Abstract
This review reports on methods used to evaluate airway clearance techniques (ACT) in adults with CF and examined data for evidence of any effect. Sixty-eight studies described ACT in adequate detail and were included in this review. Frequently reported outcomes were sputum expectoration (72%) and spirometric lung function (60%). Compared with cough alone, following any ACT, there was a trend for greater sputum wet weight, however FEV1 was not different. The mean (95% CI) within-group effect for sputum wet weight following any ACT was 12.43 g (9.28 to 15.58) (n = 30 studies) and for FEV1 was 0.03 L (−0.17 to 0.24) (n = 14 studies). Meta-regression demonstrated that, when compared with cough alone, greater sputum wet weight was reported in groups that received additional ACT by between 2.45 and 3.94 g (F3,66 = 2.97, p = 0.04). These data suggest the addition of ACT to cough alone may optimise sputum clearance; however, FEV1 lacked sensitivity to detect this change. Importantly, this review highlights the lack of appropriate measures to assess ACT efficacy.
1. Introduction
Cystic fibrosis (CF) is a life-limiting inherited disease, with progressive respiratory impairment being the leading cause of morbidity and mortality [1,2,3]. Lung disease in CF is caused by excessive viscous airway secretions and chronic infection, which reduces mucociliary clearance leading to inflammation and destruction of airway walls [4]. Airway clearance techniques (ACT) are an important component of physiotherapy care for people with CF, facilitating mucociliary clearance and reducing sputum load in the lungs with the overall goal of reducing exacerbation frequency and slowing disease progression [5,6]. Airway clearance techniques are defined as “any technique which manipulates lung volumes, gas flow, ventilation, gravity, pulmonary pressures and/or compressive forces with the goal of shearing sputum along the airway lumen towards the mouth” [7]. Commonly used ACT can be grouped according to their mechanism of action, and classified as those which (i) deliver flow to create positive pressure within the airways (positive-pressure device (PPD); e.g., non-invasive ventilation [NIV]); (ii) use a device to create positive intrinsic pressure within the airways during expiration with or without oscillation (positive expiratory pressure device (PEP); e.g., PARI PEP, Aerobika, Acapella, Flutter); (iii) do not utilise positive pressure but require the person to manipulate their breathing pattern to change flow and/or volume beyond the third generation airways, which may be applied with or without external vibration/percussion of the chest wall (other techniques (OT)); or (iv) involve spontaneous/directed coughs performed in isolation from other ACT (cough alone (C)) [8]. Several objective outcomes have been used to evaluate the immediate effects of ACT as well as the medium- and long- term effects of these techniques. However, there is little agreement about which are to be used at each time interval post ACT. The primary outcome for respiratory function recommended by the European Medicines Agency [9] and the US Food and Drugs Administration [10] for clinical trials in people with CF is spirometry, in particular, forced expiratory volume in one second (FEV1). Other examples of objective outcomes used to evaluate the immediate effects of ACT include sputum expectoration (as either wet/dry weight or volume), inflammatory markers and rheology, measurements of lung volumes, lung mechanics, diffusion capacity and various imaging modalities. Despite the common use of ACT in this clinical population, no study has attempted to comprehensively synthesise the methods used to evaluate the immediate effects of ACT in adults with CF.
Therefore, the primary aim of this review was to synthesise data from studies that had applied ACT in adults with CF to determine (i) which ACT have been used in this clinical population and (ii) what outcomes have been used to evaluate the immediate effect of ACT. An exploratory aim was to determine in this same population whether there is any evidence that ACT, when compared to cough alone, results in a measurable change, using commonly used outcomes.
2. Materials and Methods
The review has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. A protocol was not published; however, it was registered with PROSPERO (CRD42020155843).
2.1. Study Criteria
To be included in this review, studies (of any design excluding reviews) needed to have applied ACT to adults (mean age ≥ 18 years) with CF and collected data on outcomes to evaluate the effect of ACT within 60 minutes of treatment completion. Neither nebulised mucolytics nor exercise alone were considered ACT. Studies were excluded if (i) participants within a group received different ACT and individual data were unable to be obtained (e.g., a group that continued with their usual ACT, which differed between participants); (ii) they were not written in English; or (iii) they were available only as abstracts.
2.2. Search Procedure
Studies were identified by searching the following databases from inception to August 2021: the Cochrane Library, CINAHL, Embase (via OVID), PEDro and PubMed. The search strategy used for PubMed can be found in Appendix A. This was adapted for use in other databases. Reference lists from included studies were also screened.
2.3. Screening
Studies identified in the database search were imported into Covidence [12] and screened independently based on their title and abstract by two review authors (NC and KW). Studies that were unable to be excluded based on title and abstract were screened by reading the full text. Any disagreement between the two review authors about inclusion of studies was resolved via discussion with a third review author (KH).
2.4. Data Extraction and Coding
Data were extracted by NC on authors, publication year, participant characteristics, sample size, study setting, and type and duration of any ACT. The ACT performed by each group within each study was coded according to its mechanism of action as positive-pressure device (PPD), positive expiratory pressure device (PEP), other techniques (OT) or cough alone (C). For groups that used an ACT that utilised more than one mechanism of action, the group was coded according to the most ‘active’ mechanism in the hierarchy of: C < OT < PEP < PPD (e.g., a study that applied the active cycle of breathing techniques (an OT) with concurrent non-invasive ventilation (PPD) was coded as PPD). Data were extracted on objective outcomes used to evaluate the effect of the ACT at a single time point closest to (but not extending beyond) 60 minutes following treatment completion. Data were not extracted on outcomes collected to monitor patient tolerance/safety (i.e., heart rate, blood pressure or oxygen saturation) or to report the flow characteristics of an ACT. For papers published after 2005, study authors were contacted in the case of missing, incomplete or ambiguous data. Numerical data were estimated from figures using the software Graph Grabber (Quintessa; 2.0.2, Oxfordshire, UK).
2.5. Risk of Bias
For randomised controlled trials (RCT) and randomised cross-over trials (RXT), risk of bias was assessed using the Revised Cochrane Risk of Bias tool for randomised trials (RoB 2) [13]. This tool assesses risk of bias across six domains and rates each domain and the overall study as being at low, high or unclear risk of bias. Data were presented using a data visualisation tool [14]. Risk of bias was assessed for RCT and RXT only as other study designs included are expected to have high or unclear risk of bias.
2.6. Data Analysis
For the primary aims, data on the type of ACT and the outcomes used to evaluate the immediate effect of the ACT were summarised as a narrative synthesis. Further information regarding data analyses of the primary outcomes can be found in the online supplement.
