Real-World Experience of Chronic Hepatitis C-Related Compensated Liver Cirrhosis Treated with Glecaprevir/Pibrentasvir: A Multicenter Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment, Efficacy and Safety Evaluation
2.3. Statistical Analyses
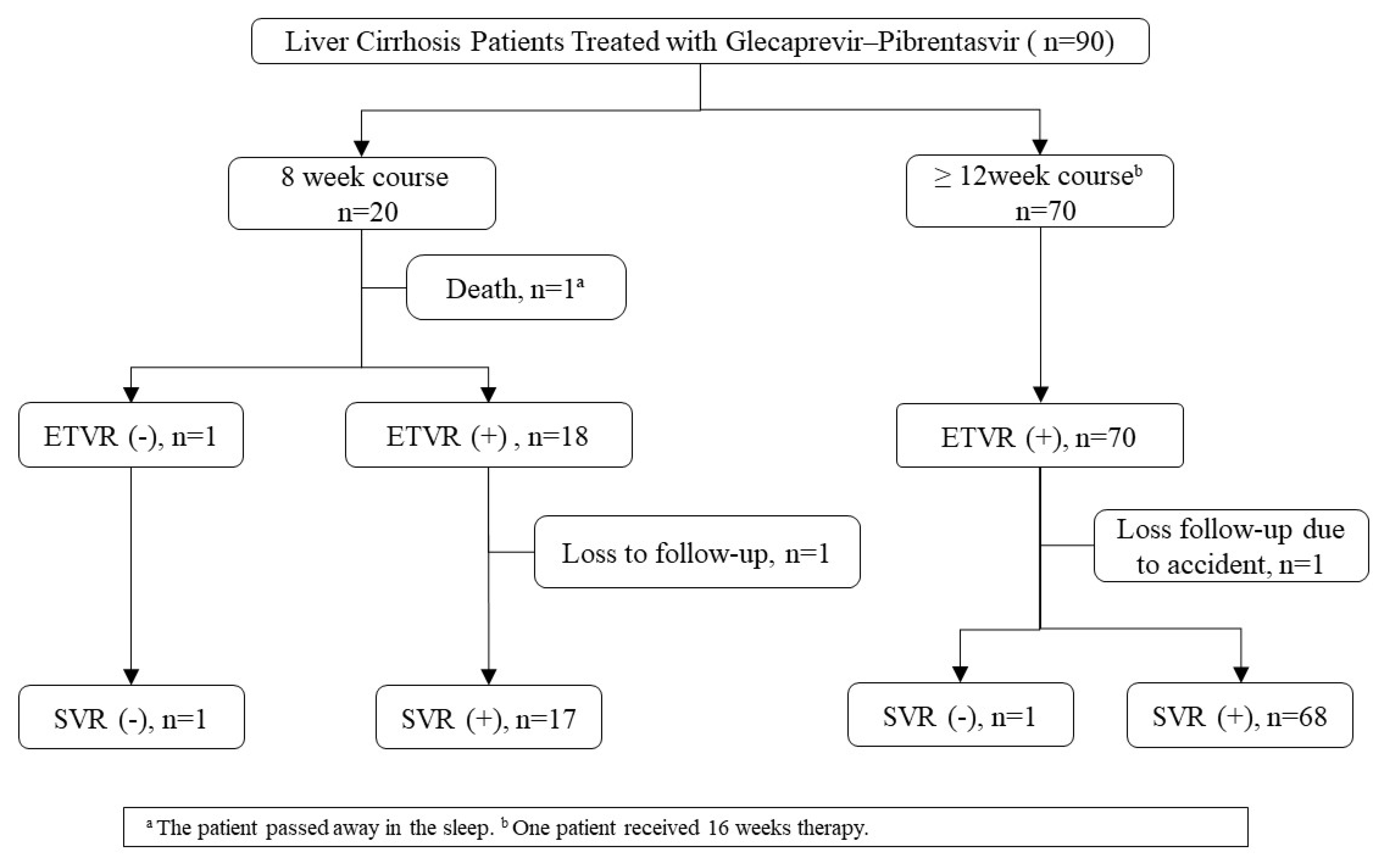
3. Results
3.1. General Characteristics of the Study Population
3.2. The Safety Profile of the Treatment
3.3. Laboratory Change Achieving SVR12
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yen, H.H.; Su, P.Y.; Liu, I.L.; Zeng, Y.H.; Huang, S.P.; Hsu, Y.C.; Hsu, P.K.; Chen, Y.Y. Retrieval of lost patients in the system for hepatitis C microelimination: A single-center retrospective study. BMC Gastroenterol. 2021, 21, 209–216. [Google Scholar] [CrossRef]
- Su, P.Y.; Yen, H.H.; Hsu, Y.C.; Wu, S.S.; Kor, C.T.; Su, W.W. Rapid virological response assessment by Abbott RealTime hepatitis C virus assay for predicting sustained virological responses in patients with hepatitis C virus genotype 1 treated with pegylated-interferon and ribavirin. Kaohsiung J. Med. Sci. 2016, 32, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, H.H.; Shih, K.L.; Lin, T.T.; Su, W.W.; Soon, M.S.; Liu, C.S. Decreased mitochondrial deoxyribonucleic acid and increased oxidative damage in chronic hepatitis C. World J. Gastroenterol. 2012, 18, 5084–5089. [Google Scholar] [CrossRef] [PubMed]
- Roudot-Thoraval, F. Epidemiology of hepatitis C virus infection. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101596. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, C.F.; Liu, C.J.; Dai, C.Y.; Liang, C.C.; Huang, J.F.; Hung, P.H.; Tsai, H.B.; Tsai, M.K.; Chen, S.I.; et al. Pegylated interferon-alpha2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: A randomized trial. Ann. Intern. Med. 2013, 159, 729–738. [Google Scholar] [CrossRef]
- Burki, T. Eliminating hepatitis C. Lancet Infect. Dis. 2019, 19, 246–247. [Google Scholar] [CrossRef]
- Hsu, S.J.; Chiu, M.C.; Fang, Y.J.; Yang, T.H.; Yu, J.J.; Chen, C.C.; Kuo, C.C.; Lee, J.Y.; Chen, C.H.; Chen, D.S.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in Asian patients with chronic hepatitis C. J. Formos. Med. Assoc. 2019, 118, 1187–1192. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, C.Y.; Tsai, S.J.; Chen, Y.C. Anti-hepatitis C virus therapy in chronic kidney disease patients improves long-term renal and patient survivals. World J. Clin. Cases 2019, 7, 1270–1281. [Google Scholar] [CrossRef]
- Su, P.Y.; Chen, Y.Y.; Yen, H.H.; Huang, S.P.; Liu, I.L.; Zeng, Y.H.; Hsu, Y.C.; Siao, F.Y. Strategy for the Micro-Elimination of Hepatitis C among Patients with Diabetes Mellitus-A Hospital-Based Experience. J. Clin. Med. 2021, 10, 2509. [Google Scholar] [CrossRef]
