Hunger and Satiety Peptides: Is There a Pattern to Classify Patients with Prader-Willi Syndrome?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Methods
2.3. Statistical Methods
3. Results
3.1. Fasting Study
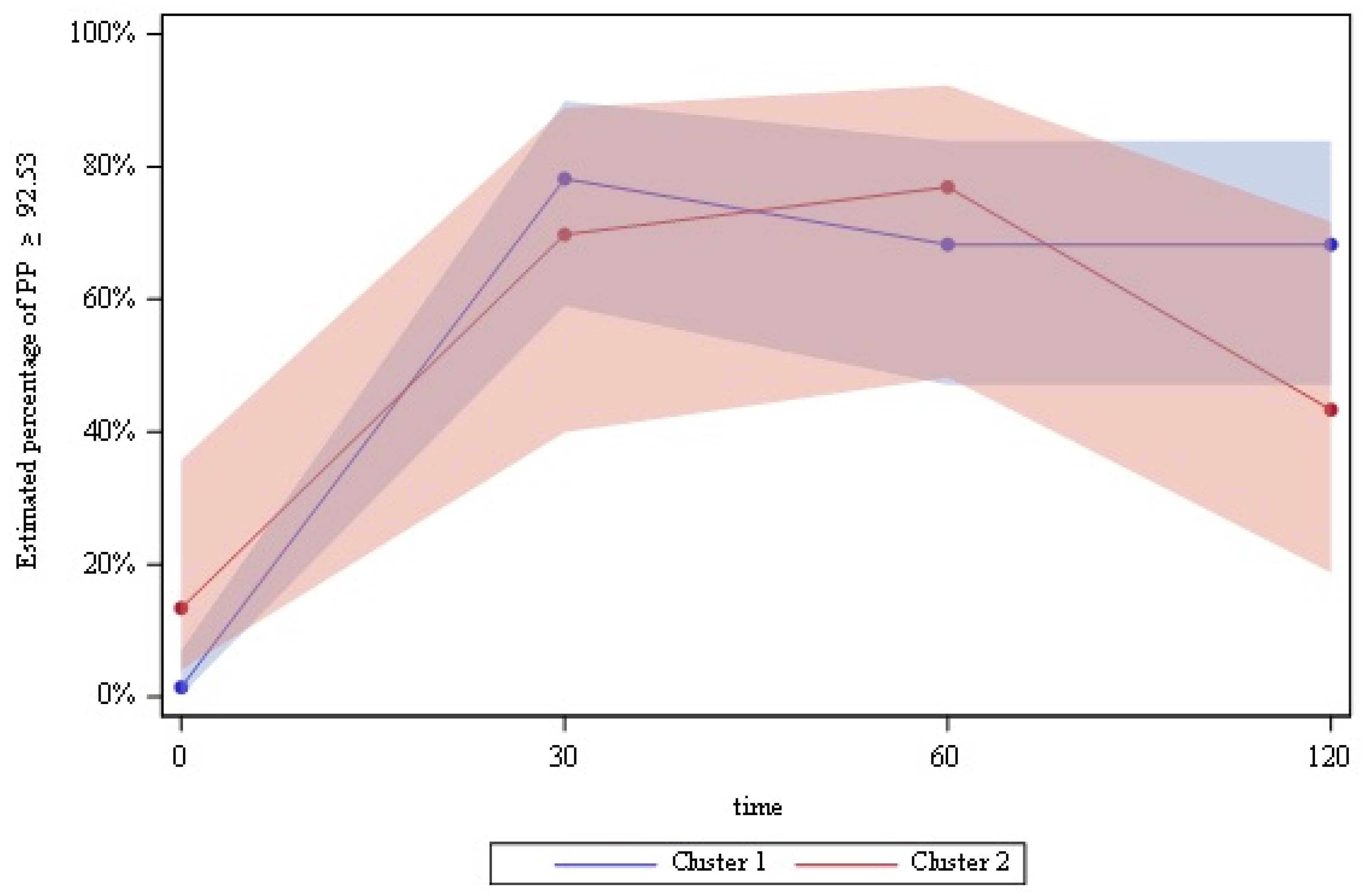
3.2. Postprandial Study
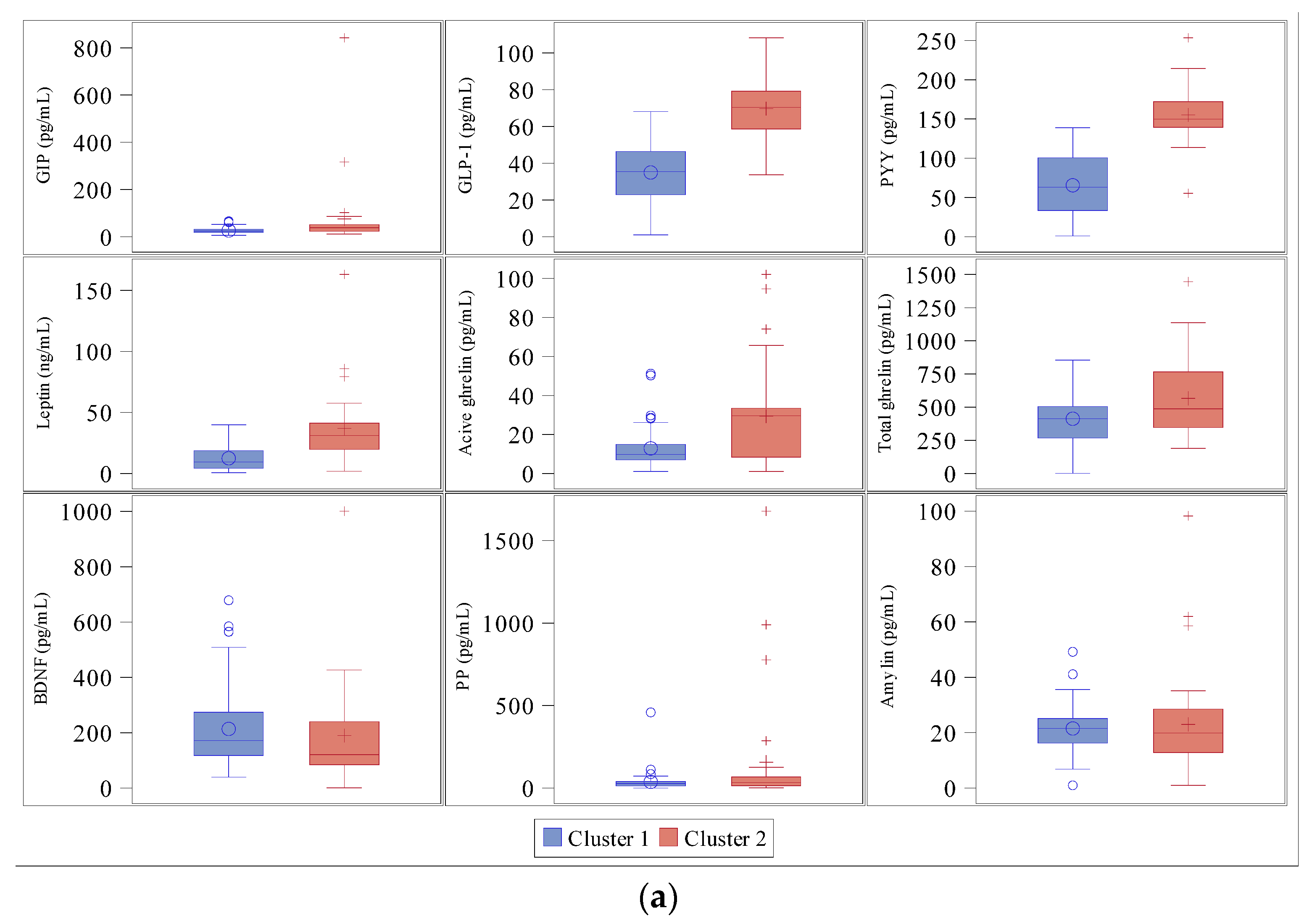
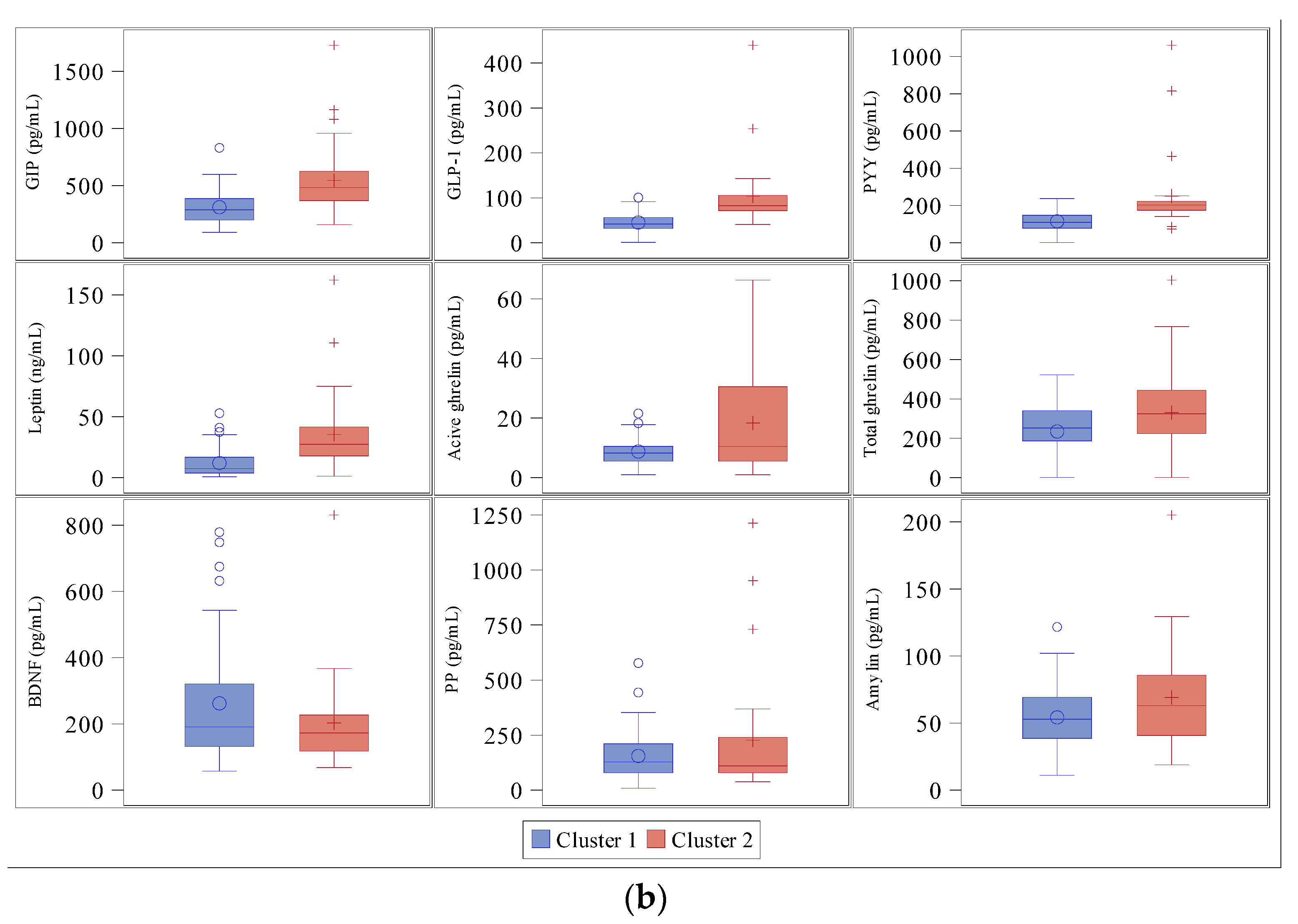
3.3. Cluster Analysis
3.4. Classification Tree
- Leave 1: Leptin < 15.3 ng/mL;
- Leave 3: Leptin ≥ 15.3 ng/mL and GLP-1 < 52.5 pg/mL;
- Leave 4: Leptin ≥ 15.3 ng/mL and GLP-1 ≥ 52.5 pg/mL.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2011, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Cummings, D.E.; Clement, K.; Purnell, J.Q.; Vaisse, C.; Foster, K.E.; Frayo, R.S.; Schwartz, M.W.; Basdevant, A.; Weigle, D.S. Elevated plasma ghrelin levels in Prader–Willi syndrome. Nat. Med. 2002, 8, 643–644. [Google Scholar] [CrossRef]
- Del Parigi, A.; Tschöp, M.; Heiman, M.L.; Salbe, A.D.; Vozarova, B.; Sell, S.M.; Bunt, J.C.; Tataranni, P.A. High Circulating Ghrelin: A Potential Cause for Hyperphagia and Obesity in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5461–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haqq, A.M.; Farooqi, S.; O’Rahilly, S.; Stadler, D.; Rosenfeld, R.G.; Pratt, K.L.; la Franchi, S.H.; Purnell, J.Q. Serum Ghrelin Levels Are Inversely Correlated with Body Mass Index, Age, and Insulin Concentrations in Normal Children and Are Markedly Increased in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 174–178. [Google Scholar] [CrossRef]
