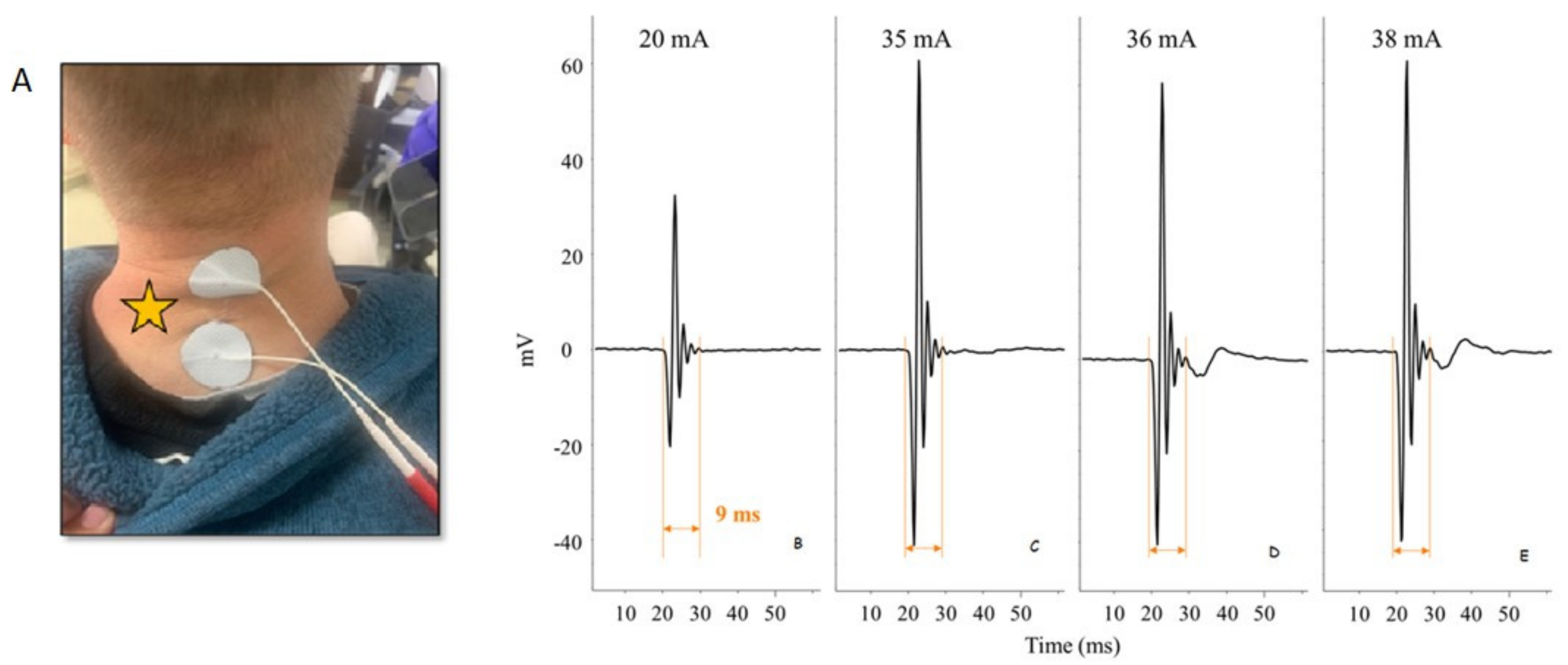
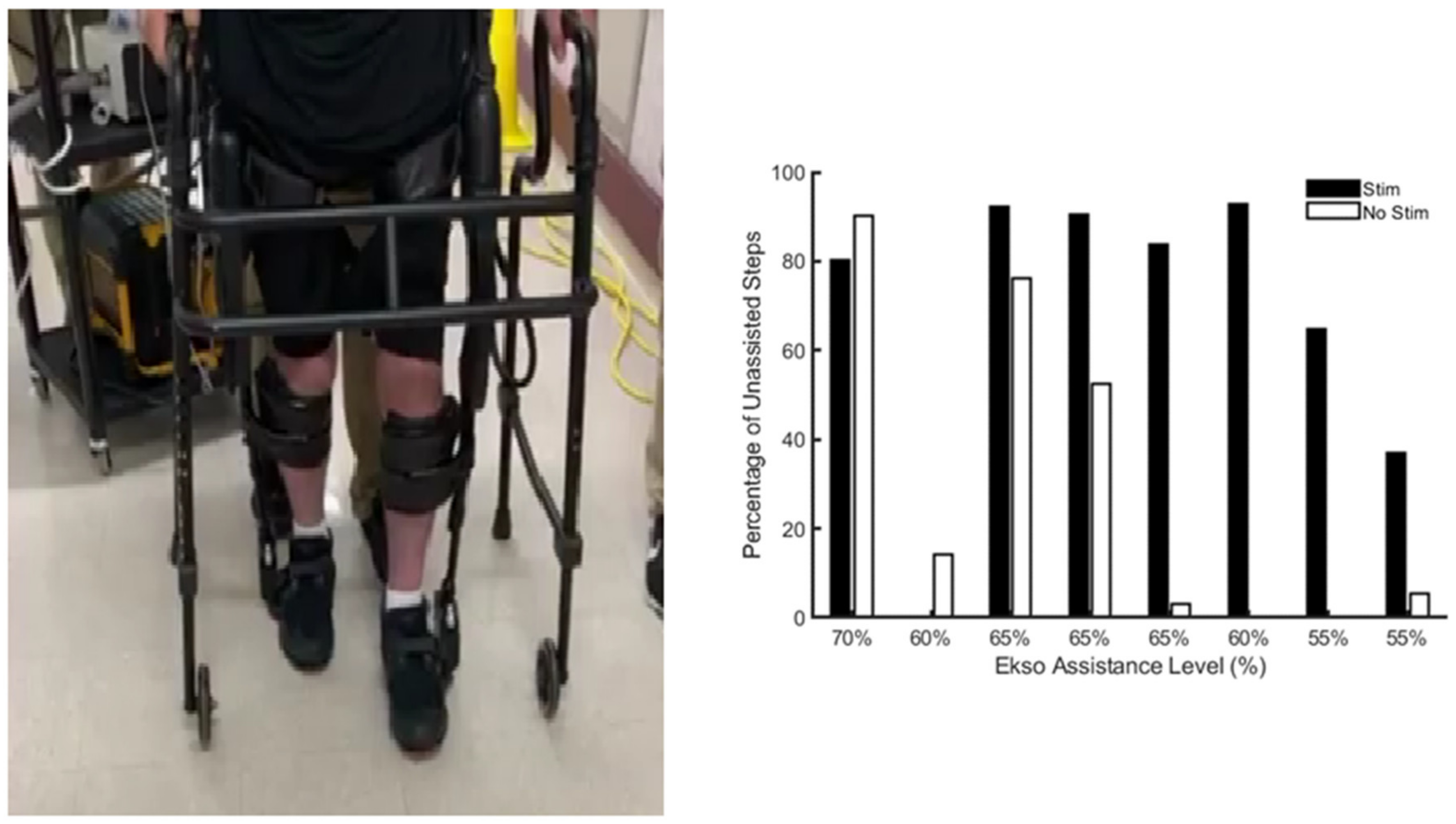
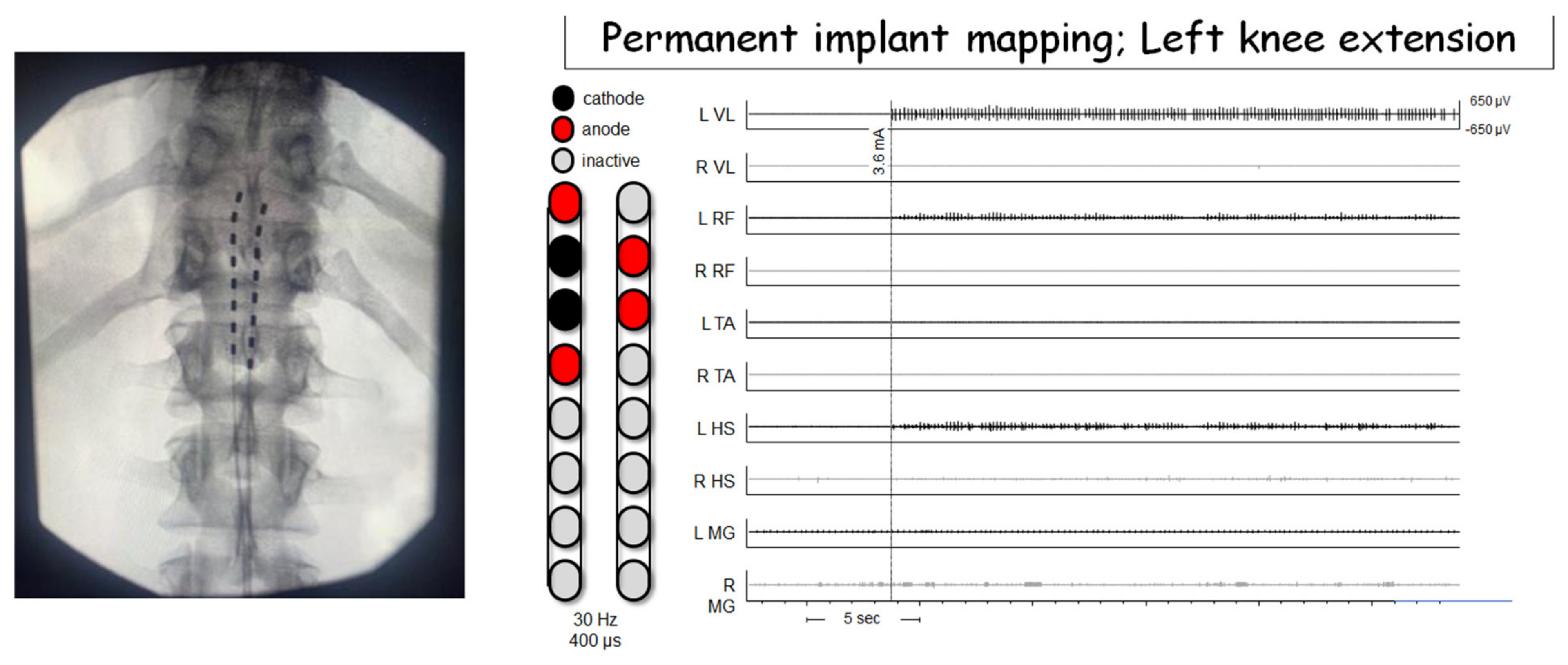
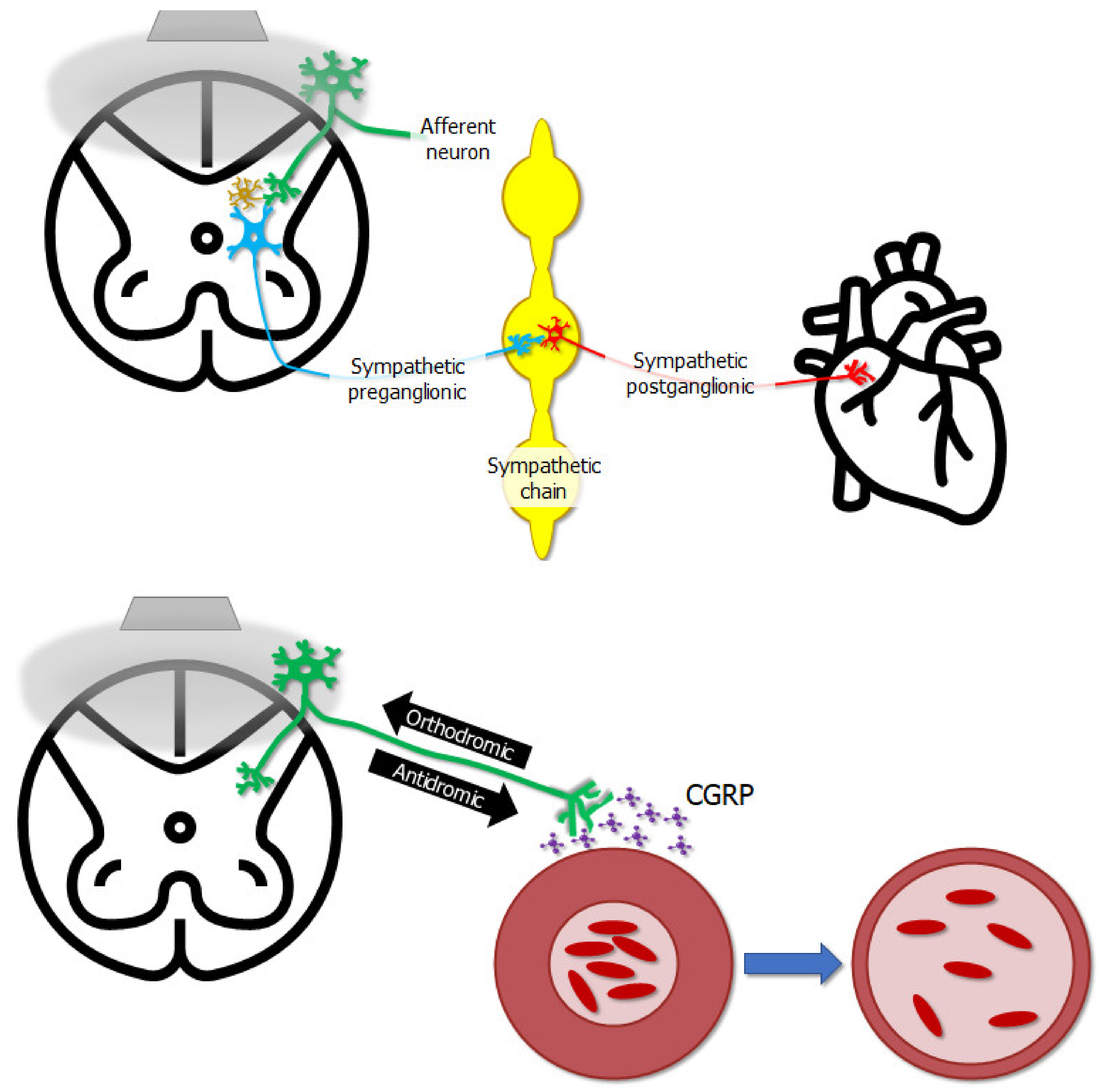
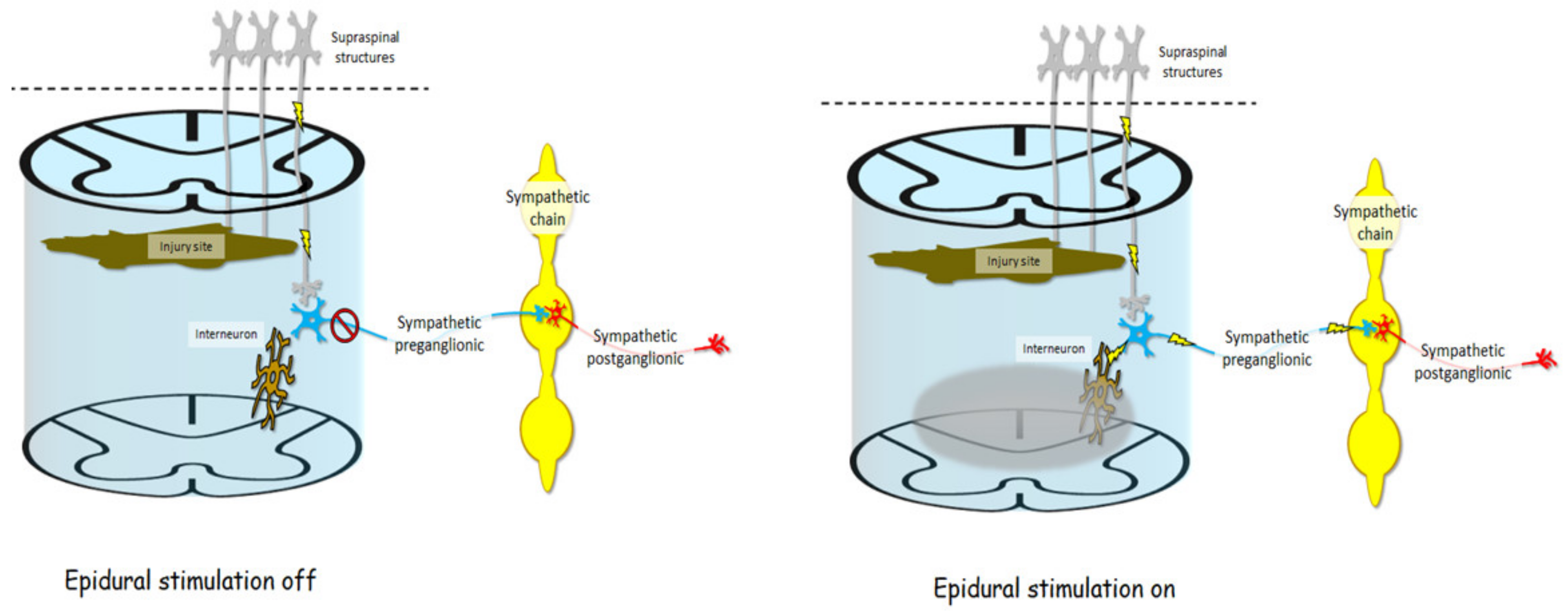
| Approaches of Delivering Electrical Stimulation | Studies | Major Findings |
|---|---|---|
| Non-invasive Approaches | Gorgey et al. [5] | NMES plus testosterone group significantly increased in total body lean mass, whole muscle mass, and knee extensor cross-sectional area and accompanied with increase in basal metabolic rate. |
| Demchak et al. [35] | The cross-sectional area of the vastus lateralis muscle increased 63% more than the non-stimulated leg. | |
| Dolbow et al. [36] | A 5.7% increase in leg lean mass and a 2.4% decrease in BF% following 8 weeks of high-intensity FES training. | |
| Fornusek et al. [37] | Increase in thig girth following six weeks of low-cadence FES cycling | |