- Yen, H.H.; Su, P.Y.; Huang, S.P.; Wu, L.; Hsu, T.C.; Zeng, Y.H.; Chen, Y.Y. Evaluation of non-alcoholic fatty liver disease in patients with inflammatory bowel disease using controlled attenuation parameter technology: A Taiwanese retrospective cohort study. PLoS ONE 2021, 16, e0252286. [Google Scholar] [CrossRef]
- Brown, R.S., Jr.; Buti, M.; Rodrigues, L.; Chulanov, V.; Chuang, W.L.; Aguilar, H.; Horvath, G.; Zuckerman, E.; Carrion, B.R.; Rodriguez-Perez, F.; et al. Glecaprevir/pibrentasvir for 8weeks in treatment-naive patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J. Hepatol. 2020, 72, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Brau, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Yang, S.S.; Peng, C.Y.; Lin, W.T.; Liu, C.J.; Su, T.H.; Tseng, T.C.; Chen, P.J.; Chen, D.S.; Kao, J.H. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection and severe renal impairment. J. Viral Hepat. 2020, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Lee, S.S.; Valdes, J.; Lens, S.; Ghalib, R.; Aguilar, H.; Felizarta, F.; Hassanein, T.; Hinrichsen, H.; Rincon, D.; et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): A single-arm, open-label, multicentre phase 3 trial. Lancet Infect. Dis. 2017, 17, 1062–1068. [Google Scholar] [CrossRef]
- Lampertico, P.; Carrion, J.A.; Curry, M.; Turnes, J.; Cornberg, M.; Negro, F.; Brown, A.; Persico, M.; Wick, N.; Porcalla, A.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J. Hepatol. 2020, 72, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, T.; Naumann, U.; Stoehr, A.; Sick, C.; John, C.; Teuber, G.; Schiffelholz, W.; Mauss, S.; Lohmann, K.; Konig, B.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: Data from the German Hepatitis C-Registry. Aliment. Pharmacol. Ther. 2019, 49, 1052–1059. [Google Scholar] [CrossRef]
- Yen, H.-H.; Wu, P.-Y.; Su, P.-Y.; Yang, C.-W.; Chen, Y.-Y.; Chen, M.-F.; Lin, W.-C.; Tsai, C.-L.; Lin, K.-P. Performance Comparison of the Deep Learning and the Human Endoscopist for Bleeding Peptic Ulcer Disease. J. Med. Biol. Eng. 2021, 41, 504–513. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver, Clinical Practice Guidelines Panel: Chair. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Flamm, S.L.; Kort, J.; Marx, S.E.; Strezewski, J.; Dylla, D.E.; Bacon, B.; Curry, M.P.; Tsai, N.; Wick, N. Effectiveness of 8-Week Glecaprevir/Pibrentasvir for Treatment-Naive, Compensated Cirrhotic Patients with Chronic Hepatitis C Infection. Adv. Ther. 2020, 37, 2267–2274. [Google Scholar] [CrossRef] [Green Version]