2.7. Exploratory Analysis
Where possible, studies that compared one type of ACT (i.e., PPD, PEP or OT) with C were included in a meta-analysis (RevMan Web, Cochrane; 5.1.2, Copenhagen, Denmark) [15] to derive the pooled between-group mean difference and 95% confidence interval (CI). A random-effects model was used unless there was no statistical heterogeneity (i.e., I2 = 0), in which case a fixed-effects model was chosen. Sensitivity analyses were conducted when I2 approached or exceeded substantial heterogeneity (i.e., 50%). Given that earlier work in this area has reported that there is significant heterogeneity in the types of ACT and outcomes utilised as well as limited data described in sufficient detail for meta-analysis [5,16,17,18], exploratory analyses were undertaken to determine whether the mechanism of action for the ACT moderated the magnitude of any within-group changes. To do this, where data were reported for the same outcome, in the same units of measurement, across ≥ four studies, pooled estimates of the within-group change were provided via a three-level meta-analysis (R package, Metafor; 4.0.3, Maastricht, The Netherlands) [19]. Meta-regression analysis was performed for sputum wet-weight, dry-weight and FEV1, as 10 or more studies reported these outcomes, in the same units of measurement [20], to determine whether the magnitude of within-group change was moderated by the mechanism of action of the ACT (i.e., PPD, PEP, OT or C) or clinical stability of the study sample (i.e., acute exacerbation, stable or mixed/no information). As these analyses included data from RXT, a three-level meta-regression was undertaken to account for dependency among effects within primary studies. For all analyses p < 0.05 was used to denote significance.
3. Results
3.1. Study Characteristics
A total of 2048 records were identified. The flow of studies has been summarised in Figure 1.
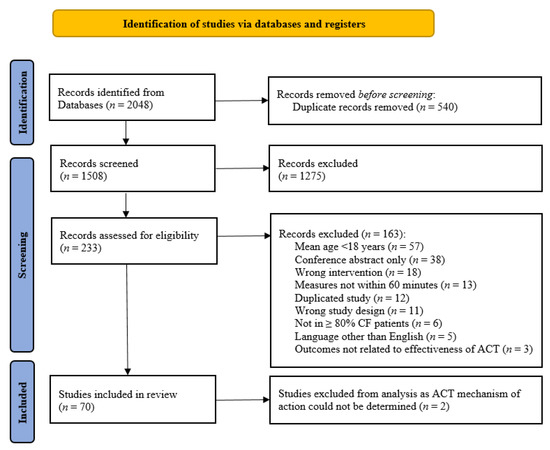
Figure 1.
PRISMA study flow diagram [21].
Of the 70 included studies, 3 (4%) were RCT, 57 (81%) were RXT and 10 (14%) were single-group interventional studies (Table 1). Thirty-five studies (50%) were published prior to year 2000. Data were available on 1204 participants (685 (57%) males; (mean ± SD) age ranged between 18 ± 4 and 36 ± 17 years; FEV1 ranged between 25 ± 6 and 76% predicted (SD not reported for the latter)).

Table 1.
Summary of studies included in systematic review.
The assessment of risk of bias for RCT and RXT are presented in Figure S1. Two of the three RCT were judged as having an unclear risk of bias and the third was judged as having a low risk of bias. For the RXT, 6 (11%) were judged as having low risk of bias, 31 (54%) were judged as having a high risk of bias and 20 (35%) were judged as having an unclear risk of bias. All RXT were judged as having a low risk of bias in regard to measurement of the outcome and all but one RXT were judged as having low risk of bias due to missing outcome data. The most common domains for high or unclear risk of bias in RXT were related to bias arising in the selection of the reported results (n = 49 studies; 86%), from the randomisation process (n = 39 studies; 68%) and from deviations from intended interventions (n = 28 studies; 59%).
3.2. Coding According to Mechanism of Action
The ACT were described in adequate detail in 68 (97%) studies (with a total of 150 intervention group’s coded accordingly). Nine (13%) studies utilised PPD, 29 (43%) utilised PEP, 51 (75%) utilised OT and 17 (25%) utilised C.
3.3. Description of Outcomes
Of the 68 studies that had their ACT coded, all reported data on at least one objective outcome within 60 min following completion of the ACT (Figure 2). Those most frequently reported measures were the amount of sputum expectorated, expressed as weight or volume (49 (72%) studies) and spirometry, in particular FEV1 (41 (60%) studies).
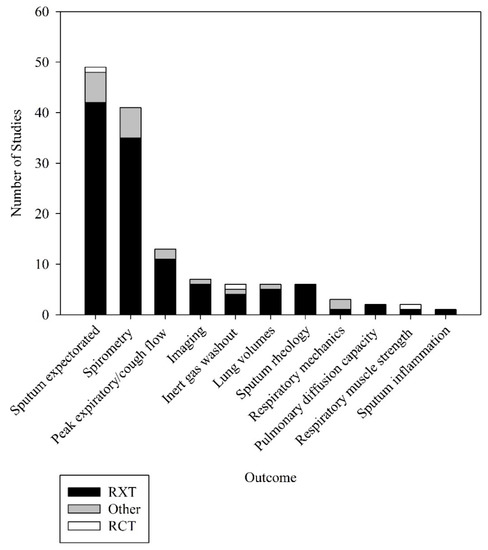
Figure 2.
Objective outcomes used to measure effectiveness of airway clearance techniques. Other = other study designs apart from RXT and RCT; RCT = randomised controlled trials; RXT = randomised crossover trials.
3.4. Magnitude of Between-Group Change
There were sufficient data from RCT and RXT to conduct a meta-analysis to estimate the effect of any ACT on the outcomes of sputum wet weight, dry weight and FEV1. These data are presented in the online supplement (Figures S2–S4). There was considerable heterogeneity across studies in regard to measures reported and types of ACT conducted, which limited data that were able to be pooled across studies. The pooled data demonstrated no between-group difference in any of the comparisons for sputum wet weight, dry weight or FEV1 (p > 0.05 for all).
3.5. Magnitude of Within-Group Change
3.5.1. Sputum Expectoration
Of the 49 studies that reported the amount of sputum expectorated following ACT, 32 (65%) had data that could be used in the meta-analyses. The pooled estimate (95% CI) for sputum wet weight, dry weight and volume were 12.43 g (9.28 to 15.58) (70 groups, 30 studies, n = 487; Figure 3a), 0.42 g (0.19 to 0.66) (28 groups, 11 studies, n = 201; Figure 3b), and 8.01 mL (2.18 to 13.84) (9 groups, 4 studies, n = 79; Figure 3c), respectively.
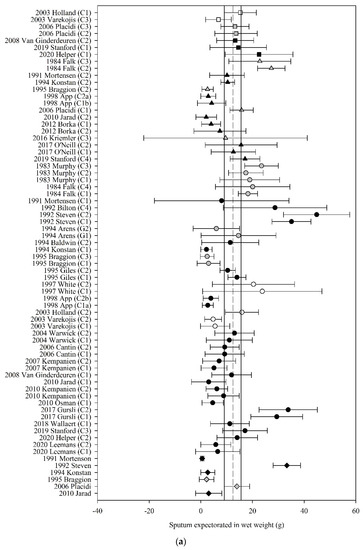
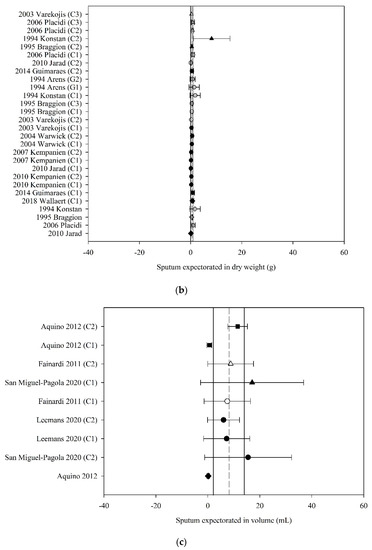
Figure 3.