| Gorgey et al. [40] | Spacing the frequency of the sessions per week or limit the sessions to 2x per week may be effective strategy to reduce low frequency fatigue. | |
| Bochkezanian et al. [42] | Higher intensity resistance training produces strong muscle contractions and significant gains in muscle strength and muscle mass with improvement in reported measures of spasticity after SCI. | |
| van der Scheer et al. [49] | FES cycling has a moderate–high certainty for improvements in muscle health, referring to muscle mass, fiber-type composition. | |
| Houston et al. [57] | A closed-loop FES system was recently combined with visual feedback to improve standing balance. | |
| Vette et al. [63] | FES can facilitate completion of visual feedback balance training by targeting the dorsiflexor and plantar flexor muscles. | |
| Gauthier et al. [64] | The feasibility of incorporating FES into perturbation-based balance training to ensure retraining-reactive postural control. | |
| Less Invasive approaches | ||
| Gerasimenko et al. [69] | Stepping pattern in a gravity-eliminated position by using non-invasive transcutaneous electrical stimulation to the spinal cord. | |
| Benavides et al. [70] | TSS had an excitatory effect at the spinal level as measured by cervico-medullary-evoked potentials and an inhibitory effect at the cortical level as measured by motor-evoked potentials. | |
| Gad et al. [73] | After eight training sessions with TSS, combined with training over four weeks for 1–2 h per session, maximum voluntary handgrip forces increased by 325% in the presence of stimulation and 225% when grip strength was tested without stimulation in chronic tetraplegia. | |
| Gad et al. [74] | TSS may complement the work of the exoskeleton by providing motor drive and improve inter-limb coordination. The findings suggested that locomotor spinal networks can be neuromodulated with applications with non-invasive TSS. | |