- Kweh, F.A.; Miller, J.L.; Sulsona, C.R.; Wasserfall, C.; Atkinson, M.; Shuster, J.J.; Goldstone, A.P.; Driscoll, D.J. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am. J. Med. Genet. Part. A 2014, 167, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Kuppens, R.J.; Diène, G.; Bakker, N.E.; Molinas, C.; Faye, S.; Nicolino, M.; Bernoux, D.; Delhanty, P.J.D.; van der Lely, A.J.; Allas, S.; et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader–Willi syndrome. Endocrine 2015, 50, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Tomita, T.; Greeley, G.; Watt, L.; Doull, V.; Chance, R. Protein Meal-Stimulated Pancreatic Polypeptide Secretion in Prader–Willi Syndrome of Adults. Pancreas 1989, 4, 395–400. [Google Scholar] [CrossRef]
- Zipf, W.B.; O’Dorisio, T.M.; Cataland, S.; Sotos, J. Blunted pancreatic polypeptide responses in children with obesity of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 1981, 52, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Zipf, W.B.; O’Dorisio, T.M.; Cataland, S.; Dixon, K. Pancreatic Polypeptide Responses to Protein Meal Challenges in Obese but Otherwise Normal Children and Obese Children with Prader-Willi Syndrome*. J. Clin. Endocrinol. Metab. 1983, 57, 1074–1080. [Google Scholar] [CrossRef]
- Giménez-Palop, O.; Giménez-Pérez, G.; Mauricio, D.; González-Clemente, J.; Potau, N.; Berlanga, E.; Trallero, R.; Laferrere, B.; Caixas, A. A lesser postprandial suppression of plasma ghrelin in Prader?Willi syndrome is associated with low fasting and a blunted postprandial PYY response. Clin. Endocrinol. 2007, 66, 198–204. [Google Scholar] [CrossRef]
- Butler, M.G.; Bittel, U.C.; Talebizadeh, Z. Plasma peptide YY and ghrelin levels in infants and children with Prader-Willi syndrome. J. Pediatr. Endocrinol. Metab. 2004, 17, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, C.; E Rigamonti, A.; Luce, A.; Cappa, M.; Cella, S.G.; Berini, J.; Sartorio, A.; E Müller, E.; Salvatoni, A. Children with Prader–Willi syndrome exhibit more evident meal-induced responses in plasma ghrelin and peptide YY levels than obese and lean children. Eur. J. Endocrinol. 