- Gane, E.; Poordad, F.; Zadeikis, N.; Valdes, J.; Lin, C.W.; Liu, W.; Asatryan, A.; Wang, S.; Stedman, C.; Greenbloom, S.; et al. Safety and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1-6 Hepatitis C Virus Infections and Compensated Liver Disease. Clin. Infect. Dis. 2019, 69, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.H.; Su, P.Y.; Zeng, Y.H.; Liu, I.L.; Huang, S.P.; Hsu, Y.C.; Chen, Y.Y.; Yang, C.W.; Wu, S.S.; Chou, K.C. Glecaprevir-pibrentasvir for chronic hepatitis C: Comparing treatment effect in patients with and without end-stage renal disease in a real-world setting. PLoS ONE 2020, 15, e0237582. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Su, P.Y.; Liu, I.L.; Zeng, Y.Y.; Huang, S.P.; Hsu, Y.C.; Yang, C.W.; Chen, Y.Y. Direct-acting antiviral treatment for Hepatitis C Virus in geriatric patients: A real-world retrospective comparison between early and late elderly patients. PeerJ 2021, 9, e10944. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Yeo, Y.H.; Wei, M.T.; Ogawa, E.; Enomoto, M.; Lee, D.H.; Iio, E.; Lubel, J.; Wang, W.; Wei, B.; et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Klinker, H.; Naumann, U.; Rössle, M.; Berg, T.; Bondin, M.; Lohmann, K.; Koenig, B.; Zeuzem, S.; Cornberg, M. Glecaprevir/pibrentasvir for 8 weeks in patients with compensated cirrhosis: Safety and effectiveness data from the German Hepatitis C-Registry. Liver Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wyles, D.; Poordad, F.; Wang, S.; Alric, L.; Felizarta, F.; Kwo, P.Y.; Maliakkal, B.; Agarwal, K.; Hassanein, T.; Weilert, F.; et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology 2018, 67, 514–523. [Google Scholar] [CrossRef]
- Panel, A.-I.H.G. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef] [Green Version]
- Ghany, M.G.; Morgan, T.R.; Panel, A.-I.H.C.G. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leustean, A.; Popescu, C.; Nichita, L.; Tiliscan, C.; Arama, V. Dynamics of APRI and FIB-4 in HCV cirrhotic patients who achieved SVR after DAA therapy. Exp. Ther. Med. 2021, 21, 99–108. [Google Scholar] [CrossRef]
- Knop, V.; Mauss, S.; Goeser, T.; Geier, A.; Zimmermann, T.; Herzer, K.; Postel, N.; Friedrich-Rust, M.; Hofmann, W.P.; German Hepatitis, C.R. Dynamics of liver stiffness by transient elastography in patients with chronic hepatitis C virus infection receiving direct-acting antiviral therapy-Results from the German Hepatitis C-Registry. J. Viral Hepat. 2020, 27, 690–698. [Google Scholar] [CrossRef]
- Rosato, V.; Ascione, A.; Nevola, R.; Fracanzani, A.L.; Piai, G.; Messina, V.; Claar, E.; Coppola, C.; Fontanella, L.; Lombardi, R.; et al. Factors affecting long-term changes of liver stiffness in direct-acting anti-hepatitis C virus therapy: A multicentre prospective study. J. Viral Hepat. 2021. [Google Scholar] [CrossRef]
