Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Neurological Examination
2.3. Ophthalmological Evaluation
- absence of a mean refractive error > ±3.00 spherical equivalent;
- IOP less than 18 mmHg;
- absence of corneal or lens opacities;
- absence of square-wave jerks, saccadic intrusions and nystagmus in primary position of gaze that can influence the ability to maintain a stable fixation during the mfERG recordings (see below Section 2.5);
- Sd-OCT image with quality signal strength index > 40 (see below Section 2.6);
- absence of other systemic diseases (i.e., diabetes, systemic hypertension, rheumatologic disorders) or intake of drugs that may influence the retinal function.
2.4. Visual Acuity Assessment
2.5. Macular Functional Assessment
2.6. Macular Morphological Assessment
2.7. Assessment of RGCs Function and Neural Conduction along the Visual Pathways
2.8. Morphological Evaluation of RGCs Axons
2.9. Data Analysis
3. Results
3.1. BCVA Data
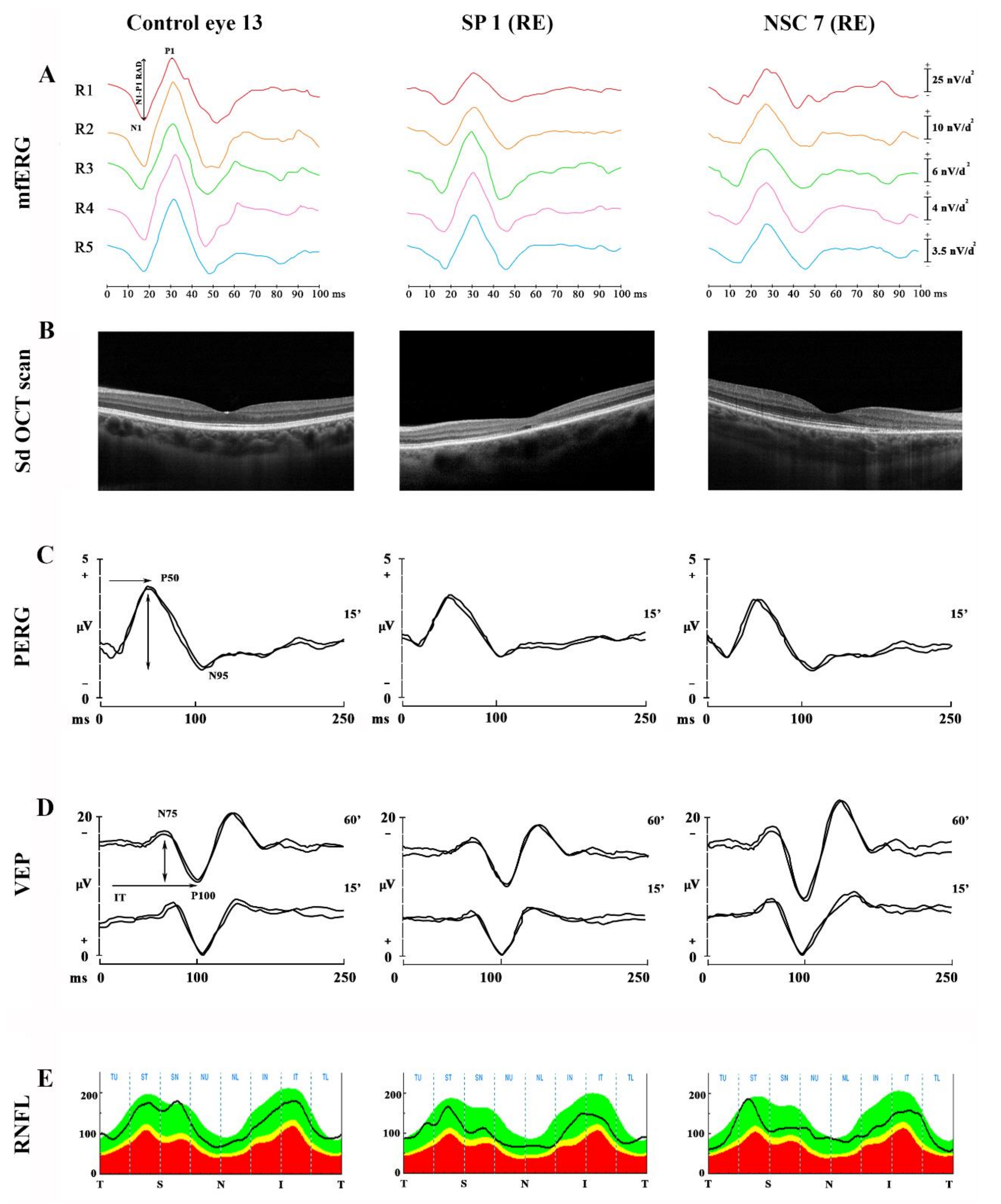
3.2. Macular Functional Data (mfERG Ring Analysis)
3.3. Macular Morphological (MT and MV) Data
3.4. Data on RGCs Function (PERG) and Neural Conduction along the Visual Pathways (VEP)
3.5. RNFL-T Data
4. Discussion
4.1. Macular Functional and Morphological Changes in SP and NSC Patients
4.2. Functional and Morphological Changes of RGCs and Their Axons
4.3. Visual Pathways’ Function in SP and NSC Subjects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCA-ATXN1 | spinocerebellar ataxia type 1 |
| ATXN1 | ataxin-1 gene |
| FO | fundus oculi |
| Sd-OCT | spectral domain-optical coherence tomography |
| mfERG | multifocal electroretinogram |
| PERG | pattern reversal electroretinogram |
| VEP | visual evoked potentials |
| ADSCAs | autosomal dominant spinocerebellar ataxias |
| SCAs | spinocerebellar ataxias |
| SCA-ATXN7 | spinocerebellar ataxia type 7 |
| RGCs | retinal ganglion cells |
| BCVA | best corrected visual acuity |
| SARA | scale for the assessment and rating of ataxia |
| IOP | intraocular pressure |
| ETDRS | early treatment diabetic retinopathy study |
| DTL | Dawson–Trick–Litzkow |
| RAD | response amplitude density |
| MT | macular thickness |
| WR-MT | whole retina macular thickness |
| IR-MT | inner retina macular thickness |
| OR-MT | outer retina macular thickness |
| MV | macular volume |
| WR-MV | whole retina macular volume |
| IR-MV | inner retina macular volume |
| OR-MV | outer retina macular volume |
| INL | inner nuclear layer |
| OPL | outer plexiform layer |
| IPL | inner plexiform layer |
| IT | implicit time |
| A | Amplitude |
| RNFL-T | retinal nerve fibers layer thickness |
| SP | symptomatic patient with neurological signs |
| NSC | not symptomatic carriers without neurological signs |
| RE | right eye |
