Reverse Shoulder Arthroplasty with Bony and Metallic versus Standard Bony Reconstruction for Severe Glenoid Bone Loss. A Retrospective Comparative Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Data Collection, and Ethical Committee Approval
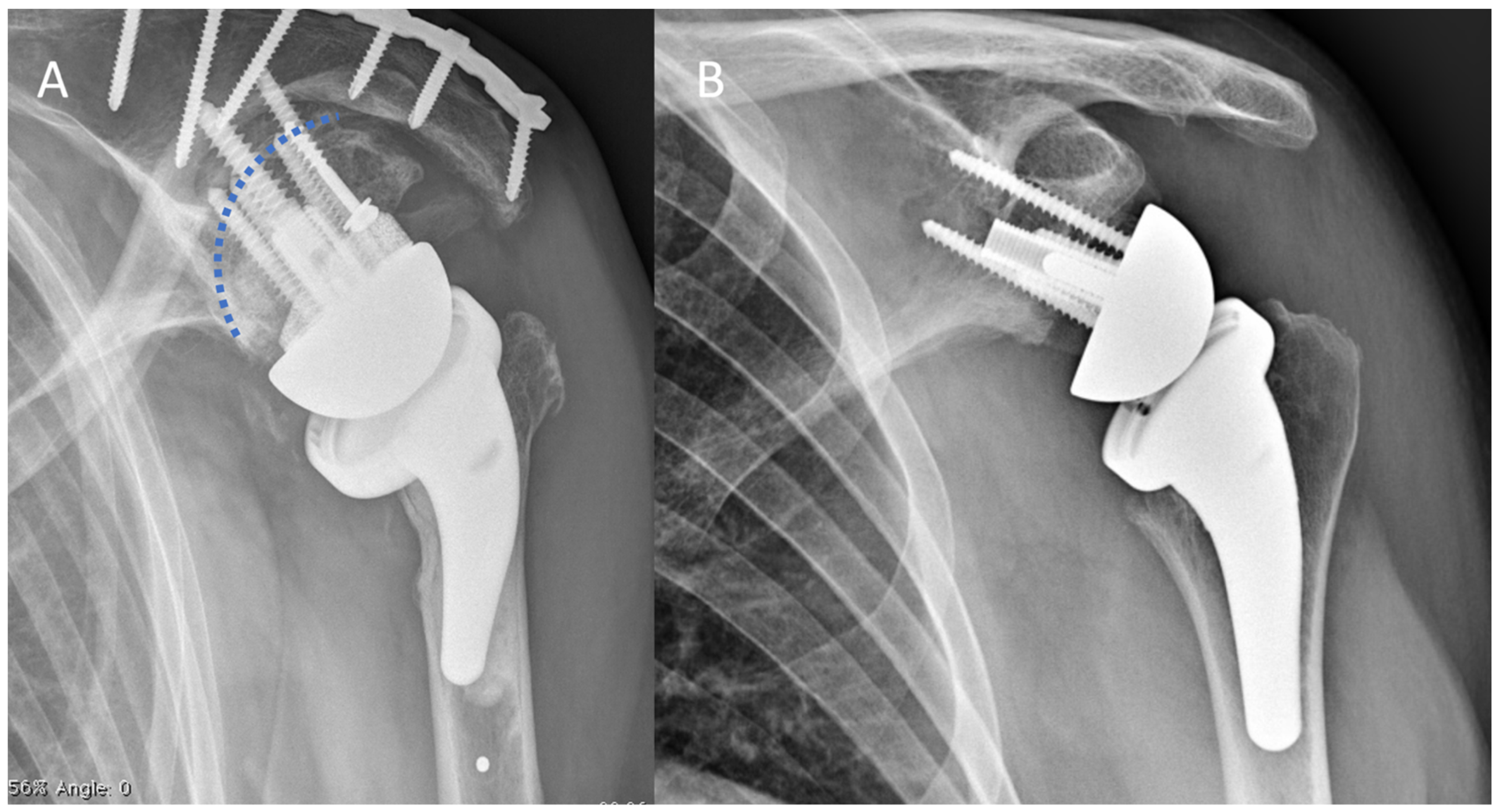
2.2. Surgical Technique and Implant Design
2.3. Postoperative Rehabilitation
2.4. Study Variables
2.5. Clinical Evaluation
2.6. Radiological Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankle, M.A.; Teramoto, A.; Luo, Z.P.; Levy, J.C.; Pupello, D. Glenoid morphology in reverse shoulder arthroplasty: Classification and surgical implications. J. Shoulder Elb. Surg. 2009, 18, 874–885. [Google Scholar] [CrossRef]
- Klein, S.M.; Dunning, P.; Mulieri, P.; Pupello, D.; Downes, K.; Frankle, M.A. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J. Bone Jt. Surg. Am. 2010, 92, 1144–1154. [Google Scholar] [CrossRef]
- Blix, M. Die Länge und die Spannung des Muskels. 1 Skandinavisches. Arch. Physiol. 1892, 3, 295–318. [Google Scholar]
- Lädermann, A.; Denard, P.J.; Boileau, P.; Farron, A.; Deransart, P.; Terrier, A.; Ston, J.; Walch, G. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int. Orthop. 2015, 39, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Roche, C.P.; Diep, P.; Hamilton, M.; Crosby, L.A.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Routman, H.D. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull. Hosp. Jt. Dis. 2013, 71, 284–293. [Google Scholar]
- Boileau, P.; Watkinson, D.; Hatzidakis, A.M.; Hovorka, I. Neer Award 2005: The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 527–540. [Google Scholar] [CrossRef]
- Frankle, M.A.; Siegal, S.; Pupello, D.R.; Gutierrez, S.; Griewe, M.; Mighell, M.A. 11: Coronal plane tilt angle affects risk of catastrophic failure in patients treated with a reverse shoulder prosthesis. J. Shoulder Elb. Surg. 2007, 16, e46. [Google Scholar] [CrossRef]
- Lädermann, A.; Tay, E.; Collin, P.; Piotton, S.; Chiu, C.H.; Michelet, A.; Charbonnier, C. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Jt. Res. 2019, 8, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Lädermann, A.; Collin, P.; Denard, P.J. Range of motion after reverse shoulder arthroplasty: Which combinations of humeral stem and glenosphere work best? Obere Extrem. 2020, 15, 172–178. [Google Scholar] [CrossRef]
- Endell, D.; Imiolczyk, J.-P.; Grob, A.; Moroder, P.; Scheibel, M. Full-wedge metallic reconstruction of glenoid bone deficiency in reverse shoulder arthroplasty. Obere Extrem. 2020, 15, 213–216. [Google Scholar] [CrossRef]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 160–164. [Google Scholar] [CrossRef]
