Automated Measurements of Ankle-Brachial Index: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
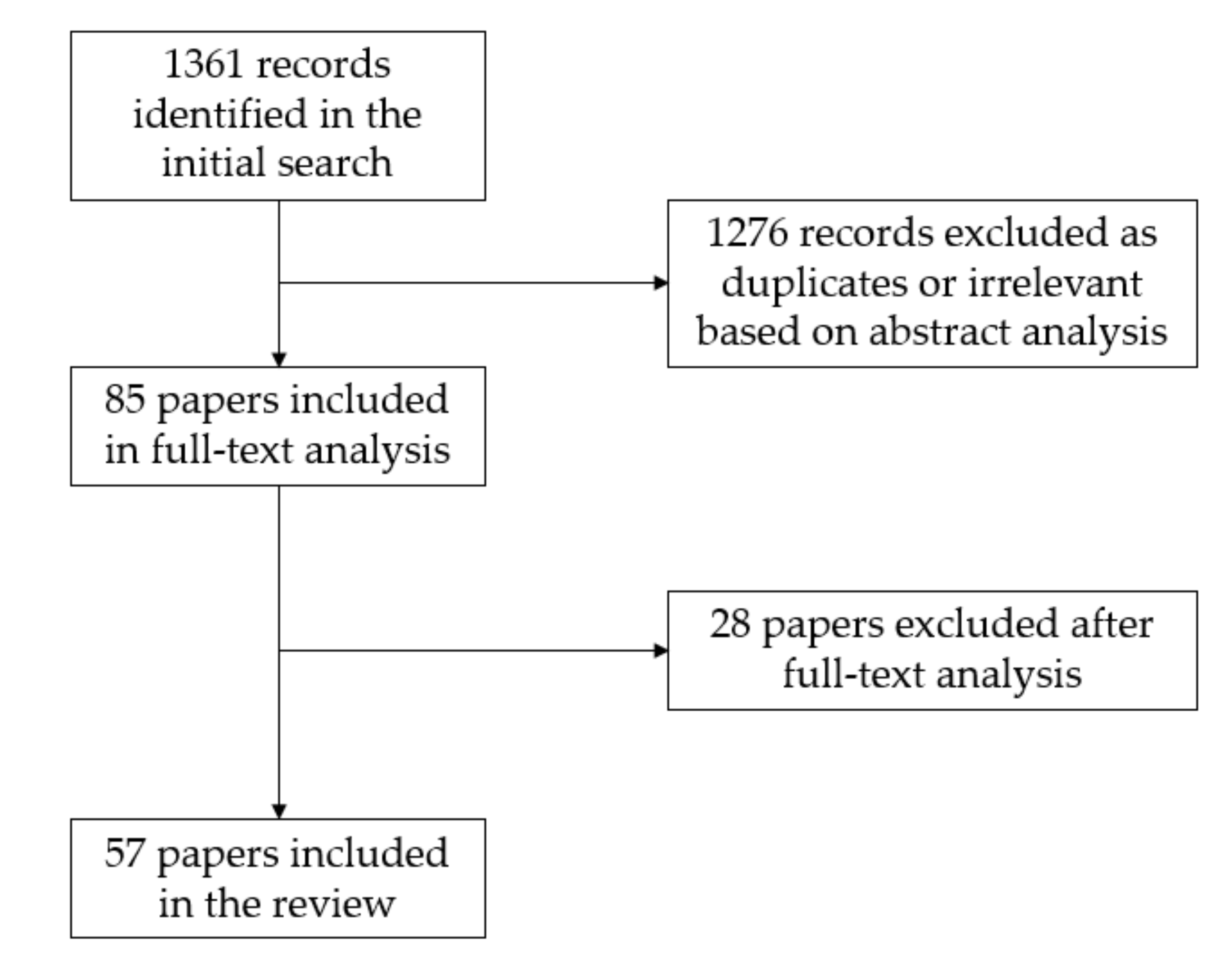
3.1. Literature Search Results
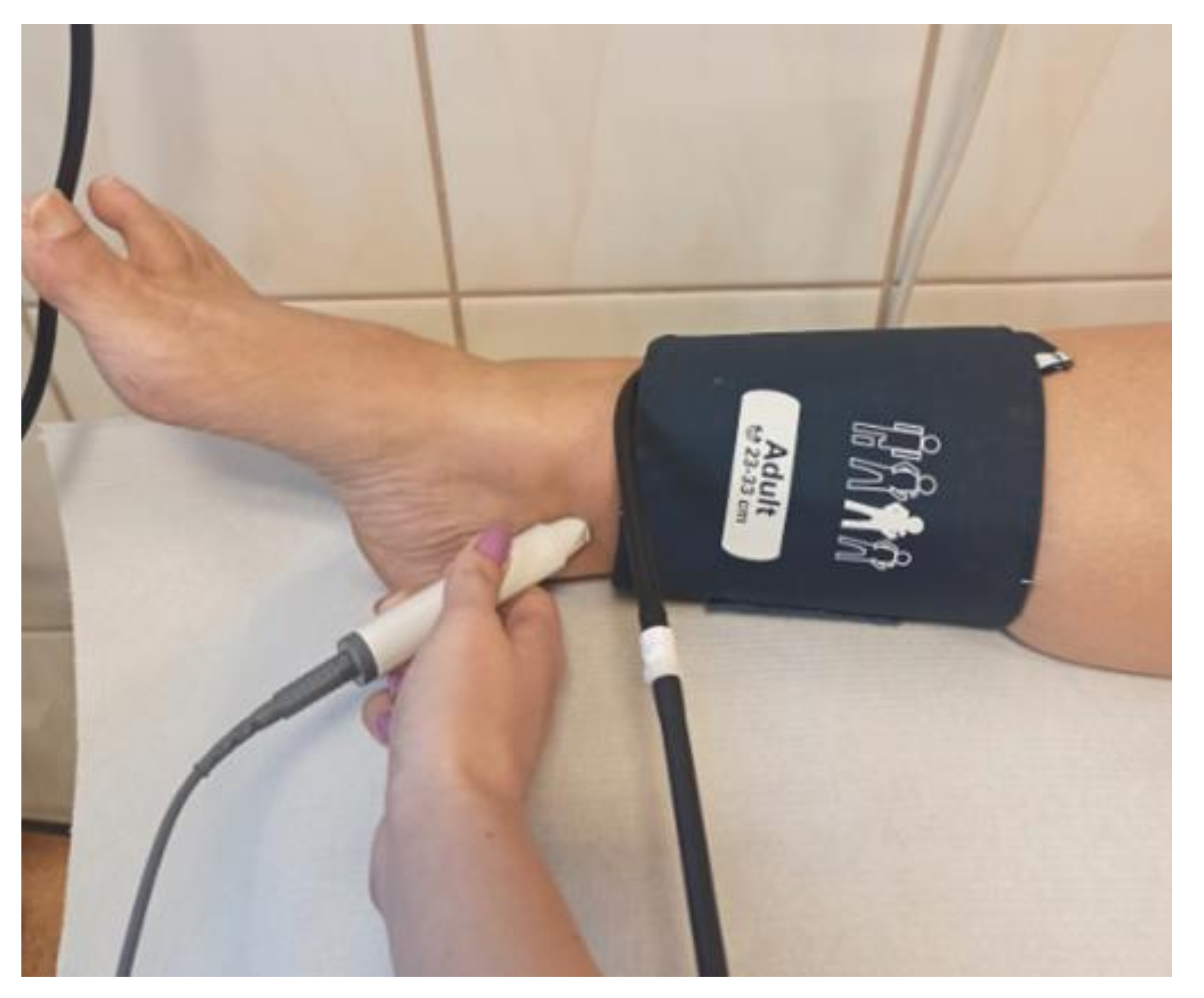
3.2. Automatic Oscillometric Devices
3.3. Automated Plethysmographic Devices
4. Discussion
4.1. Accuracy of Automated Devices
4.2. Resting ABI limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasgupta, A.; Mazumdar, A. Peripheral Artery Disease in the Lower Extremities—Prevalence and Epidemiology. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-16/Peripheral-artery-disease-in-the-lower-extremities-prevalence-and-epidemiology (accessed on 5 May 2020).