- Petta, S.; Adinolfi, L.E.; Fracanzani, A.L.; Rini, F.; Caldarella, R.; Calvaruso, V.; Camma, C.; Ciaccio, M.; Di Marco, V.; Grimaudo, S.; et al. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J. Hepatol. 2018, 69, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Perrella, A.; Guarino, M.; De Luca, M.; Piai, G.; Coppola, N.; Pafundi, P.C.; Ciardiello, F.; Fasano, M.; Martinelli, E.; et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: A prospective multicentre study. J. Transl. Med. 2019, 17, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers 2020, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Narciso, V.; Nevola, R.; Rinaldi, L.; Calvaruso, V.; Staiano, L.; Di Marco, V.; et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis 2020, 296, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 90) | Eight-Week Course (n = 20) | ≥Twelve-Week Course (n = 70) | p-Values | |
|---|---|---|---|---|
| Gender, n/N (%) | 52/90 (57.8%) | 10/20 (50.0%) | 42/70 (60.0%) | 0.425 |
| Age, y, mean ± SD | 65 ± 12 | 69 ± 11 | 63 ± 12 | 0.104 |
| HCC, n/N (%) | 15/90 (16.7%) | 7/20 (35.0%) | 8/70 (11.4%) | 0.036 |
| Active HCC *, n/N (%) | 6/15 (40%) | 3/7 (42.9%) | 3/8 (37.5%) | 1.000 |
| ESRD, n/N (%) | 16/90 (17.8%) | 2/20 (10.0%) | 14/70 (20.0%) | 0.508 |
| PWID, n/N (%) | 8/90 (8.9%) | 0 | 8/70 (11.4%) | 0.191 |
| DM, n/N (%) | 27/90 (30.0%) | 6/20 (30.0%) | 21/70 (30.0%) | 1.000 |
| HTN, n/N (%) | 37/90 (41.1%) | 6/20 (30.0%) | 31/70 (44.3%) | 0.252 |
| HBV, n/N (%) | 7/90 (7.8%) | 3/20 (15.0%) | 4/70 (5.7%) | 0.181 |
| HCV Genotype/N (%) | 0.046 | |||
| Type 1 | 36/90 (40.0%) | 12/20 (60.0%) | 24/70 (34.3%) | 0.070 |
| Type 2 | 32/90 (35.6%) | 4/20 (20.0%) | 28/70 (40.0%) | 0.167 |
| Type 3 | 12/90 (13.3%) | 0/20 (0.0%) | 12/70 (17.1%) | 0.061 |
| Type 6 | 3/90 (3.3%) | 1/20 (5.0%) | 2/70 (2.9%) | 0.534 |
| Unclassified type | 7/90 (7.8%) | 3/20 (15.0%) | 4/70 (5.7%) | 0.181 |
| Prior intervention using interferon | 11/90 (12.2%) | 0 | 11/70 (15.7%) | 0.114 |
| Failure | 9/90 (10.0%) | 0 | 9/70 (12.9%) | |
| Interrupted | 2/90 (2.2%) | 0 | 2/70 (2.9%) | |
| HCV viral load, Log IU/mL, median (IQR) | 5.85 (4.9–6.37) | 5.85 (4.5–6.34) | 5.85 (5.1–6.37) | 0.522 |
| GOT (AST), U/L, median (IQR) | 54 (34–117) | 91 (53–162) | 45 (31–104) | 0.004 |
| GPT, U/L, median (IQR) | 55 (33–138) | 86 (43–185) | 54 (30–133) | 0.055 |
| Platelet count, ×10³/μL, median (IQR) | 125 (92–155) | 137 (94–200) | 120 (92–152) | 0.277 |
| Hb, g/dL, median (IQR) | 13.1 (10.2–14) | 13.1 (11.9–14) | 13.1 (10.1–14) | 0.512 |
| Albumin, g/dL, mean ± SD | 3.7 ± 0.42 | 3.75 ± 0.4 | 3.68 ± 0.43 | 0.552 |
| Bilirubin-T, mg/dL, median (IQR) | 0.7 (0.51–0.94) | 0.68 (0.52–0.9) | 0.71 (0.51–0.95) | 0.473 |