| LE | left eye |
| RPE | retinal pigmented epithelium |
| EZ | ellipsoid zone |
References
- Durr, A. Autosomal dominant cerebellar ataxias: Polyglutamine expansions and beyond. Lancet Neurol. 2010, 9, 885–894. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.T.; Chung, M.Y.; Banfi, S.; Kwiatkowski, T.J., Jr.; Servadio, A.; Beaudet, A.L.; McCall, A.E.; Duvick, L.A.; Ranum, L.P.; Zoghbi, H.Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993, 4, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.C.; Bowman, A.B.; Jafar-Nejad, P.; Lim, J.; Richman, R.; Fryer, J.D.; Hyun, E.D.; Duvick, L.A.; Orr, H.T.; Botas, J.; et al. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell 2006, 127, 1335–1347. [Google Scholar] [CrossRef]
- Kim, E.; Lee, Y.; Choi, S.; Song, J.J. Structural basis of the phosphorylation dependent complex formation of neurodegenerative disease protein Ataxin-1 and RBM17. Biochem. Biophys. Res. Commun. 2014, 449, 399–404. [Google Scholar] [CrossRef]
- Vaclavik, V.; Borruat, F.X.; Ambresin, A.; Munier, F.L. Novel maculopathy in patients with spinocerebellar ataxia type 1 autofluorescence findings and functional characteristics. JAMA Ophthalmol. 2013, 131, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Michalik, A.; Martin, J.J.; Van Broeckhoven, C. Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur. J. Hum. Genet. 2004, 12, 2–15. [Google Scholar] [CrossRef]
- Abe, T.; Tsuda, T.; Yoshida, M.; Wada, Y.; Kano, T.; Itoyama, Y.; Tamai, M. Macular degeneration associated with aberrant expansion of trinucleotide repeat of the SCA7 gene in 2 Japanese families. Arch. Ophthalmol. 2000, 118, 1415–1421. [Google Scholar] [CrossRef]
- Kouno, R.; Kawata, A.; Yoshida, H.; Ikeda, S.; Suda, M.; Hirai, S. A family of SCA1 with pigmentary macular dystrophy. Rinsho Shinkeigaku. 1999, 39, 649–652. [Google Scholar]
- Lebranchu, P.; Le Meur, G.; Magot, A.; David, A.; Verny, C.; Weber, M.; Milea, D. Maculopathy and spinocerebellar ataxia type A new association? J. Neuro-Ophthalmol. 2013, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Matsumura, K.; Shimizu, S.; Ichikawa, Y.; Ochiai, K.; Goto, J.; Tsuji, S.; Shimizu, T. Pigmentary macular dystrophy in spinocerebellar ataxia type 1. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishiguchi, K.M.; Aoki, M.; Nakazawa, T.; Abe, T. Macular degeneration as a common cause of visual loss in spinocerebellar ataxia type 1 (SCA1) patients. Ophthalmic Genet. 2019, 40, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Oertel, F.C.; Zeitz, O.; Rönnefarth, M.; Bereuter, C.; Motamedi, S.; Zimmermann, H.G.; Kuchling, J.; Grosch, A.S.; Doss, S.; Browne, A.; et al. Functionally Relevant Maculopathy and Optic Atrophy in Spinocerebellar Ataxia Type 1. Mov. Disord. Clin. Pract. 2020, 7, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Abe, K.; Aoki, M.; Itoyama, Y.; Tamai, M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch. Ophthalmol. 1997, 115, 231–236. [Google Scholar] [CrossRef]