Pooled estimates–sputum expectoration: (a) sputum wet weight (grams); (b) sputum dry weight (grams); (c) sputum volume (millilitres). C_ = crossover intervention, G_ = RCT group, g = grams, ■ = positive pressure device (PPD), ▲ = positive expiratory pressure device (PEP), ● = other techniques (OT), ♦ = cough alone (C), closed symbol = stable population, open symbol = acute population, grey symbol = mixed population or nil info, dashed line = pooled estimate, solid vertical line = 95% CI, all individual data presented as mean ± SD.
Meta-regression revealed that the effect of ACT on sputum wet weight was moderated by the mechanism of action of ACT (F3,66 = 2.97, p = 0.04) such that those ACT that were grouped as PPD, PEP and OT produced a higher amount of wet weight sputum compared with C alone by 2.45 to 3.94 g; mean (95% CI) PPD 12.75 g (8.61 to 16.88); PEP 13.76 g (10.16 to 17.36); OT 12.27 g (9.02 to 15.52); C 9.82 g (6.00 to 13.65). The estimate of the effect of ACT on sputum wet weight was not moderated by the clinical stability of the study participants (F3,66 = 0.11, p = 0.96). The estimate of the effect of ACT on sputum dry weight was neither moderated by the mechanism of action of the ACT (F3,24 = 0.11, p = 0.95) nor by the clinical stability of the participants (F2,25 = 0.65, p = 0.53). Meta-regression could not be undertaken for sputum volume due to inadequate study numbers.
3.5.2. Spirometric Measures of Lung Function
Of the 41 studies that reported FEV1 before and after ACT, 22 (53%) had data that could be used in the meta-analyses. The pooled estimate (95% CI) for change in FEV1 following ACT, expressed in litres, was 0.03 L (−0.17 to 0.24) (30 groups, 14 studies, n = 218; Figure 4a) and expressed as percent predicted, was 1.44% (0.06 to 2.83) (19 groups, 11 studies, n = 172; Figure 4b).
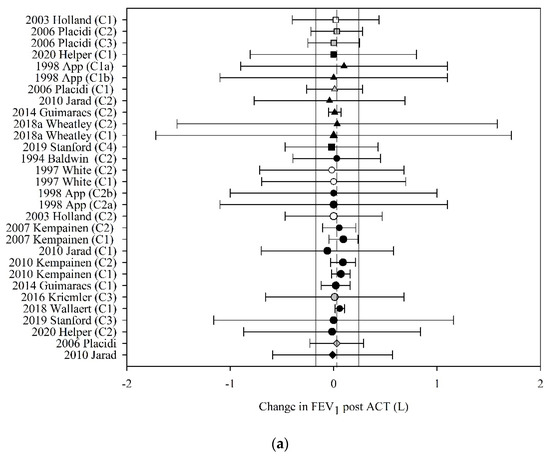
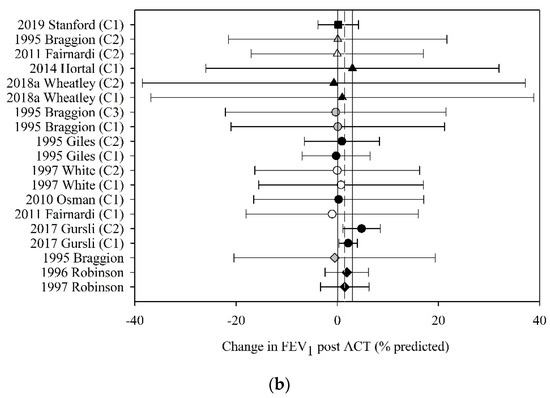
Figure 4.
Pooled estimate-FEV1: (a) FEV1 (litres); (b) FEV1 (% predicted). ACT = airway clearance techniques, C_ = crossover intervention, L = litres, ■ = positive pressure device (PPD), % predicted = percent predicted, ▲ = positive expiratory pressure device (PEP), ● = other techniques (OT), ♦ = cough alone (C), closed symbol = stable population, open symbol = acute population, grey symbol = mixed population or nil info, dashed line = pooled estimate, solid vertical line = 95% CI, all individual data presented as mean ± SD.
Meta-regression revealed that the effect of ACT on FEV1, expressed in litres and percent predicted, was neither moderated by the mechanism of action of the ACT (F3,26 = 0.01, p = 1.00; F3,15 = 0.21, p = 0.89, respectively) nor by the clinical stability of the study participants (F4,25 < 0.01, p = 1.00; F3,62 = 0.10, p = 0.96, respectively).
3.5.3. Static Lung Volumes
Of the six studies that reported residual volume (RV) before and after ACT, four (57%) had data that could be used in the meta-analyses. The pooled estimate (95% CI) for change in RV following ACT, expressed in litres, was −0.14 L (−0.72 to 0.44) (eight groups, four studies, n = 67; Figure 5). Meta-regression could not be undertaken for RV due to inadequate study numbers.
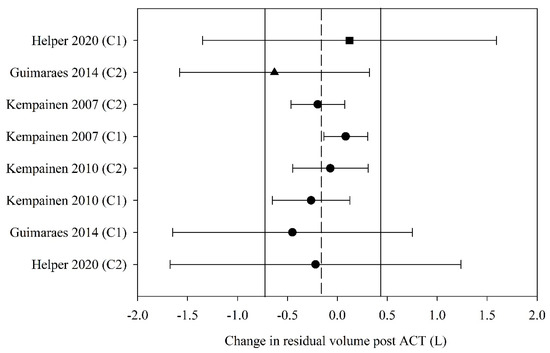
Figure 5.
Pooled estimate–residual volume (litres). ACT = airway clearance techniques, C_ = crossover intervention, L = litres, ■ = positive pressure device (PPD), ▲ = positive expiratory pressure device (PEP), ● = other techniques (OT), closed symbol = stable population, dashed line = pooled estimate, solid vertical line = 95% CI, all individual data presented as mean ± SD.
3.5.4. Other Outcomes
Of the six studies that reported measures of sputum rheology and one study that reported measures of sputum inflammatory biomarkers before and after ACT, none had data that could be used in the meta-analyses. Thirteen studies reported peak expiratory/cough flow, six reported measures using inert gas washout, three reported measures of respiratory mechanics, two reported measures of pulmonary diffusion capacity, two reported measures of respiratory muscle strength and seven reported measures quantified through lung imaging before and after ACT. None had data that could be used in the meta-analyses.
4. Discussion
This review synthesised data from studies that were conducted in adults with CF and had evaluated the effects of any ACT within 60 minutes of treatment completion. The main findings were that in adults with CF (i) there was large variability in ACT applied and outcomes used to evaluate their effect; (ii) robust data from randomised trials were lacking which precluded useful meta-analyses of between-group differences; (iii) the magnitude of the immediate within-group change of ACT on sputum wet weight was moderated by the mechanism of action of the ACT such that PPD, PEP and OT appeared to generate more sputum than cough alone; and (iv) FEV1 and RV do not appear to be responsive to change immediately following ACT.