| Creatinine, mg/dL, median (IQR) | 1.06 (0.8–2.63) | 1.06 (0.72–1.96) | 1.07 (0.8–4) | 0.578 |
| Prothrombin time (INR), mean ± SD | 1.03 ± 0.08 | 1.04 ± 0.06 | 1.03 ± 0.09 | 0.746 |
| APRI, median (IQR) | 1.309 (0.664–2.847) | 1.82 (1.107–3.517) | 1.042 (0.63–2.629) | 0.046 |
| FIB4, median (IQR) | 4.32 (2.39–7.41) | 6.82 (3.75–7.36) | 3.87 (2.36–7.41) | 0.071 |
| HCV RNA < LLOQ a | All Patients (N = 90) | Eight-Week Course (N = 20) | ≥Twelve-Week Course (N = 70) | p-Values | |||
|---|---|---|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | ||
| ETVR | |||||||
| ITT | 88/90 (97.8) | 92.2–99.7 | 18/20 (90) | 68.3–98.8 | 70/70 (100) | 94.9–100 | 0.047 |
| PP | 88/89 (98.9) | 93.9–100 | 18/19 (94.7) | 73.9–99.9 | 70/70 (100) | 94.9–100 | 0.213 |
| Reason for non-ETVR, n | |||||||
| Death ᵇ | 1 | 1 | 0 | 0.222 | |||
| SVR12 | |||||||
| ITT | 85/90 (94.4) | 87.5–98.2 | 17/20 (85) | 62.1–96.8 | 68/70 (97.1) | 90.0–99.6 | 0.071 |
| PP | 85/87 (97.7) | 92.0–99.7 | 17/18 (94.4) | 72.6–99.9 | 68/69 (98.6) | 92.3–100 | 0.373 |
| Reason for non-SVR12, n | |||||||
| Death b | 1 | 1 | 0 | 0.222 | |||
| Lost to follow-up | 2 | 1 | 1 | 0.397 | |||
| All Patients | Eight-Week Course | ≥Twelve-Week Course | p-Values | |
|---|---|---|---|---|
| Clinical Side Effects | ||||
| Anorexia, n/N (%) | 11/90 (12.2%) | 2/20 (10.0%) | 9/70 (12.9%) | 1.000 |
| Malaise, n/N (%) | 7/90 (7.8%) | 3/20 (15.0%) | 4/70 (5.7%) | 0.181 |
| Abdominal discomfort, n/N (%) | 7/90 (7.8%) | 2/20 (10.0%) | 5/70 (7.1%) | 0.649 |
| Pruritus, n/N (%) | 7/90 (7.8%) | 0 | 7/70 (10.0%) | 0.341 |
| Insomnia, n/N (%) | 6/90 (6.7%) | 1/20 (5.0%) | 5/70 (7.1%) | 1.000 |
| Dizziness, n/N (%) | 4/90 (4.4%) | 1/20 (5.0%) | 3/70 (4.3%) | 1.000 |
| GERD, n/N (%) | 1/90 (1.1%) | 1/20 (5.0%) | 0 | 0.222 |
| Laboratory Side Effects | ||||
| GOT, n/N (%) | 0.016 | |||
| <3× elevation | 83/90 (92.2%) | 16/20 (80.0%) | 67/70 (95.7%) | |
| 3–5× elevation | 5/90 (5.6%) | 2/20 (10.0%) | 3/70 (4.3%) | |
| ≥5× elevation | 2/90 (2.2%) | 2/20 (10.0%) | 0 | |
| GPT, n/N (%) | 0.238 | |||
| <3× elevation | 84/90 (93.3%) | 17/20 (85.0%) | 67/70 (95.7%) | |
| 3–5× elevation | 4/90 (4.4%) | 2/20 (10.0%) | 2/70 (2.9%) | |
| ≥5× elevation | 2/90 (2.2%) | 1/20 (5.0%) | 1/70 (1.4%) | |
| Bilirubin, n/N (%) | 0.437 | |||
| <1.5× elevation | 75/90 (83.3%) | 15/20 (75.0%) | 60/70 (85.7%) | |
| 1.5–3× elevation | 13/90 (14.4%) | 4/20 (20.0%) | 9/70 (12.9%) | |
| ≥3×elevation | 2/90 (2.2%) | 1/20 (5.0%) | 1/70 (1.4%) | |
| CTCAE Hemoglobin, n/N (%) | 0.367 | |||
| G0 | 28/79 (35.4%) | 7/17 (41.2%) | 21/62 (33.9%) | |
| G1 | 29/79 (36.7%) | 8/17 (47.1%) | 21/62 (33.9%) | |
| G2 | 17/79 (21.5%) | 2/17 (11.8%) | 15/62 (24.2%) | |
| G3 | 5/79 (6.3%) | 0 | 5/62 (8.1%) | |
