Amplified Vasodilatation within the Referred Pain Zone of Trigger Points Is Characteristic of Gluteal Syndrome—A Type of Nociplastic Pain Mimicking Sciatica
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Participant Characteristics
2.3. Method
SP Test
- (1)
- Trigger point examination based on the palpatory diagnostic criteria according to Travell and Simons [23].
- (2)
- Thermal side-to-side comparison of the patients at rest to exclude a possible neuropathic pain component (confirmed there was a temperature decrease of more than 0.5 °C if in the pain subarea compared to the opposite side).
- (3)
- Examination towards the autonomic response of the trigger points at the daily complaint area that is coincident with the referred pain zone for the examined muscle.
- (4)
- Numerical analysis of the thermograms.
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
A Detailed Description of the SP Test Parameters Depending on the Disease and Test Phase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Stewart, J.D. Focal Peripheral Neuropathies, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; Volume 317. [Google Scholar]
- Available online: https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673&navItemNumber=677 (accessed on 19 May 2021).
- Simons, D.G.; Travell, J.G.; Simons, L. Myofascial Pain and Dysfunction: The Trigger Point Manual, 3rd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Shah, J.P.; Danoff, J.V.; Desai, M.J.; Parikh, S.; Nakamura, L.Y.; Phillips, T.M.; Gerber, L.H. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch. Phys. Med. Rehabil. 2008, 89, 16–23. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A Delphi study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Quintner, J.L.; Bove, G.M.; Cohen, M.L. A critical evaluation of the trigger point phenomenon. Rheumatology 2015, 54, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorupska, E.; Atarowska, M.; Samborski, W. Sympathetic Nervous System activity—a new concept of the complicated etiology of low back pain radiates distally at the extremities. J. Med. Sci. 2014, 1, 53–56. [Google Scholar] [CrossRef]
- Freynhagen, R.; Rolke, R.; Baron, R.; Tölle, T.R.; Rutjes, A.K.; Schu, S.; Treede, R.D. Pseudoradicular and radicular low-back pain—A disease continuum rather than different entities? Answers from quantitative sensory testing. Pain 2008, 135, 65–74. [Google Scholar] [CrossRef]
- Hofstee, D.J.; Gijtenbeek, J.M.; Hoogland, P.H.; van Houwelingen, H.C.; Kloet, A.; Lötters, F.; Tans, J.T. Westeinde Sciatica Trial: Randomized controlled study of bed rest and physiotherapy for acute sciatica. J. Neurosurg. 2002, 96, 45–49. [Google Scholar] [CrossRef]
- van Rijn, J.C.; Klemetso, N.; Reitsma, J.B.; Majoie, C.B.; Hulsmans, F.J.; Peul, W.C.; Bossuyt, P.M.; Den Heeten, G.J.; Stam, J. Symptomatic and asymptomatic abnormalities in patients with lumbosacral radicular syndrome: Clinical examination compared with MRI. Clin. Neurol. Neurosurg. 2006, 108, 553–557. [Google Scholar] [CrossRef]