- Cunningham, G.; Lädermann, A.; Denard, P.J.; Kherad, O.; Burkhart, S.S. Correlation Between American Shoulder and Elbow Surgeons and Single Assessment Numerical Evaluation Score After Rotator Cuff or SLAP Repair. Arthroscopy 2015, 31, 1688–1692. [Google Scholar] [CrossRef]
- Collin, P.; Matsukawa, T.; Denard, P.J.; Gain, S.; Lädermann, A. Pre-operative factors influence the recovery of range of motion following reverse shoulder arthroplasty. Int. Orthop. 2017, 41, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Bercik, M.J.; Kruse, K., II; Yalizis, M.; Gauci, M.O.; Chaoui, J.; Walch, G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J. Shoulder Elb. Surg. 2016, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Walch, G.; Badet, R.; Boulahia, A.; Khoury, A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J. Arthroplast. 1999, 14, 756–760. [Google Scholar] [CrossRef]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Mole, D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J. Bone Jt. Surg. Br. 2004, 86, 388–395. [Google Scholar] [CrossRef]
- Melis, B.; DeFranco, M.; Lädermann, A.; Mole, D.; Favard, L.; Nerot, C.; Maynou, C.; Walch, G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J. Bone Jt. Surg. Br. 2011, 93, 1240–1246. [Google Scholar] [CrossRef]
- Simovitch, R.; Flurin, P.H.; Wright, T.; Zuckerman, J.D.; Roche, C.P. Quantifying success after total shoulder arthroplasty: The minimal clinically important difference. J. Shoulder Elb. Surg. 2018, 27, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Chang, B.; Nguyen, J.T.; Dines, D.M.; Gulotta, L.V. What Change in American Shoulder and Elbow Surgeons Score Represents a Clinically Important Change After Shoulder Arthroplasty? Clin. Orthop. Relat. Res. 2016, 474, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascione, F.; Routman, H.D. Severe Glenoid Erosion (B2, B3, C, E2, E3) Treated with RSA. In Complex and Revision Shoulder Arthroplasty: An Evidence-Based Approach to Evaluation and Management, 1st ed.; Tashjian, R.Z., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 59–73. [Google Scholar] [CrossRef]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony increased-offset reversed shoulder arthroplasty: Minimizing scapular impingement while maximizing glenoid fixation. Clin. Orthop. Relat. Res. 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Neyton, L.; Boileau, P.; Nove-Josserand, L.; Edwards, T.B.; Walch, G. Glenoid bone grafting with a reverse design prosthesis. J. Shoulder Elb. Surg. 2007, 16, S71–S78. [Google Scholar] [CrossRef]