| CTCAE Thrombocytopenia, n/N (%) | 0.303 | |||
| G0 | 25/78 (32.1%) | 8/17 (47.1%) | 17/61 (27.9%) | |
| G1 | 38/78 (48.7%) | 6/17 (35.3%) | 32/61 (52.5%) | |
| G2 | 11/78 (14.1%) | 3/17 (17.6%) | 8/61 (13.1%) | |
| G3 | 4/78 (5.1%) | 0 | 4/61 (6.6%) |
| Item | Baseline | After SVR12 | p-Value |
|---|---|---|---|
| GOT (AST), U/median (IQR) | 55 (36–117) | 25 (20–31) | <0.001 |
| GPT, U/L, median (IQR) | 55 (33–138) | 20 (14–28) | <0.001 |
| Platelet count, ×10³/μL, median (IQR) | 120 (90–152) | 122 (94–177) | 0.219 |
| Hb, g/dL, median (IQR) | 12.9 (10.2–13.9) | 13 (10.3–14.3) | 0.404 |
| Prothrombin time (INR), median (IQR) | 1.03 (0.98–1.08) | 1.02 (0.97–1.06) | 0.011 |
| Albumin, g/dL, median (IQR) | 3.7 (3.5–4) | 3.9 (3.7–4.2) | <0.001 |
| Bilirubin-T, mg/dL, median (IQR) | 0.7 (0.51–0.94) | 0.6 (0.45–0.8) | 0.005 |
| Creatinine, mg/dL, median (IQR) | 1.07 (0.79–2.66) | 1.03 (0.81–2.77) | 0.464 |
| FIB4, median (IQR) | 4.49 (2.39–7.44) | 2.86 (1.84–4.6) | <0.001 |
| APRI, median (IQR) | 1.309 (0.665–2.853) | 0.504 (0.317–0.826) | <0.001 |
| MELD score, median (IQR) | 6.905 (4.31–13.457) | 5.47 (3.065–13.81) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.-Y.; Chen, Y.-Y.; Lai, J.-H.; Chen, H.-M.; Yao, C.-T.; Liu, I.-L.; Zeng, Y.-H.; Huang, S.-P.; Hsu, Y.-C.; Wu, S.-S.; et al. Real-World Experience of Chronic Hepatitis C-Related Compensated Liver Cirrhosis Treated with Glecaprevir/Pibrentasvir: A Multicenter Retrospective Study. J. Clin. Med. 2021, 10, 5236. https://doi.org/10.3390/jcm10225236
Su P-Y, Chen Y-Y, Lai J-H, Chen H-M, Yao C-T, Liu I-L, Zeng Y-H, Huang S-P, Hsu Y-C, Wu S-S, et al. Real-World Experience of Chronic Hepatitis C-Related Compensated Liver Cirrhosis Treated with Glecaprevir/Pibrentasvir: A Multicenter Retrospective Study. Journal of Clinical Medicine. 2021; 10(22):5236. https://doi.org/10.3390/jcm10225236
Chicago/Turabian StyleSu, Pei-Yuan, Yang-Yuan Chen, Jun-Hung Lai, Hung-Ming Chen, Chih-Ta Yao, I-Ling Liu, Ya-Huei Zeng, Siou-Ping Huang, Yu-Chun Hsu, Shun-Sheng Wu, and et al. 2021. "Real-World Experience of Chronic Hepatitis C-Related Compensated Liver Cirrhosis Treated with Glecaprevir/Pibrentasvir: A Multicenter Retrospective Study" Journal of Clinical Medicine 10, no. 22: 5236. https://doi.org/10.3390/jcm10225236
APA StyleSu, P.-Y., Chen, Y.-Y., Lai, J.-H., Chen, H.-M., Yao, C.-T., Liu, I.-L., Zeng, Y.-H., Huang, S.-P., Hsu, Y.-C., Wu, S.-S., Siao, F.-Y., & Yen, H.-H. (2021). Real-World Experience of Chronic Hepatitis C-Related Compensated Liver Cirrhosis Treated with Glecaprevir/Pibrentasvir: A Multicenter Retrospective Study. Journal of Clinical Medicine, 10(22), 5236. https://doi.org/10.3390/jcm10225236