2010, 162, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, A.P.; Patterson, M.; Kalingag, N.; Ghatei, M.A.; Brynes, A.E.; Bloom, S.R.; Grossman, A.B.; Korbonits, M. Fasting and Postprandial Hyperghrelinemia in Prader-Willi Syndrome Is Partially Explained by Hypoinsulinemia, and Is Not Due to Peptide YY3–36Deficiency or Seen in Hypothalamic Obesity Due to Craniopharyngioma. J. Clin. Endocrinol. Metab. 2005, 90, 2681–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irizarry, K.A.; Bain, J.; Butler, M.G.; Ilkayeva, O.; Muehlbauer, M.; Haqq, A.M.; Freemark, M. Metabolic profiling in Prader-Willi syndrome and nonsyndromic obesity: Sex differences and the role of growth hormone. Clin. Endocrinol. 2015, 83, 797–805. [Google Scholar] [CrossRef]
- Haqq, A.M.; Muehlbauer, M.; Svetkey, L.P.; Newgard, C.B.; Purnell, J.Q.; Grambow, S.C.; Freemark, M.S. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): Association with insulin sensitivity and circulating satiety peptide hormones. Clin. Endocrinol. 2007, 67, 944–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haqq, A.M.; Muehlbauer, M.J.; Newgard, C.B.; Grambow, S.; Freemark, M. The Metabolic Phenotype of Prader-Willi Syndrome (PWS) in Childhood: Heightened Insulin Sensitivity Relative to Body Mass Index. J. Clin. Endocrinol. Metab. 2011, 96, E225–E232. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, A.P.; Thomas, E.; Brynes, A.E.; Castroman, G.; Edwards, R.; Ghatei, M.A.; Frost, G.; Holland, A.J.; Grossman, A.B.; Korbonits, M.; et al. Elevated Fasting Plasma Ghrelin in Prader-Willi Syndrome Adults Is Not Solely Explained by Their Reduced Visceral Adiposity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2004, 89, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Purtell, L.; Sze, L.; Loughnan, G.; Smith, E.; Herzog, H.; Sainsbury, A.; Steinbeck, K.; Campbell, L.V.; Viardot, A. In adults with Prader–Willi syndrome, elevated ghrelin levels are more consistent with hyperphagia than high PYY and GLP-1 levels. Neuropeptides 2011, 45, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigamonti, A.E.; Bini, S.; Grugni, G.; Agosti, F.; De Col, A.; Mallone, M.; Cella, S.G.; Sartorio, A. Unexpectedly increased anorexigenic postprandial responses of PYY and GLP-1 to fast ice cream consumption in adult patients with Prader-Willi syndrome. Clin. Endocrinol. 2014, 81, 542–550. [Google Scholar] [CrossRef]
- Lee, H.J.; Choe, Y.H.; Lee, J.H.; Sohn, Y.B.; Kim, S.J.; Park, S.W.; Son, J.S.; Kim, S.W.; Jin, D.-K. Delayed Response of Amylin Levels after an Oral Glucose Challenge in Children with Prader-Willi Syndrome. Yonsei Med. J. 2011, 52, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.C.; Muehlbauer, M.J.; Cui, H.N.; Newgard, C.B.; Haqq, A.M. Lower Brain-Derived Neurotrophic Factor in Patients with Prader-Willi Syndrome Compared to Obese and Lean Control Subjects. J. Clin. Endocrinol. Metab. 2010, 95, 3532–3536. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Esteba-Castillo, S.; Novell, R.; Giménez-Palop, O.; Coronas, R.; Gabau, E.; Corripio, R.; Baena, N.; Viñas-Jornet, M.; Guitart, M.; et al. Lack of Postprandial Peak in Brain-Derived Neurotrophic Factor in Adults with Prader-Willi Syndrome. PLoS ONE 2016, 11, e0163468. [Google Scholar] [CrossRef] [Green Version]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigerlová, E.; Diene, G.; Conte-Auriol, F.; Molinas, C.; Gennero, I.; Salles, J.-P.; Arnaud, C.; Tauber, M. Hyperghrelinemia Precedes Obesity in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 2800–2805. [Google Scholar] [CrossRef]