Studies exploring the immediate effects of ACT in adults with CF were heterogeneous in terms of both the types of ACT and outcomes used to evaluate their effect. This finding corroborates those of previous systematic reviews that have sought to explore the effect of ACT in CF [5,16,17,91,92,93,94], bronchiectasis [95] and chronic obstructive pulmonary disease (COPD) [96]. The dates of publication presented in Table 1 suggest that ACT have evolved over the years, with a move from passive techniques such as postural drainage and percussion/vibration (commonly published in the 1970s, 1980s and early 1990s) to techniques such as PEP, oscillating PEP and autogenic drainage (commonly published in the mid to late 1990 s and 2000 s). There were two reasons for this. First, evidence emerged that in people with chronic lung disease, techniques such as postural drainage and percussion may lead to deleterious effects such as bronchospasm [97], desaturation [98] and gastro-oesophageal reflux [99]. Second, in contrast with percussion/vibration, the use of techniques such as PEP, oscillating PEP and autogenic drainage encourage people with CF to be more independent with their airway clearance, aligning with the principles of chronic disease self-management [93]. Despite being able to identify this trend in the type of ACT that were used, the exact nature of the ACT used were often described in insufficient detail that would allow replication. This reflects the fact that approximately 73% of the studies included in this review were published prior to 2014, when the importance of reporting intervention protocols and implementation fidelity was specifically articulated by the Template for Intervention Description and Replication (TIDieR) checklist [100].
Similar to earlier reviews of ACT in CF that have been published between 2015 and 2020 [16,17,91,92,93,94], there was limited capacity to complete a meta-analysis of between-group differences. The only previous review that completed a meta-analysis of studies in regard to sputum wet and dry weight pooled data across four studies and included data from two groups from within one crossover study (one RCT and three RXT). This earlier analysis reported no differences in wet or dry sputum weight when oscillating devices were compared to “conventional physiotherapy”, which comprised different groupings of postural drainage, percussion, vibration and clapping [16]. While the present study did not demonstrate that ACT produced more sputum when compared with cough alone, the mean summary effect statistic for all meta-analyses favoured the ACT group. Statistical heterogeneity was found in the meta-analyses of between-group changes in sputum wet weight when comparing PEP versus C and OT versus C. Due to this, a sensitivity analyses was run to reduce the statistical heterogeneity (I2 reduced to 0%), yet this did not change the finding of no between-group differences in sputum wet weight for the same comparisons. It is unlikely that robust RCT will become available to improve our precision around the estimate of this effect. This is because the strong physiological basis for these techniques, coupled with data from laboratory and animal studies [101,102], and enduring anecdotal evidence has resulted in acceptance by the clinical community that ACT are effective in people with CF. In fact, routine use of ACT in the management of adults with CF is recommended as a standard of care in national and international guidelines [6,103,104] and is likely to explain why most studies investigating ACT are RXT. Given the lack of data to support one ACT over all others, in clinical practice, ACT continue to be selected based on therapist and patient preference, the specific needs of the patient and accessibility of devices [16].
As future RCT of ACT in adults with CF are unlikely, a novel analysis was instead undertaken to explore possible moderators of within-group differences in the most commonly reported outcomes. The pooled estimate for sputum wet weight was 12.42 g [9.28 to 15.58]. The magnitude of this effect is difficult to interpret, as the minimal clinically important difference (MCID) for this outcome, in this population, has not been determined. Nevertheless, an important finding of this study was that compared with cough alone, greater sputum wet weight was reported by studies which applied any ACT. There are two important caveats to consider when interpreting this result. First, studies in this review were generally of low quality and at moderate-to-high risk of bias. Second, although sputum wet weight is a commonly used outcome that is easily interpretable by clinicians, its use as an outcome has been criticised. This is due to the estimate of wet weight sputum being potentially confounded by expectorated saliva and the use of muco-active medications (e.g., hypertonic saline). Nevertheless, these analyses suggest that ACT, when considered together, appear to offer additional benefit in terms of the amount of sputum expectorated, over and above cough alone.
Finally, this study suggests that any immediate (i.e., less than 60 min) effect of ACT on FEV1 and RV is likely to be negligible. The MCID for FEV1 has been difficult to define in the CF population due to disease heterogeneity and age-related cofounders as a result of the growing lung; MCID values ranging from a 5–10% improvement have been proposed [105], which is greater than the change reported in this study. Our pooled estimate for change in FEV1 of 0.03 L falls short of the lower limit of the range of MCID for FEV1 of 0.10 L proposed for the management of other obstructive diseases including COPD [106]. This supports earlier work that has questioned the appropriateness of using FEV1 in studies evaluating ACT in adults with CF [105,107,108,109,110]. Despite these concerns, as mentioned previously FEV1 is the only measure of respiratory function that is recommended as an outcome by the European Medicines Agency [9] and the US Food and Drugs Administration [10] for clinical trials in people with CF. It appears that changes in FEV1 are not likely following ACT, irrespective of the type of ACT applied or the clinical stability of the study population. Similarly, the pooled estimate for change in RV of −0.14 L estimated in this study falls well below the MCID proposed for emphysema management of between −0.31 and −0.43 L [111]. One possible alternative outcome which has shown promise in measuring CF related lung disease and identifying treatment effects in patients with CF is lung clearance index measured via multiple breath washout (MBW) [105]; however, further evidence is needed before this outcome can be utilised as an adjunct or surrogate measure to FEV1.
Strengths, Limitations and Future Directions
Compared with earlier reviews in this area, the current study (i) extracted data across the largest number of studies (n = 68), (ii) described the variety in ACT and outcomes used, (iii) completed analyses of both between and within-group differences in sputum wet weight and FEV1 and (iv) completed meta-regression on within-group differences in sputum volume, dry weight and RV. A limitation of this study is the exclusion of studies published in any language other than English. Further, techniques such as postural drainage, percussion, autogenic drainage and external chest wall oscillation devices were grouped as OT; PEP and oscillatory PEP were grouped together. We accept that the mechanisms of action for these techniques are likely distinct, however, given the high risk of bias and serious inconsistency, indirectness and imprecision across the included studies, a pragmatic approach of grouping these techniques was chosen to ensure sufficient studies were included in the meta-regression. Although this choice of coding has not allowed us to look at the individual effects of ACT, it allowed us to look at ACT as a whole compared to cough alone. It is clear that future studies in this area need to report the intervention (and the implementation fidelity) according to the TIDieR guidelines [100].
5. Conclusions
The most common outcomes used to measure the effectiveness of ACT are not sensitive to the effects of ACT. More work is needed to explore alternative measures of respiratory function that can be used in RXT to compare the immediate effects of different ACT in adults with CF.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10225280/s1, Figure S1: Risk of Bias analyses; Figure S2: Between-group differences sputum wet weight; Figure S3: Between-group differences sputum dry weight, Figure S4: Between-group differences FEV1.