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Guralnik, J.M.; Ferrucci, L.; Tian, L.; Liu, K.; Liao, Y.; Green, D.; Sufit, R.; Hoff, F.; Nishida, T.; et al. Asymptomatic Peripheral Arterial Disease Is Associated with More Adverse Lower Extremity Characteristics Than Intermittent Claudication. Circulation 2008, 117, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Davies, J.H.; Kenkre, J.; Williams, E.M. Current Utility of the Ankle-Brachial Index (ABI) in General Practice: Implications for Its Use in Cardiovascular Disease Screening. BMC Fam. Pract. 2014, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Nexøe, J.; Damsbo, B.; Lund, J.O.; Munck, A. Measurement of Blood Pressure, Ankle Blood Pressure and Calculation of Ankle Brachial Index in General Practice. Fam. Pract. 2012, 29, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hageman, D.; Pesser, N.; Gommans, L.N.M.; Willigendael, E.M.; van Sambeek, M.R.H.M.; Huijbers, E.; Snoeijen, A.; Scheltinga, M.R.M.; Teijink, J.A.W. Limited Adherence to Peripheral Arterial Disease Guidelines and Suboptimal Ankle Brachial Index Reliability in Dutch Primary Care. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Yap Kannan, R.; Dattani, N.; Sayers, R.D.; Bown, M.J. Survey of Ankle-Brachial Pressure Index Use and Its Perceived Barriers by General Practitioners in the UK. Postgrad. Med. J. 2016, 92, 322–327. [Google Scholar] [CrossRef]
- Haigh, K.J.; Bingley, J.; Golledge, J.; Walker, P.J. Barriers to Screening and Diagnosis of Peripheral Artery Disease by General Practitioners. Vasc. Med. 2013, 18, 325–330. [Google Scholar] [CrossRef]
- Lewis, P.S. Oscillometric Measurement of Blood Pressure: A Simplified Explanation. A Technical Note on Behalf of the British and Irish Hypertension Society. J. Hum. Hypertens. 2019, 33, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Khandanpour, N.; Armon, M.P.; Jennings, B.; Clark, A.; Meyer, F.J. Photoplethysmography, an Easy and Accurate Method for Measuring Ankle Brachial Pressure Index: Can Photoplethysmography Replace Doppler? Vasc. Endovasc. Surg. 2009, 43, 578–582. [Google Scholar] [CrossRef]
- Davies, J.H.; Williams, E.M. Automated Plethysmographic Measurement of the Ankle-Brachial Index: A Comparison with the Doppler Ultrasound Method. Hypertens. Res. 2016, 39, 100–106. [Google Scholar] [CrossRef] [PubMed]
- NICE. Overview. Peripheral Arterial Disease: Diagnosis and Management. Guidance. Available online: https://www.nice.org.uk/guidance/cg147 (accessed on 5 May 2020).
