Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
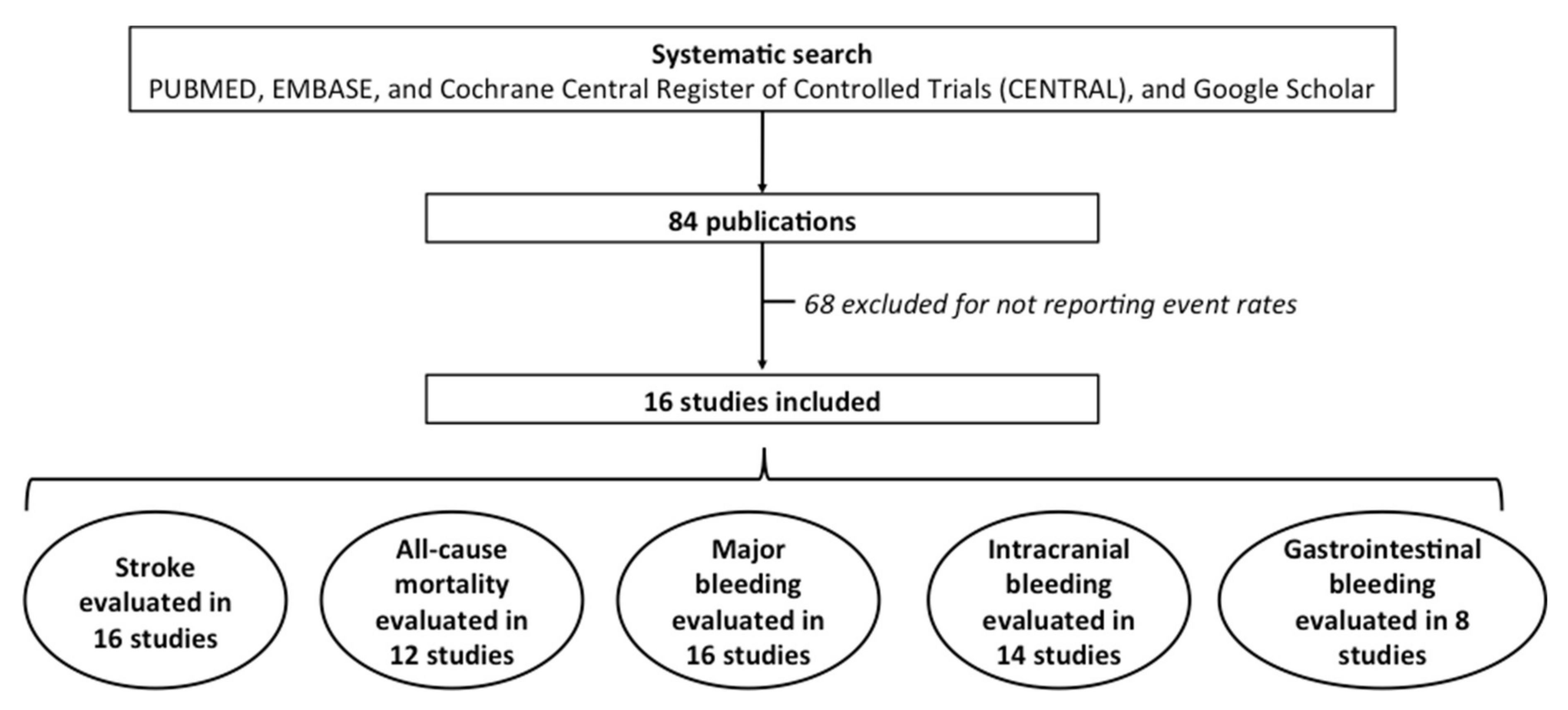
2. Material and Methods
Data Synthesis and Statistical Analyses
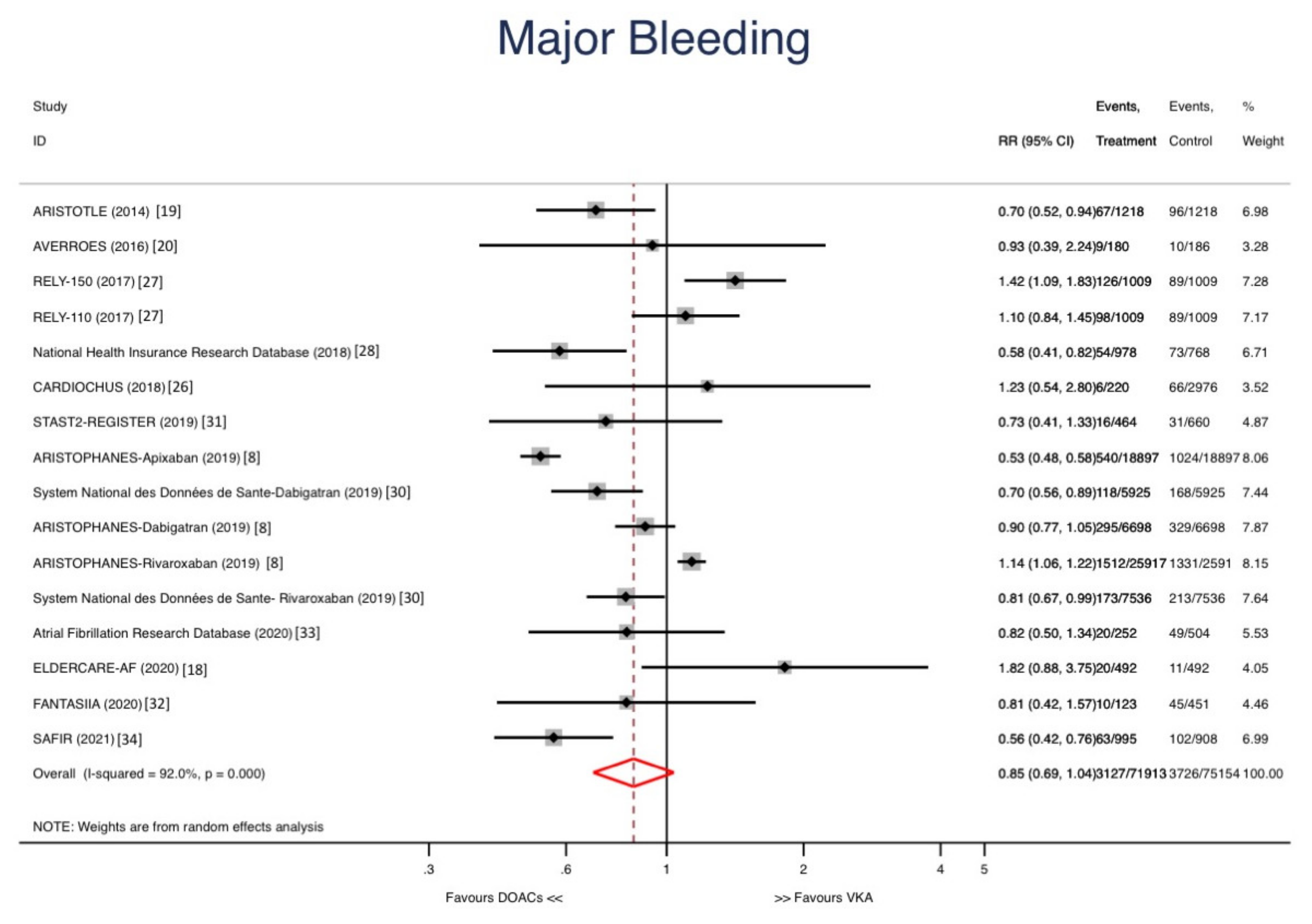
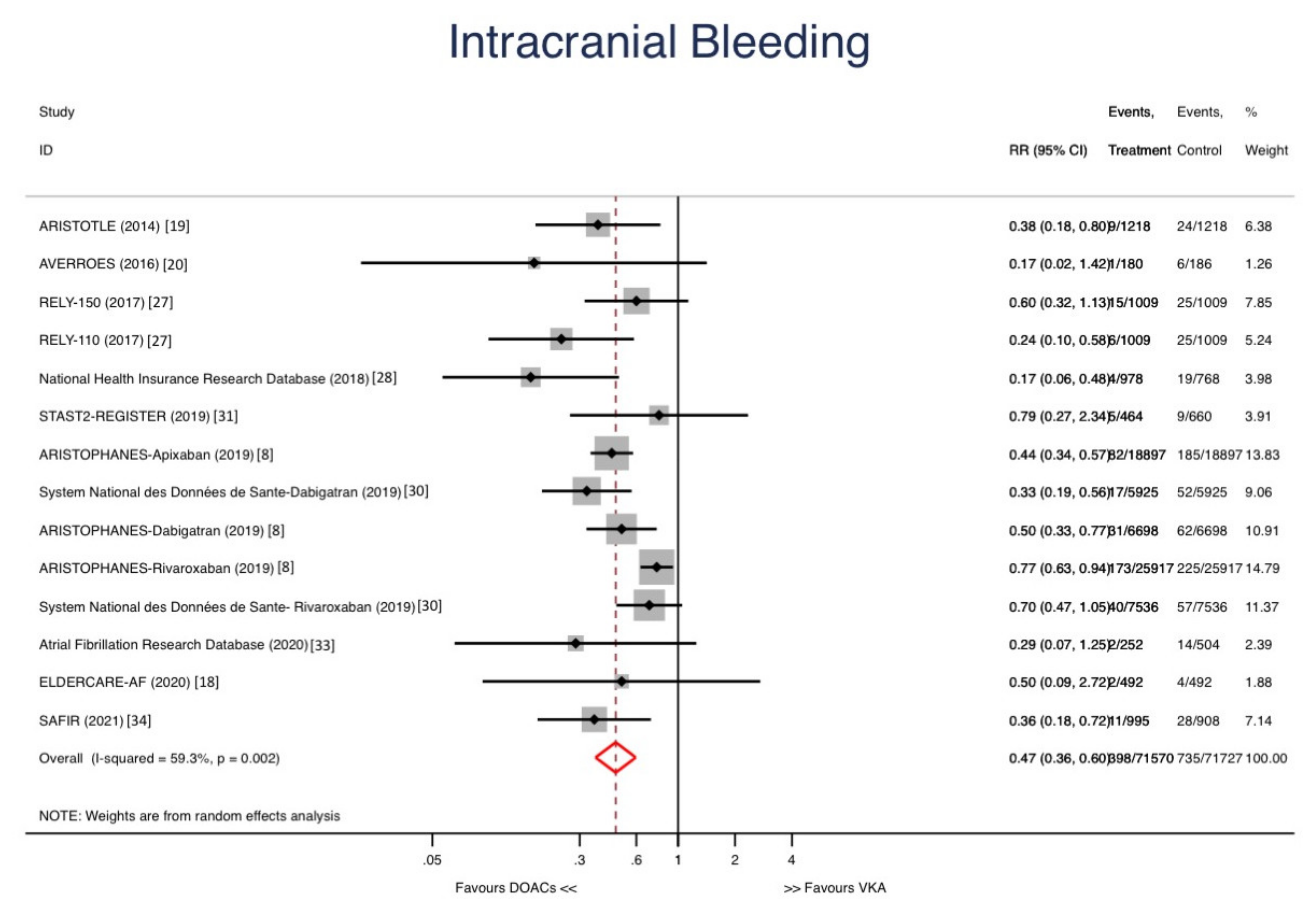
3. Results
| Study | Study Type | First Author | Year | Country | Age Criteria | Follow-Up (Days) | Adjustment | Treatment | Control | Treatment (n) | Control (n) | Mean Age | Males |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARISTOTLE [19] | RCT | Halvorsen | 2014 | World-wide | ≥80 | 900 | Randomization | Apixaban | VKA | 1218 | 1218 | 82 | 512 |