Author Contributions
N.C., V.C., J.W. and K.H. were responsible for the study conceptualisation and overall methodology. N.C., K.W., T.H. and K.H. were responsible for article selection. N.C. was responsible for data curation. D.F.G. was responsible for providing resources, software and completing formal analysis. N.C., V.C., D.F.G., E.F.S. and K.H. were involved in the interpretation of data. N.C. drafted the manuscript. N.C., V.C., J.W., D.F.G., E.F.S. and K.H. were involved in the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. However, NC is supported by a Conquer Cystic Fibrosis PhD Scholarship co-funded by the Institute for Respiratory Health and Curtin University and a CFWA Post Graduate Studentship Top Up Scholarship from Cystic Fibrosis Australia. VC is supported by a Cancer Council Western Australia Postdoctoral Fellowship. ES is supported by National Health and Medical Research Council (NHMRC) funding. KH, NC, VC and JW received the 2018 Glenn Brown Memorial Grant from the Institute for Respiratory Health.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to it being a review article.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
ACT: airway clearance techniques, C: cough alone, COPD: chronic obstructive pulmonary disease, MCID: minimally clinical important differences, FEV1: forced expiratory volume in one second, MBW: multiple breath washout, NIV: non-invasive ventilation, OT: other techniques, PEP: positive expiratory pressure device, PPD: positive-pressure device, RCT: randomised controlled trials, RV: residual volume, RXT: randomised cross-over trials, TIDieR: Template for Intervention Description and Replication.
Appendix A. Example Search Strategy for PubMed
((((((((((((((((((((((((((((((Mucociliary clearance[MeSH Terms]) OR airway clearance[Title/Abstract]) OR lung clearance[Title/Abstract]) OR chest physiotherapy[Title/Abstract]) OR chest physical therapy[Title/Abstract]) OR breathing exercises[MeSH Terms]) OR respiratory therapy[MeSH Terms]) OR flutter[Title/Abstract]) OR shak*[Title/Abstract]) OR acapella[Title/Abstract]) OR aerobika[Title/Abstract]) OR positive expiratory pressure[Title/Abstract]) OR oscillat*[Title/Abstract]) OR huff*[Title/Abstract]) OR cough[MeSH Terms]) OR percussion[MeSH Terms]) OR vibration[MeSH Terms]) OR forced expirat* AND technique[Title/Abstract]) OR postural drainage, pulmonary[MeSH Terms]) OR chest wall oscillation[MeSH Terms]) OR high frequency chest wall oscillation[MeSH Terms]) OR insufflat*[Title/Abstract]) OR exsufflat*[Title/Abstract])) OR autogenic drainage[Title/Abstract])) OR position*[Title/Abstract]) OR intrapulmonary percussive ventilation[Title/Abstract])) AND ((cystic fibrosis, pulmonary[MeSH Terms]) OR mucoviscidosis[Title/Abstract])) AND Humans[Mesh] AND English[lang] AND adult[MeSH].
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Rafeeq, M.M.; Murad, H.A.S. Cystic fibrosis: Current therapeutic targets and future approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Ruseckaite, R.; Ahern, S.; Ranger, T.; Tacey, M.; Dean, J.; Gardam, M.; Bell, S.; Burke, N. The Australian Cystic Fibrosis Data Registry Annual Report, 2016; Monash University, Department of Epidemiology and Preventive Medicine: Melbourne, Australia, 2018. [Google Scholar]
- Cohen-Cymberknoh, M.; Shoseyov, D.; Kerem, E. Managing cystic fibrosis: Strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit. Care Med. 2011, 183, 1463–1471. [Google Scholar] [CrossRef]
- Wilson, L.M.; Morrison, L.; Robinson, K.A. Airway clearance techniques for cystic fibrosis: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2019, 1, CD011231. [Google Scholar] [CrossRef]
- Button, B.M.; Wilson, C.; Dentice, R.; Cox, N.S.; Middleton, A.; Tannenbaum, E.; Bishop, J.; Cobb, R.; Burton, K.; Wood, M.; et al. Physiotherapy for cystic fibrosis in Australia and New Zealand: A clinical practice guideline. Respirology 2016, 21, 656–667. [Google Scholar] [CrossRef]
- Osadnik, C.R.; McDonald, C.F.; Holland, A.E. Airway clearance techniques in acute exacerbations of COPD: A survey of Australian physiotherapy practice. Physiotherapy 2013, 99, 101–106. [Google Scholar] [CrossRef]
- Ward, N.; Stiller, K.; Holland, A.E. Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: A survey. J. Physiother. 2019, 65, 43–50. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Clinical Development of Medicinal Products for the Treatment of Cystic Fibrosis; European Medicines Agency: London, UK, 2009. [Google Scholar]
- U.S. Food and Drug Administration. Table of Surrogate Endpoints That Were the Basis of Drug Approval or Licensure. Available online: https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure (accessed on 1 June 2021).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia; Available online: https://www.covidence.org/home (accessed on 20 October 2019).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Review Manager Web (RevMan Web). Version 3.1.2. 2021. Available online: https://revman.cochrane.org/#/myReviews (accessed on 3 March 2021).
- Morrison, L.; Milroy, S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 2020, 4, CD006842. [Google Scholar] [CrossRef]
- Warnock, L.; Gates, A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst. Rev. 2015, 12, CD001401. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Moran, F.M.; Stuart Elborn, J. Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: An overview of five Cochrane systematic reviews. Respir. Med. 2006, 100, 191–201. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 48. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Cochrane: London, UK, 2021. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- App, E.M.; Kieselmann, R.; Reinhardt, D.; Lindemann, H.; Dasgupta, B.; King, M.; Brand, P. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: Flutter vs. autogenic drainage. Chest 1998, 114, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Aquino, E.S.; Shimura, F.; Santos, A.S.; Goto, D.M.; Coelho, C.C.; de Fuccio, M.B.; Saldiva, P.H.; Lorenzi-Filho, G.; Rubin, B.K.; Nakagawa, N.K. CPAP has no effect on clearance, sputum properties, or expectorated volume in cystic fibrosis. Respir. Care 2012, 57, 1914–1919. [Google Scholar] [CrossRef]
- Arens, R.; Gozal, D.; Omlin, K.J.; Vega, J.; Boyd, K.P.; Keens, T.G.; Woo, M.S. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1994, 150, 1154–1157. [Google Scholar] [CrossRef]