- Pula, J.H.; Towle, V.L.; Staszak, V.M.; Cao, D.; Bernard, J.T.; Gomez, C.M. Retinal Nerve Fibre Layer and Macular Thinning in Spinocerebellar Ataxia and Cerebellar Multisystem Atrophy. Neuroophthalmology 2011, 35, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Joo, K.; Woo, S.J. Ophthalmic Manifestations and Genetics of the Polyglutamine Autosomal Dominant Spinocerebellar Ataxias: A Review. Fron. Neurosci. 2020, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.; Oberwahrenbrock, T.; Zimmermann, H.; Schroeter, J.; Endres, M.; Brandt, A.U.; Paul, F. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS ONE 2011, 6, 23024. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Xiao, J.; Zhang, Q.; Xu, G.; Wu, G.; Liu, T.; Luo, W. Combination of Multifocal Electroretinogram and Spectral-Domain OCT Can Increase Diagnostic Efficacy of Parkinson’s Disease. Parkinsons Dis. 2018, 4163239. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Barbano, L.; Boffa, L.; Albanese, M.; Nicoletti, C.G.; Landi, D.; Grzybowski, A.; Falsini, B.; Marfia, G.A.; Centonze, D.; et al. Functional Assessment of Outer and Middle Macular Layers in Multiple Sclerosis. J. Clin. Med. 2020, 9, 3766. [Google Scholar] [CrossRef] [PubMed]
- Kardon, R.H. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J. Neuroophthalmol. 2011, 31, 353–361. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Sadun, F.; De Negri, A.M.; Barboni, P.; Savini, G.; Borrelli, E.; La Morgia, C.; Carelli, V.; Parisi, V. Retinal function and neural conduction along the visual pathways in affected and unaffected carriers with Leber’s hereditary optic neuropathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6893–6901. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Tormene, A.P.; Vaegan, V. ISCEV standard for clinical visual evoked potentials (2009 update). Doc. Ophthalmol. 2010, 133, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Gallinaro, G.; Ziccardi, L.; Coppola, G. Electrophysiological assessment of visual function in patients with non-arteritic ischaemic optic neuropathy. Eur. J. Neurol. 2008, 15, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Perretti, A.; Santoro, L.; Lanzillo, B.; Filla, A.; De Michele, G.; Barbieri, F.; Martino, G.; Ragno, M.; Cocozza, S.; Caruso, G. Autosomal dominant cerebellar ataxia type I: Multimodal electrophysiological study and comparison between SCA1 and SCA2 patients. J. Neurol. Sci. 1996, 142, 45–53. [Google Scholar] [CrossRef]