| AVERROES [20] | RCT | Ng | 2016 | World-wide | ≥85 | 375 | Randomization | Apixaban | Aspirin | 180 | 186 | 163 | |
| CARDIOCHUS [26] | O | Rodriguez-Mañero | 2017 | Spain | ≥80 | 696 | Multivariate analyses | DOACs | VKA | 220 | 2976 | 84.6 | 1600 |
| RELY [27] | RCT | Lauw | 2017 | Word-wide | ≥80 | 730 | Randomization | Dabigatran 110 | VKA | 1009 | 1009 | 83 | 1678 |
| RELY [27] | RCT | Lauw | Word-wide | ≥80 | 730 | Randomization | Dabigatran 150 | VKA | 1009 | 1009 | 83 | 1678 | |
| National Health Insurance Research Database [28] | O | Chao | 2018 | Taiwan | ≥90 | 670 | Propensity score matching | DOACs | VKA | 978 | 768 | 92.4 | 778 |
| ARISTOPHANES [8] | O | Deitelzweig | 2019 | US | ≥80 | 243 | Propensity score matching | Apixaban | VKA | 18,897 | 18,897 | 85.3 | 5383 |
| ARISTOPHANES [8] | O | Deitelzweig | 2019 | US | ≥80 | 255 | Propensity score matching | Dabigatran | VKA | 6698 | 6698 | 84.4 | 15,069 |
| ARISTOPHANES [8] | O | Deitelzweig | 2019 | US | ≥80 | 255 | Propensity score matching | Rivaroxaban | VKA | 25,917 | 25,917 | 85.1 | 5655 |
| System National des Données de Sante- Rivaroxaban | O | Blin [29] | 2019 | France | ≥80 | 365 | Propensity score matching | Rivaroxaban | VKA | 7536 | 7536 | 88.5 | 5482 |