- Baldwin, D.R.; Hill, A.L.; Peckham, D.G.; Knox, A.J. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respir. Med. 1994, 88, 49–53. [Google Scholar] [CrossRef][Green Version]
- Bilton, D.; Dodd, M.E.; Abbot, J.V.; Webb, A.K. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respir. Med. 1992, 86, 507–511. [Google Scholar] [CrossRef]
- Bishop, J.R.; Erskine, O.J.; Middleton, P.G. Timing of dornase alpha inhalation does not affect the efficacy of an airway clearance regimen in adults with cystic fibrosis: A randomised crossover trial. J. Physiother. 2011, 57, 223–229. [Google Scholar] [CrossRef]
- Borka, P.; Gyurkovits, K.; Bodis, J. Comparative study of PEP mask and Flutter on expectoration in cystic fibrosis patients. Acta Physiol. Hung. 2012, 99, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Braggion, C.; Cappelletti, L.M.; Cornacchia, M.; Zanolla, L.; Mastella, G. Short-term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: A cross-over randomized study. Pediatr. Pulmonol. 1995, 19, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Bacon, M.; Berthiaume, Y. Mechanical airway clearance using the Frequencer electro-acoustical transducer in cystic fibrosis. Clin. Investig. Med. 2006, 29, 159–165. [Google Scholar]
- Carr, L.; Pryor, J.A.; Hodson, M.E. Self chest clapping Patients’ views and the effects on oxygen saturation. Physiotherapy 1995, 81, 753–757. [Google Scholar] [CrossRef]
- Chatham, K.; Ionescu, A.A.; Nixon, L.S.; Shale, D.J. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. Eur. Respir. J. 2004, 23, 435–439. [Google Scholar] [CrossRef]
- Darbee, J.C.; Ohtake, P.J.; Grant, B.J.; Cerny, F.J. Physiologic evidence for the efficacy of positive expiratory pressure as an airway clearance technique in patients with cystic fibrosis. Phys. Ther. 2004, 84, 524–537. [Google Scholar] [CrossRef]
- DeCesare, J.A.; Babchyck, B.M.; Colten, H.R.; Treves, S. Radionuclide assessment of the effects of chest physical therapy on ventilation in cystic fibrosis. Phys. Ther. 1982, 62, 820–827. [Google Scholar] [CrossRef]
- Dwyer, T.J.; Alison, J.A.; McKeough, Z.J.; Daviskas, E.; Bye, P.T.; Dwyer, T.J.; Alison, J.A.; McKeough, Z.J.; Daviskas, E.; Bye, P.T.P. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011, 139, 870–877. [Google Scholar] [CrossRef]
- Dwyer, T.J.; Robbins, L.; Kelly, P.; Piper, A.J.; Bell, S.C.; Bye, P.T. Non-invasive ventilation used as an adjunct to airway clearance treatments improves lung function during an acute exacerbation of cystic fibrosis: A randomised trial. J. Physiother. 2015, 61, 142–147. [Google Scholar] [CrossRef]
- Dwyer, T.J.; Zainuldin, R.; Daviskas, E.; Bye, P.T.; Alison, J.A. Effects of treadmill exercise versus Flutter(R) on respiratory flow and sputum properties in adults with cystic fibrosis: A randomised, controlled, cross-over trial. BMC Pulm. Med. 2017, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.J.; Daviskas, E.; Zainuldin, R.; Verschuer, J.; Eberl, S.; Bye, P.T.P.; Alison, J.A. Effects of exercise and airway clearance (positive expiratory pressure) on mucus clearance in cystic fibrosis: A randomised crossover trial. Eur. Resp. J. 2019, 53, 1801793. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Longo, F.; Faverzani, S.; Tripodi, M.C.; Chetta, A.; Pisi, G. Short-term effects of high-frequency chest compression and positive expiratory pressure in patients with cystic fibrosis. J. Clin. Med. Res. 2011, 3, 279–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falk, M.; Kelstrup, M.; Andersen, J.B.; Kinoshita, T.; Falk, P.; Stovring, S.; Gothgen, I. Improving the ketchup bottle method with positive expiratory pressure, PEP, in cystic fibrosis. Eur. J. Respir. Dis. 1984, 65, 423–432. [Google Scholar]
- Giles, D.R.; Wagener, J.S.; Accurso, F.J.; Butler-Simon, N. Short-term effects of postural drainage with clapping vs. autogenic drainage on oxygen saturation and sputum recovery in patients with cystic fibrosis. Chest 1995, 108, 952–954. [Google Scholar] [CrossRef][Green Version]
- Grosse-Onnebrink, J.; Mellies, U.; Olivier, M.; Werner, C.; Stehling, F. Chest physiotherapy can affect the lung clearance index in cystic fibrosis patients. Pediatr. Pulmonol. 2017, 52, 625–631. [Google Scholar] [CrossRef]
- Guimaraes, F.S.; Lopes, A.J.; Moco, V.J.R.; de Souza, F.C.; de Menezes, S.L.S. Eltgol acutely improves airway clearance and reduces static pulmonary volumes in adult cystic fibrosis patients. J. Phys. Ther. Sci. 2014, 26, 813–816. [Google Scholar] [CrossRef][Green Version]
- Gursli, S.; Sandvik, L.; Bakkeheim, E.; Skrede, B.; Stuge, B. Evaluation of a novel technique in airway clearance therapy—Specific cough technique (SCT) in cystic fibrosis: A pilot study of a series of N-of-1 randomised controlled trials. SAGE Open Med. 2017, 5, 2050312117697505. [Google Scholar] [CrossRef]
- Helper, N.; Kodesh, E.; Sokol, G.; Hakimi, R.; Vilozni, D.; Efrati, O. The benefits of mechanical insufflator-exsufflator compared to autogenic drainage in adults with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 3046–3052. [Google Scholar] [CrossRef]
- Hofmeyr, J.L.; Webber, B.A.; Hodson, M.E. Evaluation of positive expiratory pressure as an adjunct to chest physiotherapy in the treatment of cystic fibrosis. Thorax 1986, 41, 951–954. [Google Scholar] [CrossRef]
- Holland, A.E.; Denehy, L.; Ntoumenopoulos, G.; Naughton, M.T.; Wilson, J.W. Non-invasive ventilation assists chest physiotherapy in adults with acute exacerbations of cystic fibrosis. Thorax 2003, 58, 880–884. [Google Scholar] [CrossRef]
- Hordvik, N.L.; Sammut, P.H.; Judy, C.G.; Strizek, S.J.; Colombo, J.L. The effects of albuterol on the lung function of hospitalized patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 156–160. [Google Scholar] [CrossRef]
- Hortal, M.C.R.; Hjelte, L. Time point to perform lung function tests evaluating the effects of an airway clearance therapy session in cystic fibrosis. Respir. Care 2014, 59, 1537–1541. [Google Scholar] [CrossRef]
- Jarad, N.A.; Powell, T.; Smith, E. Evaluation of a novel sputum clearance technique-hydro-acoustic therapy (HAT) in adult patients with cystic fibrosis: A feasibility study. Chronic Respir. Dis. 2010, 7, 217–227. [Google Scholar] [CrossRef]
- Kempainen, R.R.; Williams, C.B.; Hazelwood, A.; Rubin, B.K.; Milla, C.E. Comparison of high-frequency chest wall oscillation with differing waveforms for airway clearance in cystic fibrosis. Chest 2007, 132, 1227–1232. [Google Scholar] [CrossRef]