- De Waele, K.; Ishkanian, S.L.; Bogarin, R.; A Miranda, C.; A Ghatei, M.; Bloom, S.R.; Pacaud, D.; Chanoine, J.-P. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader–Willi syndrome. Eur. J. Endocrinol. 2008, 159, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.M.-M.; Vanderpump, M.; Khoo, B.; Patterson, M.; Ghatei, M.A.; Goldstone, A.P. Somatostatin Infusion Lowers Plasma Ghrelin without Reducing Appetite in Adults with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 4162–4165. [Google Scholar] [CrossRef] [Green Version]
- Allas, S.; Caixàs, A.; Poitou, C.; Coupaye, M.; Thuilleaux, D.; Lorenzini, F.; Diene, G.; Crinò, A.; Illouz, F.; Grugni, G.; et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0190849. [Google Scholar] [CrossRef]
- Tauber, M.; Diene, G. Prader–Willi syndrome: Hormone therapies. Genet. Med. 2021, 181, 351–367. [Google Scholar] [CrossRef]
- Batterham, R.; Le Roux, C.; Cohen, M.A.; Park, A.J.; Ellis, S.M.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Pancreatic Polypeptide Reduces Appetite and Food Intake in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3989–3992. [Google Scholar] [CrossRef]
- Berntson, G.G.; Zipf, W.B.; O’Dorisio, T.; Hoffman, J.A.; Chance, R.E. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides 1993, 14, 497–503. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Matías, L.C.G.; Alvarez-Crespo, M.; Leiras, R.; Tovar, S.; Diéguez, C.; Mallo, F. Exendin-4 Potently Decreases Ghrelin Levels in Fasting Rats. Diabetes 2006, 56, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Senda, M.; Ogawa, S.; Nako, K.; Okamura, M.; Sakamoto, T.; Ito, S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr. J. 2012, 59, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Wang, Q.; Ran, H.; Liu, K.; Wang, Y.; Wang, J.; Liu, Y.; Chen, R.; Sun, Y.; Liu, R.; et