- Page, R.S.; Haines, J.F.; Trail, I. Impaction Bone Grafting of the Glenoid in Revision Shoulder Arthroplasty: Classification, Technical Description and Early Results. Shoulder Elb. 2017, 1, 81–88. [Google Scholar] [CrossRef]
- Steinmann, S.P.; Cofield, R.H. Bone grafting for glenoid deficiency in total shoulder replacement. J. Shoulder Elb. Surg. 2000, 9, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Thussbas, C.; Koch, M.; Seebauer, L. Management of glenoid bone defects with reverse shoulder arthroplasty-surgical technique and clinical outcomes. J. Shoulder Elb. Surg. 2018, 27, 853–862. [Google Scholar] [CrossRef]
- Neyton, L.; Walch, G.; Nove-Josserand, L.; Edwards, T.B. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J. Shoulder Elb. Surg. 2006, 15, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Wright, T.W.; Roche, C.P. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull. Hosp. Jt. Dis. 2015, 73, S129–S135. [Google Scholar]
- Levigne, C.; Boileau, P.; Favard, L.; Garaud, P.; Mole, D.; Sirveaux, F.; Walch, G. Scapular notching in reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2008, 17, 925–935. [Google Scholar] [CrossRef]
- Wong, M.T.; Langohr, G.D.G.; Athwal, G.S.; Johnson, J.A. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J. Shoulder Elb. Surg. 2016, 25, 1889–1895. [Google Scholar] [CrossRef]
- Gutierrez, S.; Greiwe, R.M.; Frankle, M.A.; Siegal, S.; Lee, W.E., 3rd. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J. Shoulder Elb. Surg. 2007, 16, S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, S.; Walker, M.; Willis, M.; Pupello, D.R.; Frankle, M.A. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2011, 20, 732–739. [Google Scholar] [CrossRef]
- Ernstbrunner, L.; Werthel, J.D.; Wagner, E.; Hatta, T.; Sperling, J.W.; Cofield, R.H. Glenoid bone grafting in primary reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Norris, T.R. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J. Bone Jt. Surg. Am. 2001, 83, 877–883. [Google Scholar] [CrossRef]
- Malhas, A.; Rashid, A.; Copas, D.; Bale, S.; Trail, I. Glenoid bone loss in primary and revision shoulder arthroplasty. Shoulder Elb. 2016, 8, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashjian, R.Z.; Granger, E.; Chalmers, P.N. Structural glenoid grafting during primary reverse total shoulder arthroplasty using humeral head autograft. J. Shoulder Elb. Surg. 2018, 27, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Houdek, M.T.; Griffith, T.; Elhassan, B.T.; Sanchez-Sotelo, J.; Sperling, J.W.; Cofield, R.H. Glenoid Bone-Grafting in Revision to a Reverse Total Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2015, 97, 1653–1660. [Google Scholar] [CrossRef]