- Chandran, V.; Jhunjhunwala, K.; Purushottam, M.; Jain, S.; Pal, P.K. Multimodal evoked potentials in spinocerebellar ataxia types 1, 2, and 3. Ann. Indian Acad. Neurol. 2014, 17, 321–324. [Google Scholar] [PubMed]
- Rakowicz, M.; Zdzienicka, E.; Poniatowska, R.; Waliniowska, E.; Sułek, A.; Jakubowska, T.; Niedzielska, K.; Rola, R.; Wierzbicka, A.; Hoffman-Zacharska, D.; et al. Ataksja rdzeniowo-mózdzkowa typu 1 i 2—porównanie oceny klinicznej, elektrofizjologicznej i rezonansu magnetycznego [Spinocerebellar ataxias type 1 and 2: Comparison of clinical, electrophysiological and magnetic resonance evaluation]. Neurol. Neurochir. Pol. 2005, 39, 263–275. [Google Scholar] [PubMed]
- Liang, L.; Chen, T.; Wu, Y. The electrophysiology of spinocerebellar ataxias. Neurophysiol. Clin. 2016, 46, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.P.; Barron, L.H.; Goudie, D.; Kelly, K.; Dow, D.; Fitzpatrick, D.R.; Brock, D.J. A general method for the detection of large CAG repeat expansions by fluorescent PCR. J. Med. Genet. 1996, 33, 1022–1026. [Google Scholar] [CrossRef]
- Cagnoli, C.; Stevanin, G.; Michielotto, C.; Promis, G.G.; Brussino, A.; Pappi, P. Large pathogenic expansions in the SCA2 and SCA7 genes can be detected by fluorescent repeat-primed polymerase chain reaction assay. J. Mol. Diagn. 2006, 8, 128–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmitz-Hubsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Hood, D.C.; Bach, M.; Brigell, M.; Keating, D.; Kondo, M.; Lyons, J.S.; Marmor, M.F.; McCulloch, D.L.; Palmowski-Wolfe, A.M. International Society For Clinical Electrophysiology of Vision (ISCEV) standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc. Ophthalmol. 2012, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Parisi, V.; Picconi, F.; Di Renzo, A.; Lombardo, M.; Frontoni, S.; Parravano, M. Early and localized retinal dysfunction in patients with type 1 diabetes mellitus studied by multifocal electroretinogram. Acta Diabetol. 2018, 55, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Ziccardi, L.; Costanzo, E.; Tedeschi, M.; Barbano, L.; Manca, D.; Di Renzo, A.; Giorno, P.; Varano, M.; Parravano, M. Macular Functional and Morphological Changes in Intermediate Age-Related Maculopathy. Invest. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016, 86, 2303–2309. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Sadun, F.; De Negri, A.M.; La Morgia, C.; Barbano, L.; Carelli, V.; Barboni, P. Functional Changes of Retinal Ganglion Cells and Visual Pathways in Patients with Chronic Leber’s Hereditary Optic Neuropathy during One Year of Follow-up. Ophthalmology 2019, 126, 1033–1044. [Google Scholar] [CrossRef]
- Celesia, G.G. Evoked potential techniques in the evaluation of visual function. J. Clin. Neurophysiol. 1984, 1, 55–76. [Google Scholar] [CrossRef]