al. Abnormal Response to the Anorexic Effect of GHS-R Inhibitors and Exenatide in Male Snord116 Deletion Mouse Model for Prader-Willi Syndrome. Endocrinology 2014, 155, 2355–2362. [Google Scholar] [CrossRef] [Green Version]
- Salehi, P.; Hsu, I.; Azen, C.G.; Mittelman, S.D.; Geffner, M.E.; Jeandron, D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr. Obes. 2016, 12, 221–228. [Google Scholar] [CrossRef]
- Sze, L.; Purtell, L.; Jenkins, A.; Loughnan, G.; Smith, E.; Herzog, H.; Sainsbury, A.; Steinbeck, K.; Campbell, L.V.; Viardot, A. Effects of a Single Dose of Exenatide on Appetite, Gut Hormones, and Glucose Homeostasis in Adults with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1314–E1319. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.-A.; Driscoll, D.J. Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. Part. A 2017, 173, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, E.; Levine, K.G.; Ferretti, C.J.; Freeman, K.; Doernberg, E.; Desilva, N.; Taylor, B.P. Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi Syndrome: A randomized controlled pilot trial. J. Psychiatr. Res. 2020, 137, 643–651. [Google Scholar] [CrossRef]
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.; Hokken-Koelega, A.C. Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol. 2020, 94, 774–785. [Google Scholar] [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: London, UK, 2011; pp. 71–100. [Google Scholar]
- Zhang, H.; Singer, B.H. Recursive Partitioning and Applications. Springer Sci. Bus. Media 2010. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, K.A.; Mager, D.R.; Triador, L.; Muehlbauer, M.J.; Haqq, A.M.; Freemark, M. Hormonal and metabolic effects of carbohydrate restriction in children with Prader-Willi syndrome. Clin. Endocrinol. 