- Malahias, M.A.; Chytas, D.; Kostretzis, L.; Brilakis, E.; Fandridis, E.; Hantes, M.; Antonogiannakis, E. Bone grafting in primary and revision reverse total shoulder arthroplasty for the management of glenoid bone loss: A systematic review. J. Orthop. 2020, 20, 78–86. [Google Scholar] [CrossRef]
- Singh, J.; Odak, S.; Neelakandan, K.; Walton, M.J.; Monga, P.; Bale, S.; Trail, I. Survivorship of autologous structural bone graft at a minimum of 2 years when used to address significant glenoid bone loss in primary and revision shoulder arthroplasty: A computed tomographic and clinical review. J. Shoulder Elb. Surg. 2021, 30, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.S.; Bohm, D.; Abdelkawi, A.; Gohlke, F. Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J. Shoulder Elb. Surg. 2014, 23, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Bonnevialle, N.; Geais, L.; Muller, J.H.; Shoulder Friends, I.; Berhouet, J. Effect of RSA glenoid baseplate central fixation on micromotion and bone stress. JSES Int. 2020, 4, 979–986. [Google Scholar] [CrossRef]



| Bony-Metallic Augmentation (n = 8 Patients) | Bony Augmentation (n = 8 Patients) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||||
| Mean | ±SD | (Range) | Mean | ±SD | (Range) | ||||
| Male sex | 3 | (37.5%) | 5 | (62.5%) | 0.619 | ||||
| Operation on dominant side | 7 | (87.5%) | 3 | (37.5%) | 0.119 | ||||
| Prior surgery | 1 | (12.5%) | 0 | (0.0%) | 1.000 | ||||
| Tobacco use | 1 | (12.5%) | 0 | (0.0%) | 1.000 | ||||
| Primary diagnosis | 0.200 | ||||||||
| Primary OA | 5 | (62.5%) | 8 | (100.0%) | |||||
| Post-traumatic arthritis | 1 | (12.5%) | 0 | (0.0%) | |||||
| Dislocation arthropathy | 1 | (12.5%) | 0 | (0.0%) | |||||
| Revision | 1 | (12.5%) | 0 | (0.0%) | |||||
| Glenoid morphology | <0.001 | ||||||||
| B1 | 1 | (12.5%) | 0 | (0.0%) | |||||
| B2 | 0 | (0.0%) | 8 | (100.0%) | |||||
| B3 | 1 | (12.5%) | 0 | (0.0%) | |||||
| C | 2 | (25.0%) | 0 | (0.0%) | |||||
| D | 2 | (25.0%) | 0 | (0.0%) | |||||
| E3 | 2 | (25.0%) | 0 | (0.0%) | |||||
| Age at index operation (yrs) | 72.1 | ±11.7 | (51.9 | −83.9) | 73.2 | ±6.8 | (61.5 | −84.1) | 0.721 |
| Body mass index | 25.4 | ±4.1 | (20.4 | −31.6) | 26.6 | ±3.0 | (23.7 | −31.0) | 0.400 |