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS) Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity Arteries Endorsed by: The European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.; Gerry, R.; Hiatt, W.R.; Jönsson, B.; et al. Measurement and Interpretation of the Ankle-Brachial Index. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on Peripheral Arterial Disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Abola, M.T.B.; Golledge, J.; Miyata, T.; Rha, S.-W.; Yan, B.P.; Dy, T.C.; Ganzon, M.S.V.; Handa, P.K.; Harris, S.; Zhisheng, J.; et al. Asia-Pacific Consensus Statement on the Management of Peripheral Artery Disease: A Report from the Asian Pacific Society of Atherosclerosis and Vascular Disease Asia-Pacific Peripheral Artery Disease Consensus Statement Project Committee. J. Atheroscler. Thromb. 2020, 27, 809–907. [Google Scholar] [CrossRef] [PubMed]
- Vinyoles, E.; Pujol, E.; Casermeiro, J.; de Prado, C.; Jabalera, S.; Salido, V. Ankle-brachial index to detect peripheral arterial disease: Concordance and validation study between Doppler and an oscillometric device. Med. Clin. 2007, 128, 92–94. [Google Scholar] [CrossRef]
- Vega, J.; Romaní, S.; Garcipérez, F.J.; Vicente, L.; Pacheco, N.; Zamorano, J.; Gómez-Barrado, J.J.; Sánchez Muñoz-Torrero, J.F. Peripheral arterial disease: Efficacy of the oscillometric method. Rev. Esp. Cardiol. 2011, 64, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Clairotte, C.; Retout, S.; Potier, L.; Roussel, R.; Escoubet, B. Automated Ankle-Brachial Pressure Index Measurement by Clinical Staff for Peripheral Arterial Disease Diagnosis in Nondiabetic and Diabetic Patients. Diabetes Care 2009, 32, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Furukawa, K.; Ohishi, W.; Takahashi, T.; Matsumoto, M.; Fujiwara, S. Comparison between Oscillometric- and Doppler-ABI in Elderly Individuals. Vasc. Health Risk Manag. 2013, 9, 89–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massmann, A.; Stemler, J.; Fries, P.; Kubale, R.; Kraushaar, L.E.; Buecker, A. Automated Oscillometric Blood Pressure and Pulse-Wave Acquisition for Evaluation of Vascular Stiffness in Atherosclerosis. Clin. Res. Cardiol. 2017, 106, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, P.; Ingrischová, M.; Krajcoviechová, A.; Palous, D.; Dolejsová, M.; Seidlerová, J.; Galovcová, M.; Bruthans, J.; Jozífová, M.; Adámková, V.; et al. A Novel Oscillometric Device for Peripheral Arterial Disease Screening in Everyday Practice. The Czech-Post MONICA Study. Int. Angiol. 2011, 30, 256–261. [Google Scholar] [PubMed]
- Nelson, M.R.; Quinn, S.; Winzenberg, T.M.; Howes, F.; Shiel, L.; Reid, C.M. Ankle-Brachial Index Determination and Peripheral Arterial Disease Diagnosis by an Oscillometric Blood Pressure Device in Primary Care: Validation and Diagnostic Accuracy Study. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Boilley, P.-Y.; Howlett, J.; Tollenaere, Q.; Miossec, A.; Guilcher, A.; Lanéelle, D.; Mahé, G. Comparison of Ankle–Brachial Index Measured with an Automatic Oscillometric Method with the Standard Continuous Doppler Method and Effect of Rest Time before the Measure in Patients with Exertional Limb Symptoms. Hypertens. Res. 2020, 43, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Conaghan, P.J.; Jenkinson, A.D.; Bishop, C.R. Comparison of Ankle-Brachial Pressure Index Measurements Using an Automated Oscillometric Device with the Standard Doppler Ultrasound Technique. ANZ J. Surg. 2003, 73, 105–108. [Google Scholar] [CrossRef]
- Kornø, M.; Eldrup, N.; Sillesen, H. Comparison of Ankle-Brachial Index Measured by an Automated Oscillometric Apparatus with That by Standard Doppler Technique in Vascular Patients. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 610–615. [Google Scholar] [CrossRef][Green Version]