- Fiorentini, A.; Maffei, L.; Pirchio, M.; Spinelli, D.; Porciatti, V. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest. Ophthalmol. Vis. Sci. 1981, 21, 490–493. [Google Scholar] [PubMed]
- Barbano, L.; Ziccardi, L.; Parisi, V. Correlations between visual morphological, electrophysiological, and acuity changes in chronic non-arteritic ischemic optic neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1297–1308. [Google Scholar] [CrossRef]
- Kumaran, D.; Balagopal, K.; Tharmaraj, R.G.A.; Aaron, S.; George, K.; Muliyil, J.; Sivadasan, A.; Danda, S.; Alexander, M.; Hasan, G. Genetic characterization of Spinocerebellar ataxia 1 in a South Indian cohort. BMC Med. Genet. 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirose, A.; Katagiri, S.; Hayashi, T.; Matsuura, T.; Nagai, N.; Fujinami, K.; Iwata, T.; Tsunoda, K. Progress of macular atrophy during 30 months’ follow-up in a patient with spinocerebellar ataxia type1 (SCA1). Doc. Ophthalmol. 2021, 142, 87–98. [Google Scholar] [CrossRef]
- Hood, D.C. Assessing retinal function with the multifocal technique. Prog. Retin. Eye Res. 2000, 19, 607–646. [Google Scholar] [CrossRef]
- Miyata, K.; Nakamura, M.; Kondo, M.; Lin, J.; Ueno, S.; Miyake, Y.; Terasaki, H. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 820–824. [Google Scholar] [CrossRef]
- Reis, A.; Mateus, C.; Viegas, T.; Florijn, R.; Bergen, A.; Silva, E.; Castelo-Branco, M. Physiological evidence for impairment in autosomal dominant optic atrophy at the pre-ganglion level. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 221–234. [Google Scholar] [CrossRef]
- Gin, T.J.; Luu, C.D.; Guymer, R.H. Central retinal function as measured by the multifocal electroretinogram and flicker perimetry in early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 9267–9274. [Google Scholar] [CrossRef]
- Parisi, V.; Perillo, L.; Tedeschi, M.; Scassa, C.; Gallinaro, G.; Capaldo, N.; Varano, M. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 2007, 27, 879–890. [Google Scholar] [CrossRef]
- Hugosson, T.; Gränse, L.; Ponjavic, V.; Andréasson, S. Macular dysfunction and morphology in spinocerebellar ataxia type 7 (SCA 7). Ophthalmic Genet. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Boquete, L.; López-Guillén, E.; Vilades, E.; Miguel-Jiménez, J.M.; Pablo, L.E.; De Santiago, L.; Ortiz Del Castillo, M.; Alonso-Rodríguez, M.C.; Morla, E.M.S.; López-Dorado, A.; et al. Diagnostic ability of multifocal electroretinogram in early multiple sclerosis using a new signal analysis method. PLoS ONE 2019, 14, 0224500. [Google Scholar] [CrossRef] [PubMed]