| Weight (kg) | 70.0 | ±11.1 | (60.0 | −85.0) | 74.8 | ±11.2 | (60.0 | −95.0) | 0.461 |
| Height (cm) | 166.6 | ±13.1 | (140.0 | −180.0) | 167.5 | ±7.9 | (155.0 | −175.0) | 0.635 |
| Follow-up (months) | 28.1 | ±15.0 | (11.0 | −51.0) | 30.7 | ±10.8 | (24.0 | −55.4) | 0.752 |
| Inclination (°) | 15.1 | ±12.0 | (0.0 | −34.0) | 9.3 | ±7.5 | (−7.0 | −17.0) | 0.528 |
| Ante retroversion (°) | −22.5 | ±21.1 | (−41.0 | −12.0) | −26.1 | ±4.7 | (−36.0 | −−21.0) | 0.494 |
| Bone loss thickness (mm) | 16.3 | ±3.8 | (11.0 | −21.0) | 12.0 | ±0.0 | (12.0 | −12.0) | 0.020 |
| Patient | Glenoid Morphology | Inclination (°) | Ante/Retroversion (°) | Bone Loss Thickness (mm) |
|---|---|---|---|---|
| Patient 1 | B2 | 10 | −27 | 12 |
| Patient 2 | B2 | 9 | −27 | 12 |
| Patient 3 | B2 | −7 | −21 | 12 |
| Patient 4 | B2 | 17 | −36 | 12 |
| Patient 5 | B2 | 9 | −23 | 12 |
| Patient 6 | B2 | 7 | −23 | 12 |
| Patient 7 | B2 | 13 | −28 | 12 |
| Patient 8 | B2 | 16 | −24 | 12 |
| Patient 9 | D | 5 | 12 | 11 |
| Patient 10 | E3 | 0 | −36 | 14 |
| Patient 11 | B1 | 10 | −10 | 12 |
| Patient 12 | C | 16 | −40 | 16 |
| Patient 13 | B3 | 27 | −37 | 21 |
| Patient 14 | D | 34 | −41 | 20 |
| Patient 15 | E3 | 23 | 3 | 20 |
| Patient 16 | C | 6 | −31 | 16 |
| Bony-Metallic Augmentation (n = 8 Patients) | Bony Augmentation (n = 8 Patients) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||||
| Mean | ±SD | (Range) | Mean | ±SD | (Range) | ||||
| Internal rotation | |||||||||
| preoperative | 0.022 | ||||||||
| Thigh | 5 | (62.5%) | 0 | (0.0%) | |||||
| Buttock | 3 | (37.5%) | 4 | (50.0%) | |||||
| Sacrum | 0 | (0.0%) | 3 | (37.5%) | |||||
| Th12 | 0 | (0.0%) | 1 | (12.5%) | |||||
| postoperative | 0.220 | ||||||||
| Buttock | 0 | (0.0%) | 1 | (12.5%) | |||||
| Sacrum | 0 | (0.0%) | 3 | (37.5%) | |||||
| L5/L3 | 4 | (50.0%) | 3 | (37.5%) | |||||
| Th12 | 2 | (25.0%) | 1 | (12.5%) | |||||
| Th7 | 2 | (25.0%) | 0 | (0.0%) | |||||
| p-value * | 0.014 | 0.090 | |||||||
| Anterior forward flexion (°) | |||||||||
| preoperative | 55.0 | ±38.5 | (0.0 | −95.0) | 101.3 | ±31.8 | (40.0 | −150.0) | 0.010 |
| postoperative | 141.3 | ±22.2 | (90.0 | −160.0) | 145.0 | ±12.0 | (130.0 | −160.0) | 0.915 |
| improvement | 86.3 | ±27.9 | (55.0 | −130.0) | 43.8 | ±25.6 | (10.0 | −90.0) | 0.013 |
| p-value * | 0.014 | 0.014 | |||||||
| Active external rotation (°) | |||||||||
| preoperative | 14.4 | ±15.5 | (0.0 | −40.0) | 10.6 | ±22.4 | (−20.0 | −45.0) | 0.545 |
| postoperative | 29.4 | ±14.7 | (15.0 | −60.0) | 49.4 | ±17.0 | (30.0 | −80.0) | 0.017 |