- Ichihashi, S.; Hashimoto, T.; Iwakoshi, S.; Kichikawa, K. Validation Study of Automated Oscillometric Measurement of the Ankle-Brachial Index for Lower Arterial Occlusive Disease by Comparison with Computed Tomography Angiography. Hypertens. Res. 2014, 37, 591–594. [Google Scholar] [CrossRef]
- Diehm, N.; Dick, F.; Czuprin, C.; Lawall, H.; Baumgartner, I.; Diehm, C. Oscillometric Measurement of Ankle-Brachial Index in Patients with Suspected Peripheral Disease: Comparison with Doppler Method. Swiss Med. Wkly. 2009, 139, 357–363. [Google Scholar] [CrossRef]
- Sinski, M.; Styczynski, G.; Szmigielski, C. Automated Oscillometric Measurement of the Ankle-Brachial Index in Patients with Coronary Artery Disease. Hypertens. Res. 2013, 36, 25–28. [Google Scholar] [CrossRef][Green Version]
- MacDougall, A.M.; Tandon, V.; Wilson, M.P.; Wilson, T.W. Oscillometric Measurement of Ankle-Brachial Index. Can. J. Cardiol. 2008, 24, 49–51. [Google Scholar] [CrossRef]
- Kollias, A.; Xilomenos, A.; Protogerou, A.; Dimakakos, E.; Stergiou, G.S. Automated Determination of the Ankle-Brachial Index Using an Oscillometric Blood Pressure Monitor: Validation vs. Doppler Measurement and Cardiovascular Risk Factor Profile. Hypertens. Res. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Herráiz-Adillo, Á.; Martínez-Vizcaíno, V.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Garrido-Miguel, M.; Notario-Pacheco, B. Diagnostic Accuracy Study of an Oscillometric Ankle-Brachial Index in Peripheral Arterial Disease: The Influence of Oscillometric Errors and Calcified Legs. PLoS ONE 2016, 11, e0167408. [Google Scholar] [CrossRef]
- Ma, J.; Liu, M.; Chen, D.; Wang, C.; Liu, G.; Ran, X. The Validity and Reliability between Automated Oscillometric Measurement of Ankle-Brachial Index and Standard Measurement by Eco-Doppler in Diabetic Patients with or without Diabetic Foot. Int. J. Endocrinol. 2017, 2017, 2383651. [Google Scholar] [CrossRef] [PubMed]
- Špan, M.; Geršak, G.; Millasseau, S.C.; Meža, M.; Košir, A. Detection of Peripheral Arterial Disease with an Improved Automated Device: Comparison of a New Oscillometric Device and the Standard Doppler Method. Vasc. Health Risk Manag. 2016, 12, 305–311. [Google Scholar] [CrossRef]
- Babaei, M.R.; Malek, M.; Rostami, F.T.; Emami, Z.; Madani, N.H.; Khamseh, M.E. Non-Invasive Vascular Assessment in People with Type 2 Diabetes: Diagnostic Performance of Plethysmographic-and-Doppler Derived Ankle Brachial Index, Toe Brachial Index, and Pulse Volume Wave Analysis for Detection of Peripheral Arterial Disease. Prim. Care Diabetes 2019. [Google Scholar] [CrossRef]
- Jönsson, B.; Laurent, C.; Eneling, M.; Skau, T.; Lindberg, L.-G. Automatic Ankle Pressure Measurements Using PPG in Ankle-Brachial Pressure Index Determination. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 395–401. [Google Scholar] [CrossRef][Green Version]
- Lewis, J.E.; Williams, P.; Davies, J.H. Non-Invasive Assessment of Peripheral Arterial Disease: Automated Ankle Brachial Index Measurement and Pulse Volume Analysis Compared to Duplex Scan. SAGE Open Med. 2016, 4. [Google Scholar] [CrossRef]
- Teren, A.; Beutner, F.; Wirkner, K.; Loeffler, M.; Scholz, M. Validity, Intra- and Inter-Observer Reliability of Automated Devices for the Assessment of Ankle Brachial Index Using Photo-Plethysmography. BMC Cardiovasc. Disord. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, S.; Chithriki, M. Arterial Pressure Measurements Using Infrared Photosensors: Comparison with CW Doppler. Clin. Physiol. 2001, 21, 129–132. [Google Scholar] [CrossRef]