- Kempainen, R.R.; Milla, C.; Dunitz, J.; Savik, K.; Hazelwood, A.; Williams, C.; Rubin, B.K.; Billings, J.L. Comparison of settings used for high-frequency chest-wall compression in cystic fibrosis. Respir. Care 2010, 55, 695–701. [Google Scholar]
- Konstan, M.W.; Stern, R.C.; Doershuk, C.F. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J. Pediatr. 1994, 124, 689–693. [Google Scholar] [CrossRef]
- Kriemler, S.; Radtke, T.; Christen, G.; Kerstan-Huber, M.; Hebestreit, H. Short-Term Effect of Different Physical Exercises and Physiotherapy Combinations on Sputum Expectoration, Oxygen Saturation, and Lung Function in Young Patients with Cystic Fibrosis. Lung 2016, 194, 659–664. [Google Scholar] [CrossRef]
- Lannefors, L.; Wollmer, P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: A comparison between postural drainage, PEP and physical exercise. Eur. Respir. J. 1992, 5, 748–753. [Google Scholar]
- Leemans, G.; Belmans, D.; Van Holsbeke, C.; Becker, B.; Vissers, D.; Ides, K.; Verhulst, S.; Van Hoorenbeeck, K. The effectiveness of a mobile high-frequency chest wall oscillation (HFCWO) device for airway clearance. Pediatr. Pulmonol. 2020, 55, 1984–1992. [Google Scholar] [CrossRef]
- Lyons, E.; Chatham, K.; Campbell, I.A.; Prescott, R.J. Evaluation of the flutter VRP1 device in young adults with cystic fibrosis. Med. Sci. Res. 1993, 21, 101–102. [Google Scholar]
- McCarren, B.; Alison, J.A. Physiological effects of vibration in subjects with cystic fibrosis. Eur. Respir. J. 2006, 27, 1204–1209. [Google Scholar] [CrossRef]
- Mentore, K.; Froh, D.K.; de Lange, E.E.; Brookeman, J.R.; Paget-Brown, A.O.; Altes, T.A. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: Assessment at baseline and after bronchodilator and airway clearance treatment. Acad. Radiol. 2005, 12, 1423–1429. [Google Scholar] [CrossRef]
- Milne, S.M.; Eales, C.J. A pilot study comparing two physiotherapy techniques in patients with cystic fibrosis. S. Afr. J. Physiother. 2004, 60, 3. [Google Scholar] [CrossRef][Green Version]
- Mortensen, J.; Falk, M.; Groth, S.; Jensen, C. The effects of postural drainage and positive expiratory pressure physiotherapy on tracheobronchial clearance in cystic fibrosis. Chest 1991, 100, 1350–1357. [Google Scholar] [CrossRef][Green Version]
- Murphy, M.B.; Concannon, D.; FitzGerald, M.X. Chest percussion: Help or hindrance to postural drainage? Ir. Med. J. 1983, 76, 189–190. [Google Scholar]
- O’Neill, K.; Moran, F.; Tunney, M.M.; Elborn, J.S.; Bradbury, I.; Downey, D.G.; Rendall, J.; Bradley, J.M. Timing of hypertonic saline and airway clearance techniques in adults with cystic fibrosis during pulmonary exacerbation: Pilot data from a randomised crossover study. BMJ Open Respir. Res. 2017, 4, e000168. [Google Scholar] [CrossRef]
- Osman, L.P.; Roughton, M.; Hodson, M.E.; Pryor, J.A. Short-term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010, 65, 196–200. [Google Scholar] [CrossRef]
- Pfleger, A.; Steinbacher, M.; Schwantzer, G.; Weinhandl, E.; Wagner, M.; Eber, E. Short-term effects of physiotherapy on ventilation inhomogeneity in cystic fibrosis patients with a wide range of lung disease severity. J. Cyst. Fibros. 2015, 14, 627–631. [Google Scholar] [CrossRef][Green Version]
- Placidi, G.; Cornacchia, M.; Polese, G.; Zanolla, L.; Assael, B.M.; Braggion, C. Chest physiotherapy with positive airway pressure: A pilot study of short-term effects on sputum clearance in patients with cystic fibrosis and severe airway obstruction. Respir. Care 2006, 51, 1145–1153. [Google Scholar]
- Pryor, J.A.; Webber, B.A.; Hodson, M.E.; Batten, J.C. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. Br. Med. J. 1979, 2, 417–418. [Google Scholar] [CrossRef]
- Pryor, J.A.; Webber, B.A. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979, 65, 304–307. [Google Scholar]
- Pryor, J.A.; Parker, R.A.; Webber, B.A. A comparison of mechanical and manual percussion as adjuncts to postural drainage in the treatment of cystic fibrosis in adolescents and adults. Physiotherapy 1981, 67, 140–141. [Google Scholar]
- Pryor, J.A.; Webber, B.A.; Hodson, M.E. Effect of chest physiotherapy on oxygen saturation in patients with cystic fibrosis. Thorax 1990, 45, 77. [Google Scholar] [CrossRef][Green Version]
- Pryor, J.A.; Webber, B.A.; Hodson, M.E.; Warner, J.O. The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. Respir. Med. 1994, 88, 677–681. [Google Scholar] [CrossRef]
- Radtke, T.; Boeni, L.; Bohnacker, P.; Maggi-Bebba, M.; Fischer, P.; Kriemler, S.; Benden, C.; Dressel, H. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: A randomized, controlled, crossover trial. Eur. Respir. J. 2018, 52, PA3418. [Google Scholar] [CrossRef]
- Robinson, M.; Regnis, J.A.; Bailey, D.L.; King, M.; Bautovich, G.J.; Bye, P.T. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 153, 1503–1509. [Google Scholar] [CrossRef]
- Robinson, M.; Hemming, A.L.; Regnis, J.A.; Wong, A.G.; Bailey, D.L.; Bautovich, G.J.; King, M.; Bye, P.T. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997, 52, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Rossman, C.M.; Waldes, R.; Sampson, D.; Newhouse, M.T. Effect of chest physiotherapy on the removal of mucus in patients with cystic fibrosis. Am. Rev. Respir. Dis. 1982, 126, 131–135. [Google Scholar] [CrossRef]
- San Miguel-Pagola, M.; Reychler, G.; Cebria, I.; Gomez-Romero, M.; Diaz-Gutierrez, F.; Herrero-Cortina, B. Impact of hypertonic saline nebulisation combined with oscillatory positive expiratory pressure on sputum expectoration and related symptoms in cystic fibrosis: A randomised crossover trial [with consumer summary. Physiotherapy 2020, 107, 243–251. [Google Scholar] [CrossRef]
- Scherer, T.A.; Barandun, J.; Martinez, E.; Wanner, A.; Rubin, E.M. Effect of high-frequency oral airway and chest wall oscillation and conventional chest physical therapy on expectoration in patients with stable cystic fibrosis. Chest 1998, 113, 1019–1027. [Google Scholar] [CrossRef]
- Sokol, G.; Vilozni, D.; Hakimi, R.; Lavie, M.; Sarouk, I.; Bar, B.E.; Dagan, A.; Ofek, M.; Efrati, O. The short-term effect of breathing tasks via an incentive spirometer on lung function compared with autogenic drainage in subjects with cystic fibrosis. Respir. Care 2015, 60, 1819–1825. [Google Scholar] [CrossRef]