- Servadio, A.; Koshy, B.; Armstrong, D.; Antalffy, B.; Orr, H.T.; Zoghbi, H.Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat. Genet. 1995, 10, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Funez, P.; Nino-Rosales, M.L.; De Gouyon, B.; She, W.C.; Luchak, J.M.; Martinez, P.; Turiegano, E.; Benito, J.; Capovilla, M.; Skinner, P.J.; et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 2000, 408, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Fiorentini, A. The pattern electroretinogram in animals and humans: Physiological and clinical applications. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1990, 67, 289–296. [Google Scholar]
- Parisi, V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin. Ophthalmol. 2003, 18, 50–57. [Google Scholar] [PubMed]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G. The uniform field and pattern ERG in macaques with experimental glaucoma: Removal of spiking activity. Invest. Ophthalmol. Vis. Sci. 2000, 41, 2797–2810. [Google Scholar]
- Doss, S.; Brandt, A.U.; Oberwahrenbrock, T.; Endres, M.; Paul, F.; Rinnenthal, J.L. Metabolic evidence for cerebral neurodegeneration in spinocerebellar ataxia type 1. Cerebellum 2014, 13, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, T.S.; Kim, I.Y.; Hong, S.; Rhim, H.; Kang, S. Polyglutamine-expanded ataxin-1 recruits Cu/Zn-superoxide dismutase into the nucleus of HeLa cells. Biochem. Biophys. Res. Commun. 2003, 307, 660–665. [Google Scholar] [CrossRef]
- Abe, T.; Abe, K.; Tsuda, T.; Itoyama, Y.; Tamai, M. Ophthalmological findings in patients with spinocerebellar ataxia type 1 are not correlated with neurological anticipation. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 722–728. [Google Scholar] [CrossRef] [PubMed]
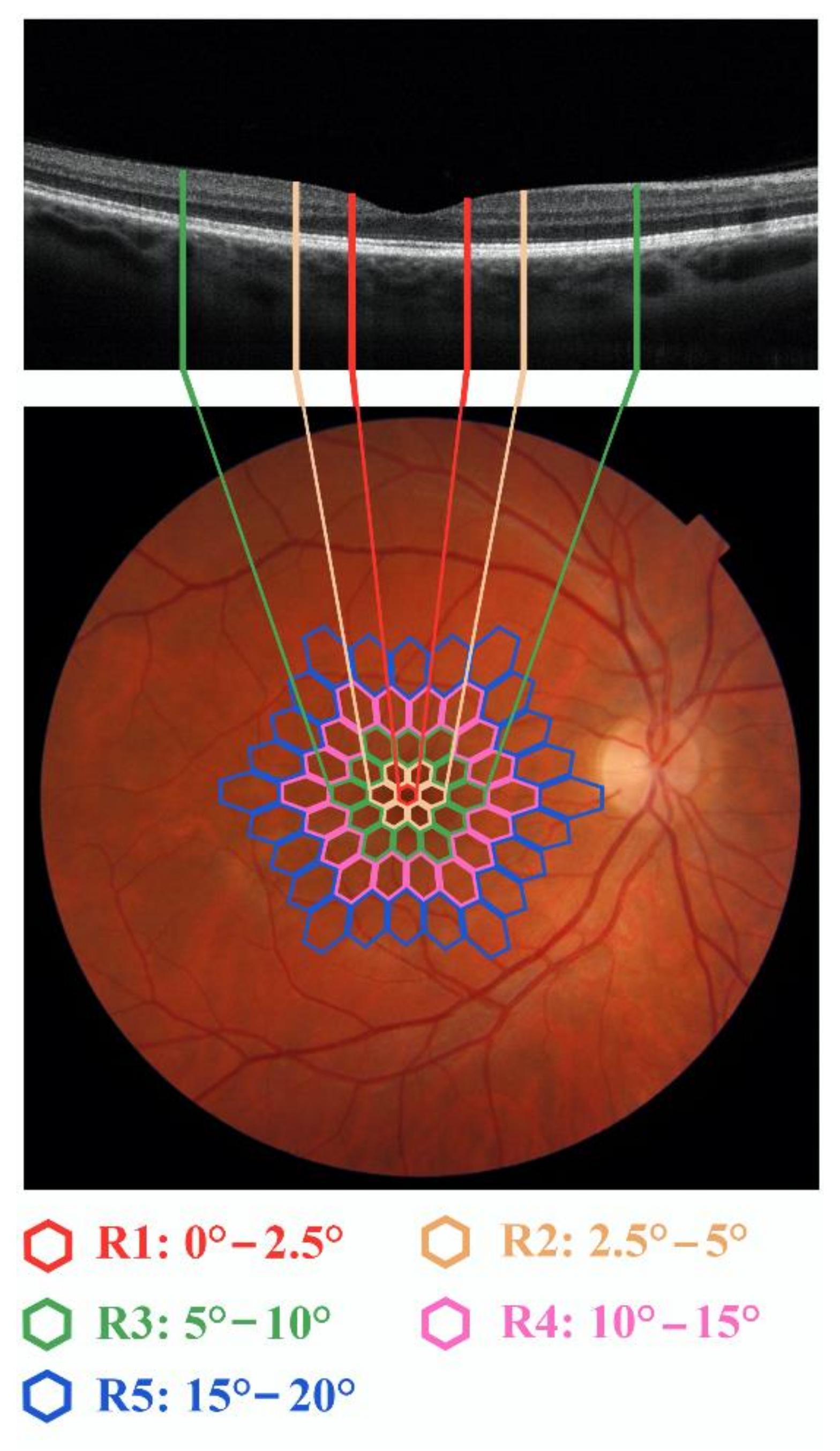
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | NSC7 | NSC8 | NSC9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (Years)/Sex | 50/M e | 68/F f | 36/M e | 51/F f | 50/F f | 50/F f | 43/M e | 47/F f | 53/M e |
| Family history | Deceased mother affected by SCA1 g | Mother of NSC7 and NSC8 | No | Deceased mother with SCA1, sister of SP5 and NSC9 | Deceased mother with SCA1, sister of SP4 and NSC9 | Deceased father with SCA1 | Mother (SP2) and sister (NSC8) affected by SCA1 | Mother (SP2) and brother (NSC7) affected by SCA1 | Deceased mother with SCA1, brother of SP4 and SP5 |
| SARA a | 17 | 29 | 10 | 10 | 10 | 8 | 0 | 0 | 0 |
| CAG triplet expansion in ATXN1 gene (number) | 58 | 51 | 44 | 44 | 62 | 63 | 51 | 51 | 44 |
| Onset age Neurological/ Visual Symptoms | 35/40 | 50/68 | 25/no visual symptoms | 40/no visual symptoms | 40/no visual symptoms | 40/no visual symptoms | Not symptomatic/no visual symptoms | Not symptomatic/ no visual symptoms | Not symptomatic/no visual symptoms |