- Arnold, C.G.; Walker, J.R.; Metter, E.J.; Young, S.; Brady, M.F. Pulse Oximeter Plethysmograph Waveform and Automated Oscillometric Sphygmomanometer for Ankle-Brachial Index Measurement. Am. J. Emerg. Med. 2021, 40, 162–165. [Google Scholar] [CrossRef]
- Beutner, F.; Teren, A.; Gielen, S.; Schuler, G.; Wirkner, K.; Tiller, D.; Loeffler, M.; Scholz, M. Automated Photoplethysmography-Based Determination of Ankle-Brachial Index: A Validation Study against Doppler Sonography. Clin. Res. Cardiol. 2012, 101, 875–883. [Google Scholar] [CrossRef]
- Alnaeb, M.E.; Boutin, A.; Crabtree, V.P.; Mikhailidis, D.P.; Seifalian, A.M.; Hamilton, G. Assessment of Lower Extremity Peripheral Arterial Disease Using a Novel Automated Optical Device. Vasc. Endovascular. Surg. 2007, 41, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Alnaeb, M.E.; Crabtree, V.P.; Boutin, A.; Mikhailidis, D.P.; Seifalian, A.M.; Hamilton, G. Prospective Assessment of Lower-Extremity Peripheral Arterial Disease in Diabetic Patients Using a Novel Automated Optical Device. Angiology 2007, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Millen, R.N.; Thomas, K.N.; Majumder, A.; Hill, B.G.; Van Rij, A.M.; Krysa, J. Accuracy and Repeatability of the Dopplex Ability. Expert Rev. Med. Devices 2018, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Van der Slegt, J.; Verbogt, N.P.; Mulder, P.G.; Steunenberg, S.L.; Steunenberg, B.E.; van der Laan, L. The Clinical Applicability of an Automated Plethysmographic Determination of the Ankle-Brachial Index after Vascular Surgery. Vascular 2016, 24, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Hawkins, M.; Barree, P.; Cawley, S.; Dayananda, S. A Comparison between a New Automatic System and Doppler Method for Obtaining Ankle Brachial Pressures. J. Foot Ankle Res. 2010, 3, O15. [Google Scholar] [CrossRef]
- Khan, S.Z.; Bin-Zafar, A.; Waris, N.; Miyan, Z.; Ulhaque, M.S.; Fawwad, A. Comparison of Ankle-Brachial Index (ABI) Measured by an Automated Oscillometric Apparatus with That by Standard Hand-Held Doppler in Patients with Type-2 Diabetes. Pak. J. Med. Sci. 2019, 35, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Herráiz-Adillo, Á.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; Pozuelo-Carrascosa, D.P.; Notario-Pacheco, B. The Accuracy of an Oscillometric Ankle-Brachial Index in the Diagnosis of Lower Limb Peripheral Arterial Disease: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2017, 71, e12994. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Adillo, Á.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Solera-Martínez, M. The Accuracy of Toe Brachial Index and Ankle Brachial Index in the Diagnosis of Lower Limb Peripheral Arterial Disease: A Systematic Review and Meta-Analysis. Atherosclerosis 2020, 315, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.; Lanting, S.; Oldmeadow, C.; Chuter, V. The Reliability of the Ankle Brachial Index: A Systematic Review. J. Foot Ankle Res. 2019, 12, 39. [Google Scholar] [CrossRef]
- Crawford, F.; Welch, K.; Andras, A.; Chappell, F.M. Ankle Brachial Index for the Diagnosis of Lower Limb Peripheral Arterial Disease. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Verberk, W.J.; Kollias, A.; Stergiou, G.S. Automated Oscillometric Determination of the Ankle-Brachial Index: A Systematic Review and Meta-Analysis. Hypertens. Res. 2012, 35, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hageman, D.; van den Houten, M.M.L.; Pesser, N.; Gommans, L.N.M.; Scheltinga, M.R.M.; Teijink, J.A.W. Diagnostic Accuracy of Automated Oscillometric Determination of the Ankle-Brachial Index in Peripheral Artery Disease. J. Vasc. Surg. 2021, 73, 652–660. [Google Scholar] [CrossRef]