| improvement | 15.0 | ±12.0 | (−10.0 | −30.0) | 38.8 | ±16.2 | (5.0 | −60.0) | 0.009 |
| p-value * | 0.027 | 0.014 | |||||||
| Constant score | |||||||||
| preoperative | 18.8 | ±7.4 | (6.0 | −30.0) | 34.5 | ±11.7 | (12.0 | −49.0) | 0.013 |
| postoperative | 75.4 | ±10.4 | (55.0 | −87.0) | 72.8 | ±14.8 | (54.0 | −91.0) | 0.792 |
| improvement | 56.6 | ±10.1 | (38.0 | −72.0) | 38.3 | ±16.7 | (17.0 | −69.0) | 0.021 |
| p-value * | 0.014 | 0.008 | |||||||
| SSV | |||||||||
| preoperative | 34.4 | ±21.9 | (15.0 | −70.0) | 36.3 | ±14.1 | (10.0 | −50.0) | 0.630 |
| postoperative | 83.8 | ±11.6 | (65.0 | −100.0) | 85.5 | ±9.2 | (70.0 | −99.0) | 0.915 |
| improvement | 49.4 | ±24.4 | (10.0 | −80.0) | 49.3 | ±16.8 | (35.0 | −85.0) | 0.958 |
| p-value* | 0.008 | 0.014 | |||||||
| ASES score | |||||||||
| preoperative | 28.6 | ±14.1 | (3.0 | −47.0) | 22.9 | ±11.8 | (10.0 | −35.0) | 0.426 |
| postoperative | 76.8 | ±11.0 | (57.0 | −95.0) | 89.1 | ±11.3 | (67.0 | −100.0) | 0.045 |
| improvement | 48.1 | ±12.2 | (31.0 | −66.0) | 66.3 | ±11.9 | (45.0 | −83.0) | 0.027 |
| p-value * | 0.014 | 0.008 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabergoj, M.; Neyton, L.; Bothorel, H.; Ho, S.W.L.; Wang, S.; Chong, X.L.; Lädermann, A. Reverse Shoulder Arthroplasty with Bony and Metallic versus Standard Bony Reconstruction for Severe Glenoid Bone Loss. A Retrospective Comparative Cohort Study. J. Clin. Med. 2021, 10, 5274. https://doi.org/10.3390/jcm10225274
Nabergoj M, Neyton L, Bothorel H, Ho SWL, Wang S, Chong XL, Lädermann A. Reverse Shoulder Arthroplasty with Bony and Metallic versus Standard Bony Reconstruction for Severe Glenoid Bone Loss. A Retrospective Comparative Cohort Study. Journal of Clinical Medicine. 2021; 10(22):5274. https://doi.org/10.3390/jcm10225274
Chicago/Turabian StyleNabergoj, Marko, Lionel Neyton, Hugo Bothorel, Sean W. L. Ho, Sidi Wang, Xue Ling Chong, and Alexandre Lädermann. 2021. "Reverse Shoulder Arthroplasty with Bony and Metallic versus Standard Bony Reconstruction for Severe Glenoid Bone Loss. A Retrospective Comparative Cohort Study" Journal of Clinical Medicine 10, no. 22: 5274. https://doi.org/10.3390/jcm10225274
APA StyleNabergoj, M., Neyton, L., Bothorel, H., Ho, S. W. L., Wang, S., Chong, X. L., & Lädermann, A. (2021). Reverse Shoulder Arthroplasty with Bony and Metallic versus Standard Bony Reconstruction for Severe Glenoid Bone Loss. A Retrospective Comparative Cohort Study. Journal of Clinical Medicine, 10(22), 5274. https://doi.org/10.3390/jcm10225274