- Capra, F.; Vanti, C.; Donati, R.; Tombetti, S.; O’Reilly, C.; Pillastrini, P. Validity of the straight-leg raise test for patients with sciatic pain with or without lumbar pain using magnetic resonance imaging results as a reference standard. J. Manip. Physiol. 2011, 34, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Endean, A.; Palmer, K.T.; Coggon, D. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: A systematic review. Spine 2011, 36, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawa, N.; Rhoda, A.; Diener, I. Accuracy of clinical neurological examination in diagnosing lumbo-sacral radiculopathy: A systematic literature review. BMC Musculoskelet. Disord. 2017, 18, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalezic, N.; Åsell, M.; Kerschbaumer, H.; Lyskov, E. Physiological reactivity to functional tests in patients with chronic low back pain. J. Musculoskelet. Pain 2007, 15, 29–40. [Google Scholar] [CrossRef]
- Nilsen, K.B.; Sand, T.; Westgaard, R.H.; Stovner, L.J.; White, L.R.; Leistad, R.B.; Helde, G.; Rø, M. Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients. Eur. J. Pain 2007, 11, 743–755. [Google Scholar] [CrossRef]
- Andary, M.T.; Stolov, W.C.; Nutter, P.B. Sympathetic skin response in fifth lumbar and first sacral radiculopathies. Electromyogr. Clin. Neurophysiol. 1993, 33, 91–99. [Google Scholar] [PubMed]
- Skorupska, E.; Rychlik, M.; Samborski, W. Validation and test-retest reliability of new thermographic technique called thermovision technique of dry needling for gluteus minimus trigger points in sciatica subjects and TrPs-negative healthy volunteers. BioMed Res. Int. 2015, 2015, 546497. [Google Scholar] [CrossRef]
- Skorupska, E.; Jokiel, M.; Rychlik, M.; Łochowski, R.; Kotwicka, M. Female Overrepresentation in Low Back-Related Leg Pain: A Retrospective Study of the Autonomic Response to a Minimally Invasive Procedure. J. Pain Res. 2020, 13, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Dybek, T.; Rychlik, M.; Jokiel, M.; Dobrakowski, P. The Automatization of a New Thermography Method Using Invasive Nociceptive Stimulation to Confirm an Autonomic Phenomenon within a Trigger Point Referred Pain Zone. Brain Sci. 2021, 11, 893. [Google Scholar] [CrossRef]
- Skorupska, E.; Rychlik, M.; Pawelec, W.; Samborski, W. Dry Needling Related Short-Term Vasodilation in Chronic Sciatica under Infrared Thermovision. Evid.-Based Complement. Altern. Med. 2015, 2015, 214374. [Google Scholar] [CrossRef]
- Skorupska, E.; Rychlik, M.; Samborski, W. Intensive vasodilatation in the sciatic pain area after dry needling. BMC Complement. Altern. Med. 2015, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd ed.; Williams & Wilkins: Baltimore, MD, USA, 1999; Volume 1. [Google Scholar]
- Hong, C. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef]