- Bulut, U.; Gunvar, T.; Guldal, A.D. Efficacy of Oscillometric Method for Screening Periferic Arterial Disease in Primary Care. Niger. J. Clin. Pract. 2020, 23, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, S.; Desormais, I.; Hashimoto, T.; Magne, J.; Kichikawa, K.; Aboyans, V. Accuracy and Reliability of the Ankle Brachial Index Measurement Using a Multicuff Oscillometric Device Versus the Doppler Method. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 462–468. [Google Scholar] [CrossRef]
- Chongthawonsatid, S.; Dutsadeevettakul, S. Validity and Reliability of the Ankle-Brachial Index by Oscillometric Blood Pressure and Automated Ankle-Brachial Index. J. Res. Med. Sci. 2017, 22, 44. [Google Scholar] [CrossRef]
- Mayr, V.; Hirschl, M.; Klein-Weigel, P.; Girardi, L.; Kundi, M. A Randomized Cross-over Trial in Patients Suspected of PAD on Diagnostic Accuracy of Ankle-Brachial Index by Doppler-Based versus Four-Point Oscillometry Based Measurements. Vasa 2019, 48, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Homza, M.; Machaczka, O.; Porzer, M.; Kozak, M.; Plasek, J.; Sipula, D. Comparison of Different Methods of ABI Acquisition for Detection of Peripheral Artery Disease in Diabetic Patients. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2019, 163, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Adillo, Á.; Mariana-Herraiz, J.Á.; Pozuelo-Carrascosa, D.P. Oscillometric and Doppler Ankle Brachial Indexes as Predictors of All-Cause Mortality in a Primary Care Population. Int. Angiol. 2019, 38, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on behalf of the American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement from the American Heart Association. Circulation 2021, 144. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Adams, E.; AbuRahma, J.; Mata, L.A.; Dean, L.S.; Caron, C.; Sloan, J. Critical Analysis and Limitations of Resting Ankle-Brachial Index in the Diagnosis of Symptomatic Peripheral Arterial Disease Patients and the Role of Diabetes Mellitus and Chronic Kidney Disease. J. Vasc. Surg. 2020, 71, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Mahe, G.; Pollak, A.W.; Liedl, D.A.; Cohoon, K.P.; Mc Carter, C.; Rooke, T.W.; Wennberg, P.W. Discordant Diagnosis of Lower Extremity Peripheral Artery Disease Using American Heart Association Postexercise Guidelines. Medicine 2015, 94, e1277. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Kinlay, S.; Gerhard-Herman, M.D. Comparison of Different Exercise Ankle Pressure Indices in the Diagnosis of Peripheral Artery Disease. Vasc. Med. 2018, 23, 541–548. [Google Scholar] [CrossRef] [PubMed]

| No | Search Term |
|---|---|
| 1 | oscillometric ABI |
| 2 | oscillometric ankle-brachial index |
| 3 | plethysmographic ABI |
| 4 | plethysmographic ankle-brachial index |
| 5 | automated ABI |
| 6 | automated ankle-brachial index |
| 7 | automatic ABI |
| 8 | automatic ankle-brachial index |
| 9 | doppler ABI vs. automatic ABI |
| 10 | doppler ankle-brachial index vs. automatic ankle-brachial index |
| 11 | doppler ABI vs. oscillometric ABI |
| 12 | doppler ankle-brachial index vs. oscillometric ankle-brachial index |
| 13 | doppler ABI vs. plethysmographic ABI |
| 14 | doppler ankle-brachial index vs. plethysmographic ankle-brachial index |
| 15 | automatic ABI validation |
| 16 | automatic ankle-brachial index validation |
| 17 | oscillometric ankle-brachial index validation |
| 18 | plethysmographic ankle-brachial index validation |
| 19 | automatic ABI validity |
| 20 | automatic ankle-brachial index validity |
| 21 | oscillometric ankle-brachial index validity |
| 22 | plethysmographic ankle-brachial index validity |
| 23 | doppler ABI vs. automated ABI |
| 24 | doppler ankle-brachial index vs. automated ankle-brachial index, |
| 25 | automated ABI validity |
| 26 | automated ankle-brachial index validity |