| O | Blin [30] | 2019 | France | ≥80 | 365 | Propensity score matching | Dabigatran | VKA | 5925 | 5925 | 88.2 | 4932 | |
| STAST2- REGISTER [31] | O | Poli | 2019 | Italy | ≥85 | 400 | Multivariate analyses | DOACs | VKA | 464 | 660 | 87.9 | 352 |
| ELDERCARE-AF [18] | RCT | Okumura | 2020 | Japan | ≥80 | 466 | Randomization | Edoxaban 15 | Placebo | 492 | 492 | 86.6 | 419 |
| FANTASIIA [32] | O | Anguita-Sanchez | 2020 | Spain | ≥80 | 1095 | Multivariate analyses | DOACs | VKA | 123 | 451 | 83.9 | 254 |
| Atrial Fibrillation Research Database [33] | O | Russo | 2020 | Italy | ≥80 | 855 | Propensity score matching | DOACs | VKA | 252 | 504 | 84.5 | 329 |
| SAFIR [34] | O | Hanon | 2021 | France | ≥80 | 286 | Propensity score matching | Rivaroxaban | VKA | 995 | 908 | 86 | 692 |
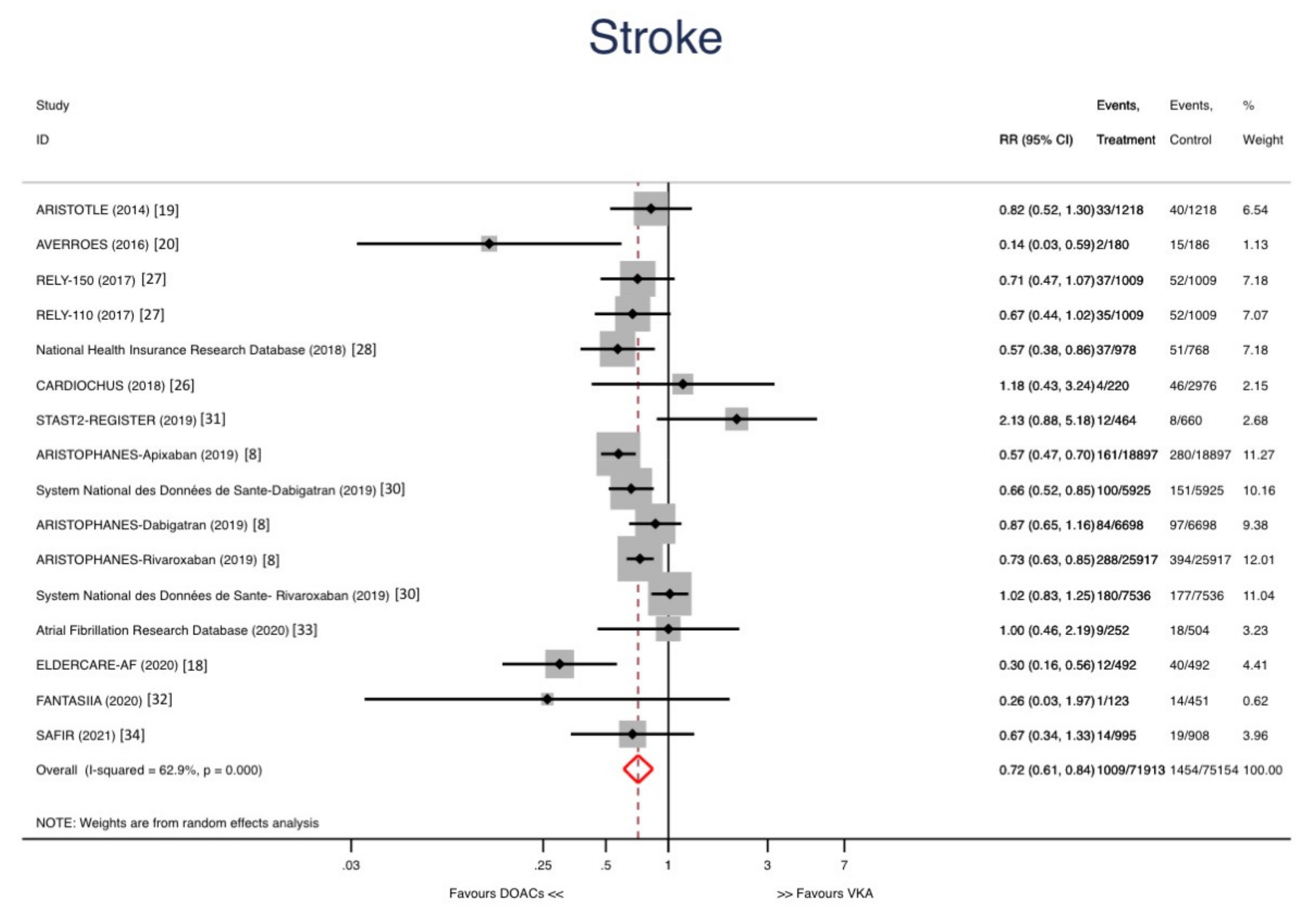
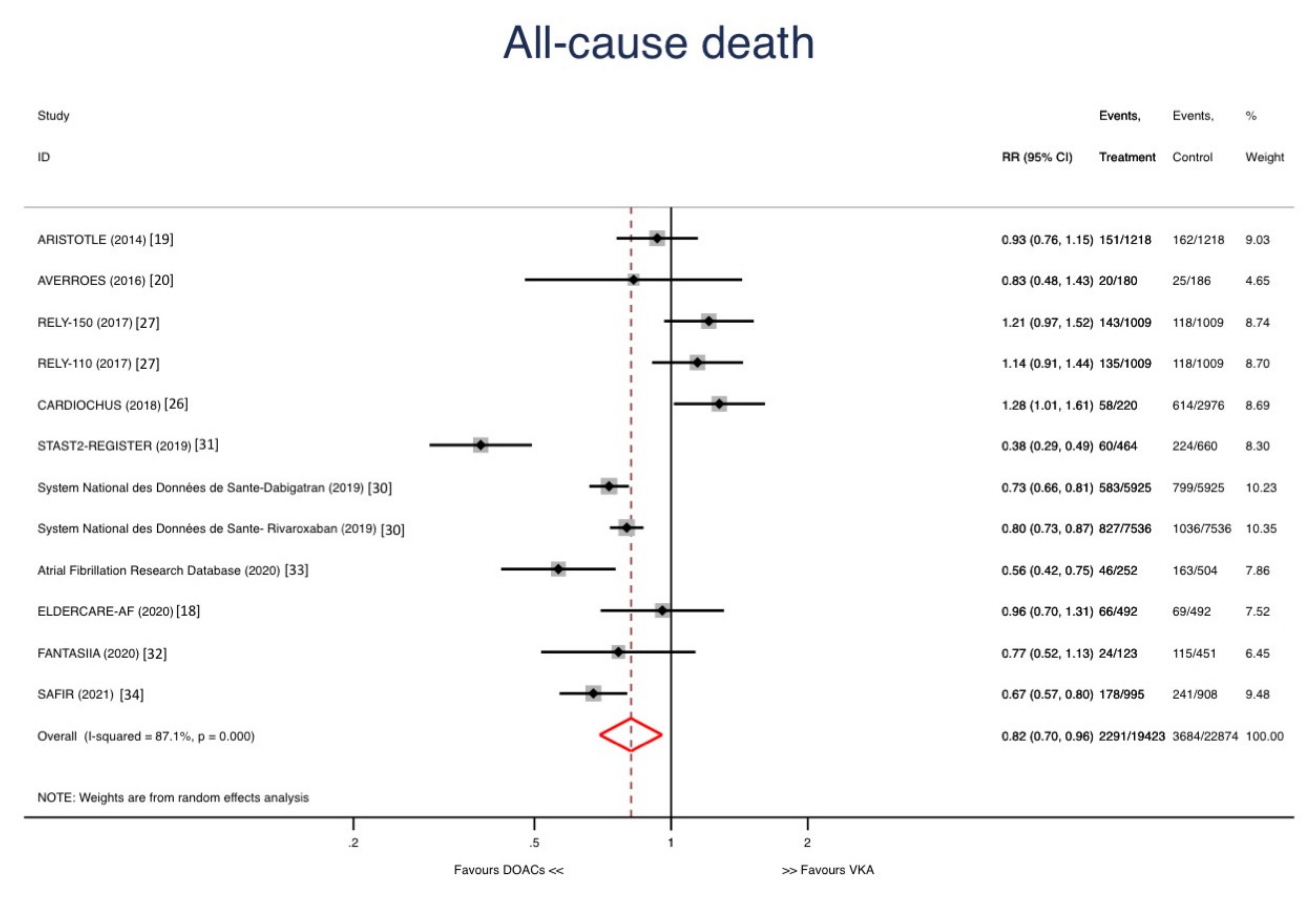
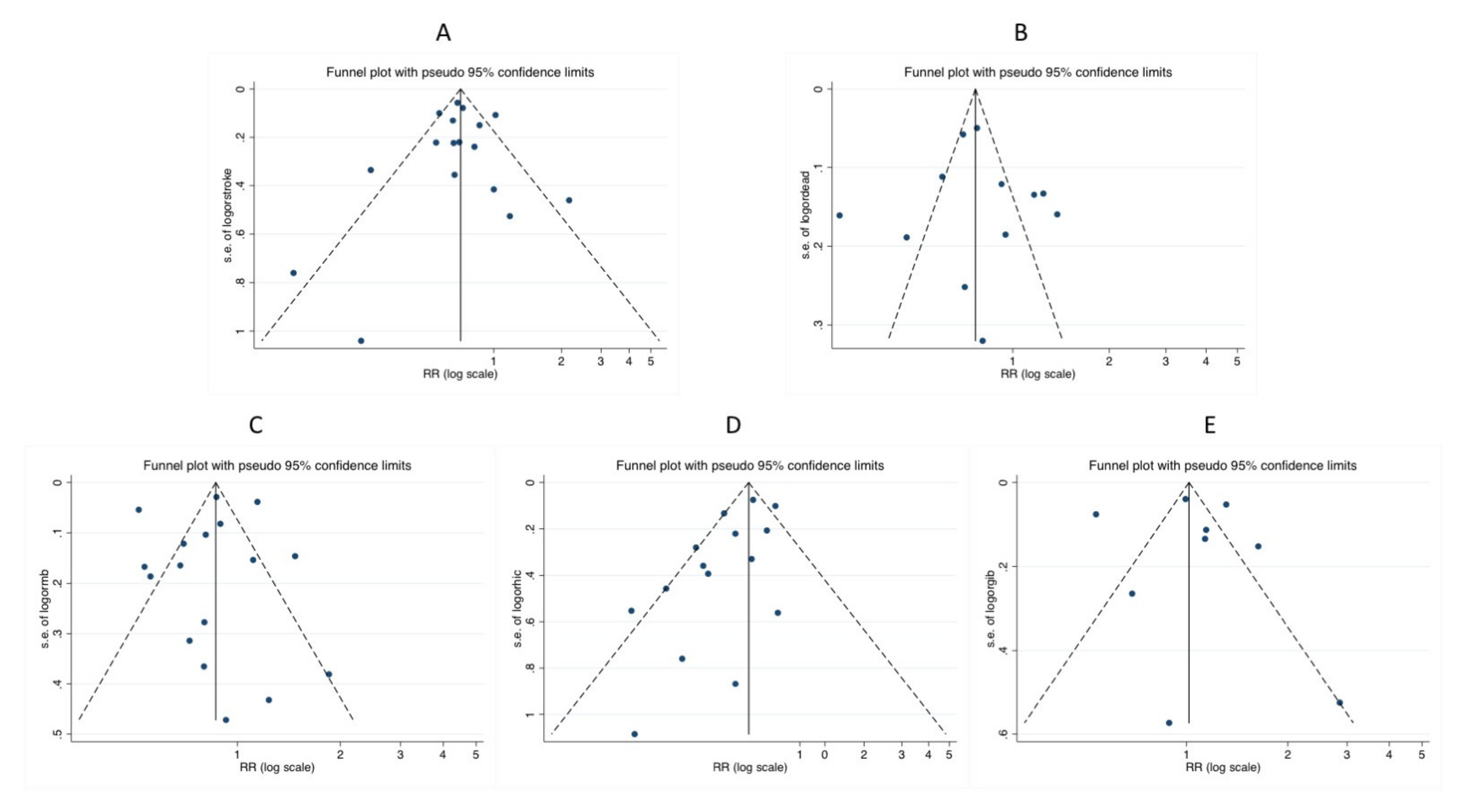
Sensitivity Analyses and Metaregression
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.C.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. J. Am. Med. Assoc. 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Sardar, P.; Chatterjee, S.; Chaudhari, S.; Lip, G.Y.H. New oral anticoagulants in elderly adults: Evidence from a meta-analysis of randomized trials. J. Am. Geriatr. Soc. 2014, 62, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fbrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Barrios, V.; Calderón, A.; Escobar, C.; de la Figuera, M. Patients with Atrial Fibrillation in a Primary Care Setting: Val-FAAP Study. Rev. Esp. Cardiol. 2012, 65, 47–53. [Google Scholar] [CrossRef]
- Gómez-Doblas, J.J.; Muñiz, J.; Martin, J.J.A.; Rodríguez-Roca, G.; Lobos, J.M.; Awamleh, P.; Permanyer-Miralda, G.; Chorro, F.J.; Anguita, M.; Roig, E. Prevalencia de fibrilación auricular en España. Resultados del estudio OFRECE. Rev. Esp. Cardiol. 2014, 67, 259–269. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 25 August 2021).