| Neurological Signs | impaired hand dexterity, mild dysarthria, dysphagia, gait ataxia | dysarthria, dysphagia, nystagmus, saccadic intrusions and severe gait ataxia | Mild dysarthria, gait ataxia, mild nystagmus | dysarthria, dysphagia, mild limb, gait ataxia | Mild dysarthria, dysphagia, hand dexterity, mild limb, gait ataxia | dysarthria, dysphagia, hand dexterity with alteration of writing, mild trunk, gait ataxia | None | None | None |
| BCVA b RE c/LE d | 0.3/0.3 | 0.1/0.1 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 | 0.0/0.0 |
| Fundus Oculi examination RE c LE d | Small parafoveal chorioretinal atrophy | Papillary pallor, macular dotted Dystrophy | Normal | Normal | Normal | Normal | Macular RPE dystrophy | Normal | Macular dystrophy |
| Normal | Papillary pallor, macular dotted dystrophy | Normal | Normal | Normal | Normal | Normal | Normal | Macular dystrophy | |
| Ishihara charts RE c LE d | 15/22 16/22 | 2/22 6/22 | 22/22 22/22 | 22/22 22/22 | 22/22 22/22 | 22/22 22/22 | 22/22 22/22 | 22/22 22/22 | 22/22 22/22 |
| R1 a N1-P1 RAD f (nV/deg2) g | R2 b N1-P1 RAD f (nV/deg2) g | R3 c N1-P1 RAD f (nV/deg2) g | R4 d N1-P1 RAD f (nV/deg2) g | R5 e N1-P1 RAD f (nV/deg2) g | |
|---|---|---|---|---|---|
| SP1 RE h | 35.85 | 17.31 | 16.85 | 10.67 | 8.49 |
| SP1 LE i | 41.45 | 15.57 | 15.47 | 12.52 | 8.77 |
| SP2 RE h | 33.78 | 14.06 | 12.67 | 8.89 | 5.65 |
| SP2 LE i | 31.73 | 13.30 | 14.77 | 12.11 | 9.81 |
| SP3 RE h | 45.28 | 19.50 | 19.02 | 9.96 | 9.38 |
| SP3 LE i | 49.61 | 20.87 | 15.29 | 11.34 | 8.76 |
| SP4 RE h | 36.68 | 23.40 | 14.55 | 10.40 | 7.65 |
| SP4 LE i | 45.01 | 28.09 | 11.70 | 10.16 | 7.80 |
| SP5 RE h | 47.40 | 35.47 | 27.54 | 12.73 | 10.88 |
| SP5 LE i | 55.38 | 28.79 | 20.60 | 13.82 | 8.82 |
| SP6 RE h | 37.29 | 27.09 | 19.00 | 10.37 | 8.10 |
| SP6 LE i | 39.34 | 23.31 | 17.78 | 11.64 | 8.08 |
| NSC7 RE h | 40.31 | 17.47 | 10.16 | 7.57 | 6.26 |
| NSC7 LE i | 40.33 | 24.78 | 13.09 | 8.99 | 6.21 |
| NSC8 RE h | 47.88 | 16.33 | 10.74 | 7.47 | 6.01 |
| NSC8 LE i | 44.47 | 16.92 | 12.29 | 6.50 | 4.70 |
| NSC9 RE h | 30.46 | 23.28 | 14.61 | 10.77 | 6.69 |
| NSC9 LE i | 28.19 | 26.55 | 16.60 | 8.13 | 4.73 |
| 95% CL l | 81.28 | 19.36 | 10.64 | 6.28 | 4.56 |
| WR a-MT b (μm) g | IR c-MT b (μm) g | OR d-MT b (μm) g | WR a-MV e (mm3) | IR c-MV e (mm3) | OR d-MV e (mm3) | RNFL-T f (μm) g | |
|---|---|---|---|---|---|---|---|
| SP1 RE h | 168 | 51 | 117 | 4.782 | 1.886 | 2.884 | 105.88 |
| SP1 LE i | 180 | 52 | 128 | 4.751 | 1.878 | 2.873 | 110.82 |
| SP2 RE h | 263 | 77 | 185 | 4.642 | 1.655 | 2.984 | 114.45 |
| SP2 LE i | 204 | 56 | 148 | 4.745 | 1.187 | 2.558 | 105.97 |
| SP3 RE h | 280 | 101 | 179 | 6.049 | 2.500 | 3.549 | 115.11 |
| SP3 LE i | 259 | 86 | 173 | 6.14 | 2.548 | 3.592 | 120.86 |
| SP4 RE h | 250 | 79 | 171 | 5.591 | 2.111 | 3.480 | 108.42 |
| SP4 LE i | 249 | 76 | 174 | 5.672 | 2.169 | 3.502 | 122.73 |
| SP5 RE h | 237 | 69 | 168 | 5.855 | 2.208 | 3.647 | 119.67 |