| 27 | automated ABI validation |
| 28 | automated ankle-brachial index validation |
| 29 | doppler ABI vs. plethysmography ABI |
| 30 | doppler ankle-brachial index vs. plethysmography ankle-brachial index |
| 31 | plethysmography ankle-brachial index validation |
| 32 | plethysmography ankle-brachial index validity |
| No. | Author/Year | Reference | Device | Sensitivity | Specificity | Author Conclusions |
|---|---|---|---|---|---|---|
| 1 | Arnold et al., 2020 [41] | Doppler ABI | Masimo Rad-97 | n/a | n/a | High level of agreement. |
| 2 | Teren et al., 2013 [39] | Doppler ABI | Vascular Explorer and Vicorder | n/a | n/a | Moderate concordance with Doppler ABI. Tendency for higher values in PPG than Doppler ABI. |
| 3 | Beutner et al., 2012 [42] | Doppler ABI | Vascular Explorer and Vicorder | 75%, 85%, 80% 1 | 96%, 89%, 98% 1 | Excellent diagnostic value. Tendency for higher values in PPG than Doppler ABI. |
| 4 | Khandanpour et al., 2009 [11] | Doppler ABI | Viasys VasoGuard MicroLite | n/a | n/a | A promising alternative to Doppler. |
| 5 | Alnaeb et al., 2008 [43] | Doppler ABI and duplex scan | Custom PPG probe | 86% | 85% | Can be used to identify patients at risk. |
| 6 | Alnaeb et al., 2007 [44] | Doppler ABI and duplex scan | Custom PPG probe | 83% | 71% | Promising technique for diabetic patient assessment. |
| 7 | Jönsson et al., 2005 [37] | Doppler ABI | Custom PPG probe | 100% | 100% | Further elaboration of the technique is motivated. |
| 8 | Sadiq et al., 2001 [40] | Doppler ABI | Healthwatch | n/a | n/a | Recommended to use on routine basis. |
| No. | Author/Year | Reference | Device | Sensitivity | Specificity | Author Conclusions |
|---|---|---|---|---|---|---|
| 1 | Babaei et al., 2019 [36] | Doppler ABI | Dopplex Ability | 20%, 40% 1 | 95.6%, 79.9% 1 | Not sufficient as standalone test. Potentially useful for identifying individuals needing further assessment after adjusting cutoff value. The study also analyzed PVW qualitative assessment, which was found to be more effective than ABI. |
| 2 | Millen et al., 2018 [45] | Doppler and plethysmography based ABI device | Dopplex Ability | 59% 2 | 86% 2 | Not accurate. The study also analyzed PVW qualitative assessment, which was found to be more effective than ABI. |
| 3 | Lewis et al., 2016 [38] | Duplex scan | Dopplex Ability | 79% | 91% | When combined with PVW analysis, can be highly accurate to rule out PAD. |
| 4 | Van der Slegt et al., 2016 [46] | Doppler ABI | Dopplex Ability | n/a | n/a | Not applicable in post-operative measurements. |
| 5 | Davies et al., 2015 [12] | Doppler ABI | Dopplex Ability | 70%, 98% 3 | 96%, 75% 3 | Unclear whether it can be used as standalone method. Potentially useful for identifying individuals needing further assessment after adjusting cutoff value. |
| 6 | Lewis et al., 2010 [47] | Doppler ABI | Dopplex Ability | n/a | n/a | Potential for PAD screening in primary care. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danieluk, A.; Chlabicz, S. Automated Measurements of Ankle-Brachial Index: A Narrative Review. J. Clin. Med. 2021, 10, 5161. https://doi.org/10.3390/jcm10215161
Danieluk A, Chlabicz S. Automated Measurements of Ankle-Brachial Index: A Narrative Review. Journal of Clinical Medicine. 2021; 10(21):5161. https://doi.org/10.3390/jcm10215161
Chicago/Turabian StyleDanieluk, Aleksandra, and Sławomir Chlabicz. 2021. "Automated Measurements of Ankle-Brachial Index: A Narrative Review" Journal of Clinical Medicine 10, no. 21: 5161. https://doi.org/10.3390/jcm10215161
APA StyleDanieluk, A., & Chlabicz, S. (2021). Automated Measurements of Ankle-Brachial Index: A Narrative Review. Journal of Clinical Medicine, 10(21), 5161. https://doi.org/10.3390/jcm10215161