- Deitelzweig, S.; Keshishian, A.; Li, X.; Kang, A.; Dhamane, A.D.; Luo, X.; Balachander, N.; Rosenblatt, L.; Mardekian, J.; Pan, X.; et al. Comparisons between Oral Anticoagulants among Older Nonvalvular Atrial Fibrillation Patients. J. Am. Geriatr. Soc. 2019, 67, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shen, N.-N.; Wu, Y.; Zhang, C.; Pan, M.-M.; Qian, Y.; Gu, Z.-C. Comparison of effectiveness and safety of direct oral anticoagulants versus vitamin-k antagonists in elderly patients with atrial fibrillation: A systematic review and cost-effectiveness analysis protocol. Ann. Transl. Med. 2020, 8, 391. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Bax, J.J.; Boriani, G.; Dan, G.A.; Fauchier, L.; Kalman, J.M.; Lane, D.A.; Lettino, M.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 9, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low-Dose Edoxaban in Very Elderly Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Atar, D.; Yang, H.; De Caterina, R.; Erol, C.; Garcia, D.; Granger, C.B.; Hanna, M.; Held, C.; Husted, S.; et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: Observations from the ARISTOTLE trial. Eur. Heart J. 2014, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Shestakovska, O.; Connolly, S.J.; Eikelboom, J.W.; Avezum, A.; Diaz, R.; Lanas, F.; Yusuf, S.; Hart, R.G. Efficacy and safety of apixaban compared with aspirin in the elderly: A subgroup analysis from the AVERROES trial. Age Ageing 2016, 45, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eikelboom, J.W.; Wallentin, L.; Connolly, S.J.; Ezekowitz, M.; Healey, J.S.; Oldgren, J.; Yang, S.; Alings, M.; Kaatz, S.; Hohnloser, S.H.; et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) Trial. Circulation 2011, 123, 2363–2372. [Google Scholar] [CrossRef] [Green Version]
- Halperin, J.L.; Hankey, G.J.; Wojdyla, D.M.; Piccini, J.P.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Singer, D.E.; Becker, R.C.; Hacke, W.; et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin k antagonism for prevention of stroke and embolism. Circulation 2014, 130, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Petidier Torregrossa, R.; Abizanda Soler, P.; Noguerón García, A.; Gonzalo Lázaro, M.; Gutiérrez Rodríguez, J.; Gil Gregorio, P.; Martín-Sánchez, F.J.; Ruíz-Artacho, P.; Duems Noriega, Ó.; Veiga Fernández, F. Oral anticoagulation therapy in the elderly population with atrial fibrillation. A review article. Rev. Esp. Geriatr. Gerontol. 2018, 53, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Mañero, M.; López-Pardo, E.; Cordero, A.; Kredieh, O.; Pereira-Vazquez, M.; Martínez-Sande, J.L.; Martínez-Gomez, A.; Peña-Gil, C.; Novo-Platas, J.; García-Seara, J.; et al. Clinical profile and outcomes in octogenarians with atrial fibrillation: A community-based study in a specific European health care area. Int. J. Cardiol. 2017, 243, 211–215. [Google Scholar] [CrossRef]
- Lauw, M.N.; Eikelboom, J.W.; Coppens, M.; Wallentin, L.; Yusuf, S.; Ezekowitz, M.; Oldgren, J.; Nakamya, J.; Wang, J.; Connolly, S.J. Effects of dabigatran according to age in atrial fibrillation. Heart 2017, 103, 1015–1023. [Google Scholar] [CrossRef]
- Chao, T.F.; Liu, C.J.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Liao, J.N.; Chung, F.P.; Chen, T.J.; et al. Oral anticoagulation in very elderly patients with atrial fibrillation: A nationwide cohort study. Circulation 2018, 138, 37–47. [Google Scholar] [CrossRef]
- Blin, P.; Fauchier, L.; Dureau-Pournin, C.; Sacher, F.; Dallongeville, J.; Bernard, M.A.; Lassalle, R.; Droz-Perroteau, C.; Moore, N. Effectiveness and Safety of Rivaroxaban 15 or 20 mg Versus Vitamin K Antagonists in Nonvalvular Atrial Fibrillation: A Population-Based New Users High-Dimensional Propensity Score Matched Cohorts Study. Stroke 2019, 50, 2469–2476. [Google Scholar] [CrossRef]
- Blin, P.; Dureau-Pournin, C.; Cottin, Y.; Bénichou, J.; Mismetti, P.; Abouelfath, A.; Lassalle, R.; Droz, C.; Moore, N. Effectiveness and safety of 110 or 150 mg dabigatran vs. vitamin K antagonists in nonvalvular atrial fibrillation. Br. J. Clin. Pharmacol. 2019, 85, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, D.; Antonucci, E.; Ageno, W.; Bertù, L.; Migliaccio, L.; Martinese, L.; Pilato, G.; Testa, S.; Palareti, G. Oral anticoagulation in very elderly patients with atrial fibrillation: Results from the prospective multicenter START2-REGISTER study. PLoS ONE 2019, 14, e0216831. [Google Scholar] [CrossRef]
- Anguita Sánchez, M.; Bertomeu Martínez, V.; Ruiz Ortiz, M.; Cequier Fillat, Á.; Roldán Rabadán, I.; Muñiz García, J.; Badimón Maestro, L.; Esteve Pastor, M.A.; Marín Ortuño, F. Direct oral anticoagulants versus vitamin K antagonists in real-world patients with nonvalvular atrial fibrillation. The FANTASIIA study. Rev. Esp. Cardiol. 2020, 73, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Attena, E.; Di Maio, M.; Mazzone, C.; Carbone, A.; Parisi, V.; Rago, A.; D’Onofrio, A.; Golino, P.; Nigro, G. Clinical profile of direct oral anticoagulants versus vitamin K anticoagulants in octogenarians with atrial fibrillation: A multicentre propensity score matched real-world cohort study. J. Thromb. Thrombolysis 2020, 49, 42–53. [Google Scholar] [CrossRef]