| SP5 LE i | 251 | 79 | 171 | 5.929 | 2.357 | 3.572 | 117.79 |
| SP6 RE h | 258 | 90 | 168 | 5.524 | 2.181 | 3.343 | 116.68 |
| SP6 LE i | 246 | 70 | 176 | 5.549 | 2.201 | 3.348 | 118.33 |
| NSC7 RE h | 260 | 81 | 180 | 6.299 | 2.618 | 3.681 | 111.03 |
| NSC7 LE i | 253 | 81 | 172 | 5.759 | 2.252 | 3.508 | 108.41 |
| NSC8 RE h | 265 | 88 | 177 | 6.204 | 2.571 | 3.632 | 120.83 |
| NSC8 LE i | 271 | 81 | 190 | 6.177 | 2.546 | 3.361 | 125.32 |
| NSC9 RE h | 242 | 70 | 164 | 5.718 | 2.239 | 3.479 | 116.08 |
| NSC9 LE i | 254 | 86 | 168 | 5.810 | 2.308 | 3.502 | 116.91 |
| 95% CL l | 238.84 | 60.01 | 162.96 | 5.150 | 1.940 | 2.890 | 104.76 |
| 15′ PERG a | 60′ VEP b | 15′ VEP a | ||||
|---|---|---|---|---|---|---|
| IT c (ms) | A d (µV) g | IT e (ms) | A f (µV) g | IT e (ms) | A f (µV) g | |
| SP1 RE h | 55 | 2.3 | 106 | 6.52 | 104 | 7.4 |
| SP1 LE i | 56 | 2.4 | 108 | 5.91 | 104 | 7.5 |
| SP2 RE h | 54 | 2.8 | 107 | 6.62 | 121 | 5.1 |
| SP2 LE i | 53 | 3.0 | 103 | 5.9 | 124 | 4.0 |
| SP3 RE h | 50 | 2.8 | 106 | 7.8 | 104 | 7.6 |
| SP3 LE i | 53 | 2.3 | 105 | 6.6 | 109 | 7.8 |
| SP4 RE h | 52 | 2.32 | 103 | 11.1 | 105 | 10.6 |
| SP4 LE i | 56 | 2.6 | 104 | 7.9 | 104 | 9.7 |
| SP5 RE h | 52 | 2.22 | 103 | 12.6 | 107 | 9.6 |
| SP5 LE i | 55 | 3.2 | 106 | 16.2 | 106 | 7.8 |
| SP6 RE h | 55 | 2.12 | 102 | 10.3 | 105 | 7.2 |
| SP6 LE i | 57 | 2.19 | 104 | 13.22 | 104 | 6.2 |
| NSC7 RE h | 56 | 2.7 | 100 | 11.5 | 99 | 8.6 |
| NSC7 LE i | 53 | 2.4 | 100 | 8.5 | 97 | 6.9 |
| NSC8 RE h | 57 | 2.21 | 103 | 6.5 | 104 | 9.7 |
| NSC8 LE i | 54 | 2.24 | 106 | 13.3 | 102 | 10.3 |
| NSC9 RE h | 52 | 2.34 | 107 | 7.7 | 108 | 8.6 |
| NSC9 LE i | 55 | 2.62 | 107 | 6.32 | 107 | 7.7 |
| 95% CL l | 58.64 | 2.08 | 107.25 | 5.67 | 111.68 | 6.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziccardi, L.; Cioffi, E.; Barbano, L.; Gioiosa, V.; Falsini, B.; Casali, C.; Parisi, V. Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs. J. Clin. Med. 2021, 10, 5271. https://doi.org/10.3390/jcm10225271
Ziccardi L, Cioffi E, Barbano L, Gioiosa V, Falsini B, Casali C, Parisi V. Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs. Journal of Clinical Medicine. 2021; 10(22):5271. https://doi.org/10.3390/jcm10225271
Chicago/Turabian StyleZiccardi, Lucia, Ettore Cioffi, Lucilla Barbano, Valeria Gioiosa, Benedetto Falsini, Carlo Casali, and Vincenzo Parisi. 2021. "Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs" Journal of Clinical Medicine 10, no. 22: 5271. https://doi.org/10.3390/jcm10225271
APA StyleZiccardi, L., Cioffi, E., Barbano, L., Gioiosa, V., Falsini, B., Casali, C., & Parisi, V. (2021). Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs. Journal of Clinical Medicine, 10(22), 5271. https://doi.org/10.3390/jcm10225271