2019, 90, 553–561. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Gross-Tsur, V.; Sabag, Y.; Nice, S.; Genstil, L.; Benarroch, F.; Constantini, N. Myokine levels after resistance exercise in young adults with Prader–Willi syndrome (PWS). Am. J. Med. Genet. Part. A 2019, 182, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Talebizadeh, Z.; Butler, M.G. Insulin resistance and obesity-related factors in Prader-Willi syndrome: Comparison with obese subjects. Clin. Genet. 2004, 67, 230–239. [Google Scholar] [CrossRef] [PubMed]


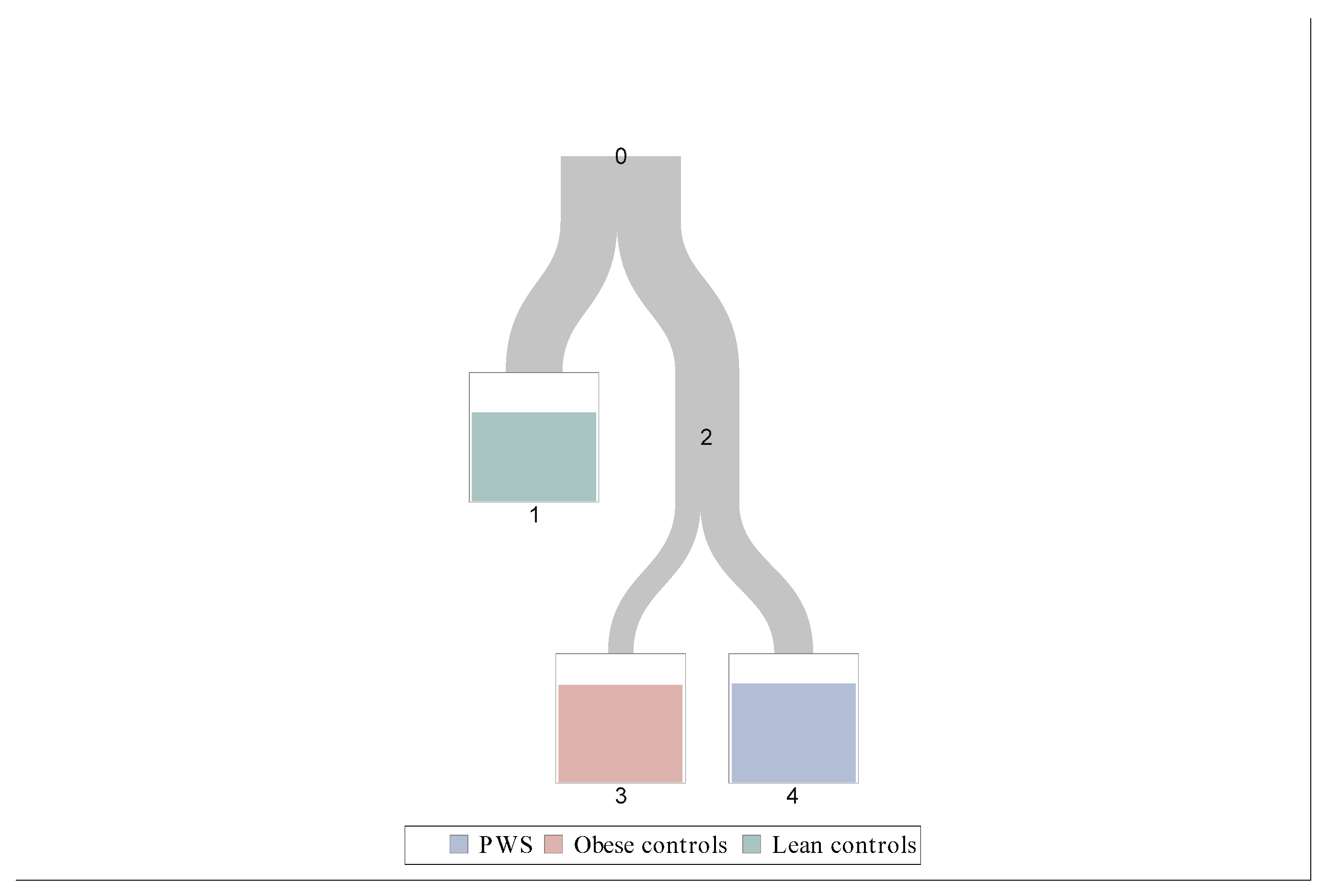
| PWS (n = 30) | Obese Controls (n = 30) | Lean Controls (n = 30) | |
|---|---|---|---|
| Sex (M/F) | 15/15 | 15/15 | 15/15 |
| Age (year) | 27.5 ± 8.02 | 28.4 ± 7.13 | 27.9 ± 7.77 |
| BMI (kg/m2) | 32.4 ± 8.14 | 33.7 ± 6.88 | 22.1 ± 2.05 *$ |
| Body fat (%) | 37.0 ± 8.39 | 35.7 ± 9.70 | 20.3 ± 7.23 *$ |
| Waist (cm) | 105.0 ± 18.3 | 105.3 ± 15.1 | 78.3 ± 7.43 *$ |
| Glucose (mg/dL) | 94.1 ± 22.0 | 98.2 ± 32.1 | 86.7 ± 6.64 $ |
| Insulin (μU/mL) | 333.7 ± 218.5 | 493.2 ± 213.7 * | 304.8 ± 134.7 $ |
| HOMA-IR | 1.69 ± 0.84 | 2.94 ± 1.57 * | 1.69 ± 0.77 $ |
| PWS | Obese Controls | Lean Controls | |
|---|---|---|---|
| BDNF (pg/mL) | 113.7 (75.6–237.7) | 187.6 (119.3–303.0) | 158.2 (118.0–275.8) |
| Leptin (ng/mL) | 35.3 (20.8–42.9) | 18.5 (12.8–25.4) * | 4.4 (2.7–9.2) *$ |
| Total ghrelin (pg/mL) | 572.4 (379.8–812.5) | 279.0 (199.4–396.2) * | 477.2 (350.7–633.6) $ |
| Active ghrelin (pg/mL) | 29.0 (1.0–35.8) | 9.5 (5.5–15.0) | 12.3 (8.6–16.0) |
| PYY (pg/mL) | 149.2 (134.2–176.6) | 101.3 (72.0–134.3) * | 34.6 (1.0–67.7) *$ |
| PP (pg/mL) | 29.9 (9.5–55.3) | 23.5 (12.5–38.3) | 31.8 (23.7–61.2) |
| GLP-1 (pg/mL) | 68.5 (55.8–77.0) | 38.1 (33.7–53.3) * | 32.5 (19.3–49.6) * |