- Hanon, O.; Vidal, J.S.; Pisica-Donose, G.; Orvoën, G.; David, J.P.; Chaussade, E.; Caillard, L.; De Jong, L.W.; Boulloche, N.; Vinsonneau, U.; et al. Bleeding risk with rivaroxaban compared with vitamin K antagonists in patients aged 80 years or older with atrial fibrillation. Heart 2021, 107, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.H.; Yang, Y.M.; Zhu, J.; Zhang, H.; Liu, Y.; Gao, X.; Yu, L.T.; Liu, L.S.; Zhao, L.; Yu, P.F.; et al. Comparison of the clinical features and outcomes in two age-groups of elderly patients with atrial fibrillation. Clin. Interv. Aging 2014, 9, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mant, J.; Hobbs, F.R.; Fletcher, K.; Roalfe, A.; Fitzmaurice, D.; Lip, G.Y.; Murray, E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): A randomised controlled trial. Lancet 2007, 370, 493–503. [Google Scholar] [CrossRef]
- Patti, G.; Lucerna, M.; Pecen, L.; Siller-Matula, J.M.; Cavallari, I.; Kirchhof, P.; De Caterina, R. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients With Atrial Fibrillation: A Sub-Analysis From the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation). J. Am. Heart Assoc. 2017, 6, e005657. [Google Scholar] [CrossRef] [Green Version]
- Fohtung, R.B.; Novak, E.; Rich, M.W. Effect of New Oral Anticoagulants on Prescribing Practices for Atrial Fibrillation in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.C.D.; St-Onge, M.; Glazer-Cavanagh, M.; Bell, L.; Kha Nguyen, J.N.; Viet-Quoc Nguyen, P.; Tannenbaum, C. The Effect of Bleeding Risk and Frailty Status on Anticoagulation Patterns in Octogenarians With Atrial Fibrillation: The FRAIL-AF Study. Can. J. Cardiol. 2016, 32, 169–176. [Google Scholar] [CrossRef]
- Maes, F.; Dalleur, O.; Henrard, S.; Wouters, D.; Scavée, C.; Spinewine, A.; Boland, B. Risk scores and geriatric profile: Can they really help us in anticoagulation decision making among older patients suffering from atrial fibrillation? Clin. Interv. Aging 2014, 9, 1091–1099. [Google Scholar]
- Sharma, M.; Cornelius, V.R.; Patel, J.P.; Davies, J.G.; Molokhia, M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation 2015, 132, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, D.; Nunes-Ferreira, A.; Rodrigues, R.; Vicente, E.; Pinto, F.J.; Ferreira, J.J. Non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: A systematic review with meta-analysis and trial sequential analysis. Arch. Gerontol. Geriatr. 2019, 81, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, H.J.; Kim, T.H.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: A systematic review and meta-analysis. J. Cardiol. 2018, 72, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, K.; Cheng, J.; Rao, S.; Xu, H.; Li, L.; Gao, Y. Efficacy and safety of direct oral anticoagulants in elderly patients with atrial fibrillation: A network meta-analysis. Front. Med. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Kailas, S.D.; Thambuluru, S.R. Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin in Prevention of Thromboembolic Events Among Elderly Patients with Atrial Fibrillation. Cureus 2016, 8, e836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadlon, A.; Tsakiris, D. Direct oral anticoagulants in the elderly: Systematic review and meta-analysis of evidence, current and future directions. Swiss Med. Wkly. 2016, 146, w14356. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanad, C.; García-Blas, S.; Torres Llergo, J.; Fernández-Olmo, R.; Díez-Villanueva, P.; Ariza-Solé, A.; Martínez-Sellés, M.; Raposeiras, S.; Ayesta, A.; Bertomeu-González, V.; et al. Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5268. https://doi.org/10.3390/jcm10225268
Bonanad C, García-Blas S, Torres Llergo J, Fernández-Olmo R, Díez-Villanueva P, Ariza-Solé A, Martínez-Sellés M, Raposeiras S, Ayesta A, Bertomeu-González V, et al. Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(22):5268. https://doi.org/10.3390/jcm10225268
Chicago/Turabian StyleBonanad, Clara, Sergio García-Blas, Javier Torres Llergo, Rosa Fernández-Olmo, Pablo Díez-Villanueva, Albert Ariza-Solé, Manuel Martínez-Sellés, Sergio Raposeiras, Ana Ayesta, Vicente Bertomeu-González, and et al. 2021. "Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 22: 5268. https://doi.org/10.3390/jcm10225268
APA StyleBonanad, C., García-Blas, S., Torres Llergo, J., Fernández-Olmo, R., Díez-Villanueva, P., Ariza-Solé, A., Martínez-Sellés, M., Raposeiras, S., Ayesta, A., Bertomeu-González, V., Tarazona Santabalbina, F., Facila, L., Vivas, D., Gabaldón-Pérez, A., Bodi, V., Nuñez, J., & Cordero, A. (2021). Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(22), 5268. https://doi.org/10.3390/jcm10225268