- Stanford, G.; Parrott, H.; Bilton, D.; Agent, P.; Banya, W.; Simmonds, N. Randomised cross-over trial evaluating the short-term effects of non-invasive ventilation as an adjunct to airway clearance techniques in adults with cystic fibrosis. BMJ Open Respir. Res. 2019, 6, e000399. [Google Scholar] [CrossRef]
- Steven, M.H.; Pryor, J.A.; Webber, B.A.; Hodson, M.R. Physiotherapy versus cough alone in the treatment of cystic fibrosis. N. Z. J. Physiother. 1992, 20, 31–37. [Google Scholar]
- Van Ginderdeuren, F.; Verbanck, S.; Van Cauwelaert, K.; Vanlaethem, S.; Schuermans, D.; Vincken, W.; Malfroot, A. Chest physiotherapy in cystic fibrosis: Short-term effects of autogenic drainage preceded by wet inhalation of saline versus autogenic drainage preceded by intrapulmonary percussive ventilation with saline. Respir 2008, 76, 175–180. [Google Scholar] [CrossRef]
- Varekojis, S.M.; Douce, F.H.; Flucke, R.L.; Filbrun, D.A.; Tice, J.S.; McCoy, K.S.; Castile, R.G. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir. Care 2003, 48, 24–28. [Google Scholar] [PubMed]
- Verboon, J.M.; Bakker, W.; Sterk, P.J. The value of the forced expiration technique with and without postural drainage in adults with cystic fibrosis. Eur. J. Respir. Dis. 1986, 69, 169–174. [Google Scholar]
- Wallaert, E.; Perez, T.; Prevotat, A.; Reychler, G.; Wallaert, B.; Le Rouzic, O. The immediate effects of a single autogenic drainage session on ventilatory mechanics in adult subjects with cystic fibrosis. PLoS ONE 2018, 13, e0195154. [Google Scholar] [CrossRef]
- Warwick, W.J.; Wielinski, C.L.; Hansen, L.G. Comparison of expectorated sputum after manual chest physical therapy and high-frequency chest compression. Biomed. Instrum. Technol. 2004, 38, 470–475. [Google Scholar]
- Webber, B.; Parker, R.; Hofmeyr, J.; Hodson, M. Evaluation of self-percussion during postural drainage using the forced expiration technique. Physiother. Pract. 1985, 1, 42–45. [Google Scholar] [CrossRef]
- Webber, B.A.; Hofmeyr, J.L.; Morgan, M.D.; Hodson, M.E. Effects of postural drainage, incorporating the forced expiration technique, on pulmonary function in cystic fibrosis. Br. J. Dis. Chest 1986, 80, 353–359. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Baker, S.E.; Daines, C.M.; Phan, H.; Martinez, M.G.; Morgan, W.J.; Snyder, E.M. Influence of the Vibralung Acoustical Percussor on pulmonary function and sputum expectoration in individuals with cystic fibrosis. Ther. Adv. Respir. Dis. 2018, 12, 1753466618770997. [Google Scholar] [CrossRef]
- White, D.; Stiller, K.; Willson, K. The role of thoracic expansion exercises during the active cycle of breathing techniques. Physiother. Theory Pract. 1997, 13, 155–161. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- McCormack, P.; Burnham, P.; Southern, K.W. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst. Rev. 2017, 10, CD009595. [Google Scholar] [CrossRef] [PubMed]
- McKoy, N.A.; Wilson, L.M.; Saldanha, I.J.; Odelola, O.A.; Robinson, K.A. Active cycle of breathing technique for cystic fibrosis. Cochrane Database Syst. Rev. 2016, 7, Cd007862. [Google Scholar] [CrossRef]
- McIlwaine, M.; Button, B.; Nevitt, S.J. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Moran, F.; Bradley, J.M.; Piper, A.J. Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst. Rev. 2017, 2, CD002769. [Google Scholar] [CrossRef]
- Franks, L.J.; Walsh, J.R.; Hall, K.; Morris, N.R. Measuring airway clearance outcomes in bronchiectasis: A review. Eur. Respir. Rev. 2020, 29, 190161. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R.; McDonald, C.F.; Jones, A.P.; Holland, A.E. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 3, CD008328. [Google Scholar] [CrossRef]
- Campbell, A.H.; O’CONNELL, J.M.; Wilson, F. The effect of chest physiotherapy upon the FEV1 in chronic bronchitis. Med. J. Aust. 1975, 1, 33–35. [Google Scholar] [CrossRef]
- McDonnell, T.; McNicholas, W.T.; FitzGerald, M.X. Hypoxaemia during chest physiotherapy in patients with cystic fibrosis. Ir. J. Med. Sci. 1986, 155, 345–348. [Google Scholar] [CrossRef]
- Button, B.M.; Heine, R.G.; Catto-Smith, A.G.; Phelan, P.D.; Olinsky, A. Postural drainage and gastro-oesophageal reflux in infants with cystic fibrosis. Arch. Dis. Child. 1997, 76, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Van der Schans, C.P.; Postma, D.S.; Koeter, G.H.; Rubin, B.K. Physiotherapy and bronchial mucus transport. Eur. Respir. J. 1999, 13, 1477–1486. [Google Scholar] [CrossRef]
- Van der Schans, C.P. Bronchial mucus transport. Respir. Care 2007, 52, 1150–1156. [Google Scholar] [PubMed]
- Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis Version 4; Cystic Fibrosis Trust: London, UK, 2020.
- Flume, P.A.; Robinson, K.A.; O’Sullivan, B.P.; Finder, J.D.; Vender, R.L.; Willey-Courand, D.B.; White, T.B.; Marshall, B.C. Cystic fibrosis pulmonary guidelines: Airway clearance therapies. Respir. Care 2009, 54, 522–537. [Google Scholar]
- Stanojevic, S.; Ratjen, F. Physiologic endpoints for clinical studies for cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2016, 15, 416–423. [Google Scholar] [CrossRef][Green Version]
- Jones, P.W.; Beeh, K.M.; Chapman, K.R.; Decramer, M.; Mahler, D.A.; Wedzicha, J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014, 189, 250–255. [Google Scholar] [CrossRef]
- Marques, A.; Cruz, J.; Jácome, C.; Oliveira, A. Outcome measures for respiratory physiotherapy in cystic fibrosis—challenges and advances. Cyst. Fibros. Light New Res. 2015, 37. [Google Scholar]
- Tiddens, H.; Puderbach, M.; Venegas, J.G.; Ratjen, F.; Donaldson, S.H.; Davis, S.D.; Rowe, S.M.; Sagel, S.D.; Higgins, M.; Waltz, D.A. Novel outcome measures for clinical trials in cystic fibrosis. Pediatr. Pulmonol. 2015, 50, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Van der Schans, C.P. Airway clearance: Assessment of techniques. Paediatr. Respir. Rev. 2002, 3, 110–114. [Google Scholar] [CrossRef]
- Stanford, G.; Cathcart, F.; Beverley, Z.; Short, C.; Jones, M.; Bilton, D.; Davies, J.C.; Simmonds, N.J. Investigating outcome measures for assessing airway clearance techniques in adults with cystic fibrosis: Protocol of a single-centre randomised controlled crossover trial. BMJ Open Respir. Res. 2020, 7, e000694. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.E.; Ten Hacken, N.H.; Klooster, K.; Boezen, H.M.; de Greef, M.H.; Slebos, D.J. The minimal important difference for residual volume in patients with severe emphysema. Eur. Respir. J. 2012, 40, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).