| GIP (pg/mL) | 30.2 (21.1–47.4) | 27.5 (18.5–35.6) | 24.8 (19.6–32.7) |
| Amylin (pg/mL) | 19.9 (7.8–28.5) | 22.4 (15.6–27.1) | 20.9 (16.2–24.2) |
| Classification Tree Leaves | PWS | Obese Controls | Lean Controls |
|---|---|---|---|
| Leave 1 | 2 (4.9%) | 10 (24.4%) | 29 (70.7%) |
| Leave 3 | 3 (16.7%) | 14 (77.8%) | 1 (5.6%) |
| Leave 4 | 22 (77.8%) | 6 (21.4%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, M.; Boixadera-Planas, E.; Blanco-Hinojo, L.; Esteba-Castillo, S.; Giménez-Palop, O.; Torrents-Rodas, D.; Pujol, J.; Corripio, R.; Deus, J.; Caixàs, A. Hunger and Satiety Peptides: Is There a Pattern to Classify Patients with Prader-Willi Syndrome? J. Clin. Med. 2021, 10, 5170. https://doi.org/10.3390/jcm10215170
Bueno M, Boixadera-Planas E, Blanco-Hinojo L, Esteba-Castillo S, Giménez-Palop O, Torrents-Rodas D, Pujol J, Corripio R, Deus J, Caixàs A. Hunger and Satiety Peptides: Is There a Pattern to Classify Patients with Prader-Willi Syndrome? Journal of Clinical Medicine. 2021; 10(21):5170. https://doi.org/10.3390/jcm10215170
Chicago/Turabian StyleBueno, Marta, Ester Boixadera-Planas, Laura Blanco-Hinojo, Susanna Esteba-Castillo, Olga Giménez-Palop, David Torrents-Rodas, Jesús Pujol, Raquel Corripio, Joan Deus, and Assumpta Caixàs. 2021. "Hunger and Satiety Peptides: Is There a Pattern to Classify Patients with Prader-Willi Syndrome?" Journal of Clinical Medicine 10, no. 21: 5170. https://doi.org/10.3390/jcm10215170
APA StyleBueno, M., Boixadera-Planas, E., Blanco-Hinojo, L., Esteba-Castillo, S., Giménez-Palop, O., Torrents-Rodas, D., Pujol, J., Corripio, R., Deus, J., & Caixàs, A. (2021). Hunger and Satiety Peptides: Is There a Pattern to Classify Patients with Prader-Willi Syndrome? Journal of Clinical Medicine, 10(21), 5170. https://doi.org/10.3390/jcm10215170