| Invasive approaches | ||
| Enrico et al. [82] | Task-specific training could regain independent standing; however, starting locomotion training resulted in interfering with their standing capabilities. | |
| Harkema S et al. [77] Angeli et al. [78] | Participants were able to initiate, maintain, and terminate the electrically enabled leg activities after implantation. | |
| Wagner et al. [79] | Targeted epidural stimulation can restore walking in persons with SCI. | |
| Gorgey et al. [33] | Exoskeleton stepping or swing phase assistance decreased to 35% while the participant walked for at least 45 min per session with epidural stimulation. | |
| Galley et al. [87] | Epidural stimulation, when delivered to the upper or mid-thoracic spinal cord, can alleviate symptoms of peripheral vascular disease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolbow, D.R.; Gorgey, A.S.; Sutor, T.W.; Bochkezanian, V.; Musselman, K. Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury. J. Clin. Med. 2021, 10, 5356. https://doi.org/10.3390/jcm10225356
Dolbow DR, Gorgey AS, Sutor TW, Bochkezanian V, Musselman K. Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury. Journal of Clinical Medicine. 2021; 10(22):5356. https://doi.org/10.3390/jcm10225356
Chicago/Turabian StyleDolbow, David R., Ashraf S. Gorgey, Tommy W. Sutor, Vanesa Bochkezanian, and Kristin Musselman. 2021. "Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury" Journal of Clinical Medicine 10, no. 22: 5356. https://doi.org/10.3390/jcm10225356
APA StyleDolbow, D. R., Gorgey, A. S., Sutor, T. W., Bochkezanian, V., & Musselman, K. (2021). Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury. Journal of Clinical Medicine, 10(22), 5356. https://doi.org/10.3390/jcm10225356