- Rivner, M.H. The neurophysiology of myofascial pain syndrome. Curr. Pain Headache Rep. 2001, 5, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Nazarewicz, J.; Verdejo-Garcia, A.; Giummarra, M.J. Sympathetic pain? A role of poor parasympathetic nervous system engagement in vicarious pain states. Psychophysiology 2015, 52, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-Z.; Kuan, T.S.; Chen, S.M. Referred pain elicited by palpation and by needling of myofascial trigger points: A comparison. Arch. Phys. Med. Rehabil. 1997, 78, 957–960. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Graven-Nielsen, T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr. Pain Headache Rep. 2003, 7, 355–361. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.G.; Wells, C.; Bouhassira, D.; Mohr Drewes, A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 2018, 22, 216–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickel, M.M.; May, E.S.; Tiemann, L.; Postorino, M.; Ta Dinh, S.; Ploner, M. Autonomic responses to tonic pain are more closely related to stimulus intensity than to pain intensity. Pain 2017, 158, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef]
- Ong, M.L.; Ng, E.Y.K. A global bioheat model with self-tuning optimal regulation of body temperature using Hebbian feedback covariance learning. Med. Phys. 2005, 32, 3819–3831. [Google Scholar] [CrossRef]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-de-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef]
- Hendrick, P.A. Neurological examination of the peripheral nervous system to diagnose lumbar spinal disc herniation with suspected radiculopathy: A systematic review and meta-analysis. Spine J. 2013, 13, 657–674. [Google Scholar] [PubMed]
- Ploner, M.; Sorg, C.; Gross, J. Brain rhythms of pain. Trends Cogn. Sci. 2017, 21, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
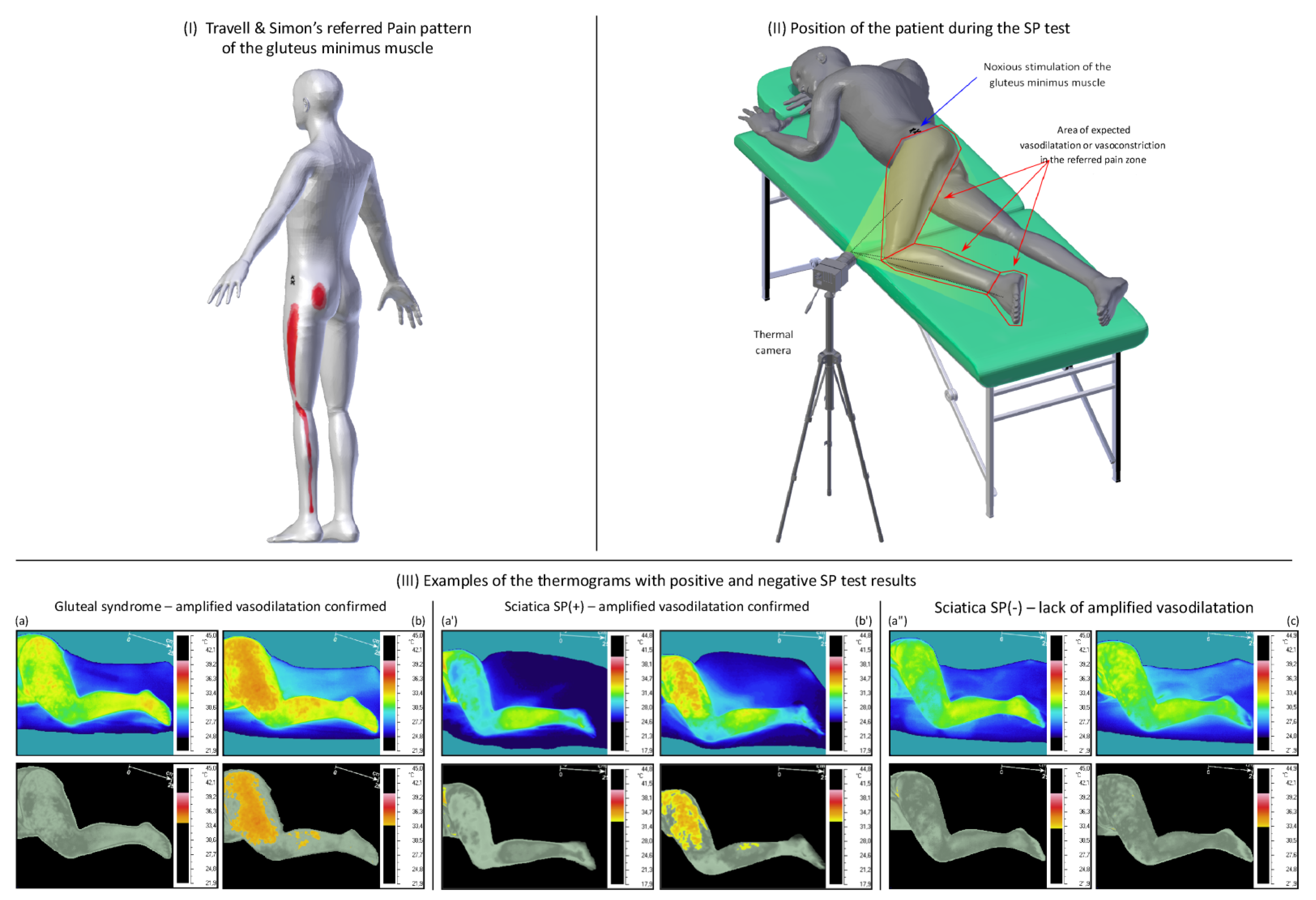
| Data | Gluteal Syndrome (n = 20) | Sciatica (n = 30) | Sig (p = 95%) |
|---|---|---|---|
| Age, mean (SD) (y) | 45.6 (± 6.0) | 45.9 (± 7.6) | * 0.895 |
| Pain intensity (VAS) (mm) | 6.1 (± 2.4) | 5.7 (± 1.5) | # 0.992 |
| Symptoms duration mean (SD)(y) | 9.6 (± 7.7) | 14.5 (± 32.2) | * 0.508 |
| 1Q/Median/3Q | 3.5/8.0/12.0 | 3.8/6.0/12.0 | |
| Leg pain above knee (%) | n = 20 (100.0%) | n = 30 (100.0%) | ** 1.000 |
| Leg pain below knee (%) | n = 9 (45.0%) | n = 10 (33.3%) | ** 0.553 |
| Leg pain below ankle (%) | n = 3 (15.0%) | n = 5 (16.7%) | ** 1.000 |
| Gluteus minimus TrPs | 20 | 0 | - |
| Gluteus medius TrPs | 5 | 0 | - |
| Gluteus maximus TrPs | 2 | 0 | - |
| Quadratus lumborum TrPs | 18 | 0 | - |
| Group of the Patients with/without Muscle-Referred Pain (SP (+/−)) (n = …) | Characteristics of the Thermograms SP(+) with Confirmed Muscle-Referred Pain | ||
|---|---|---|---|
| First Thermogram at (min/s) | Last Thermogram at (min/s) | Number of Thermograms n = … (%) | |
| GS (n = 20) | 00′9″ | 16′00″ | 290 */** (90.6) |
| SP(+) Sciatica (n = 8) | 2′12″ | 16′00″ | 66 */** (20.6) |
| SP(−) Sciatica (n = 22) | absence | absence | 0 (0.0) |
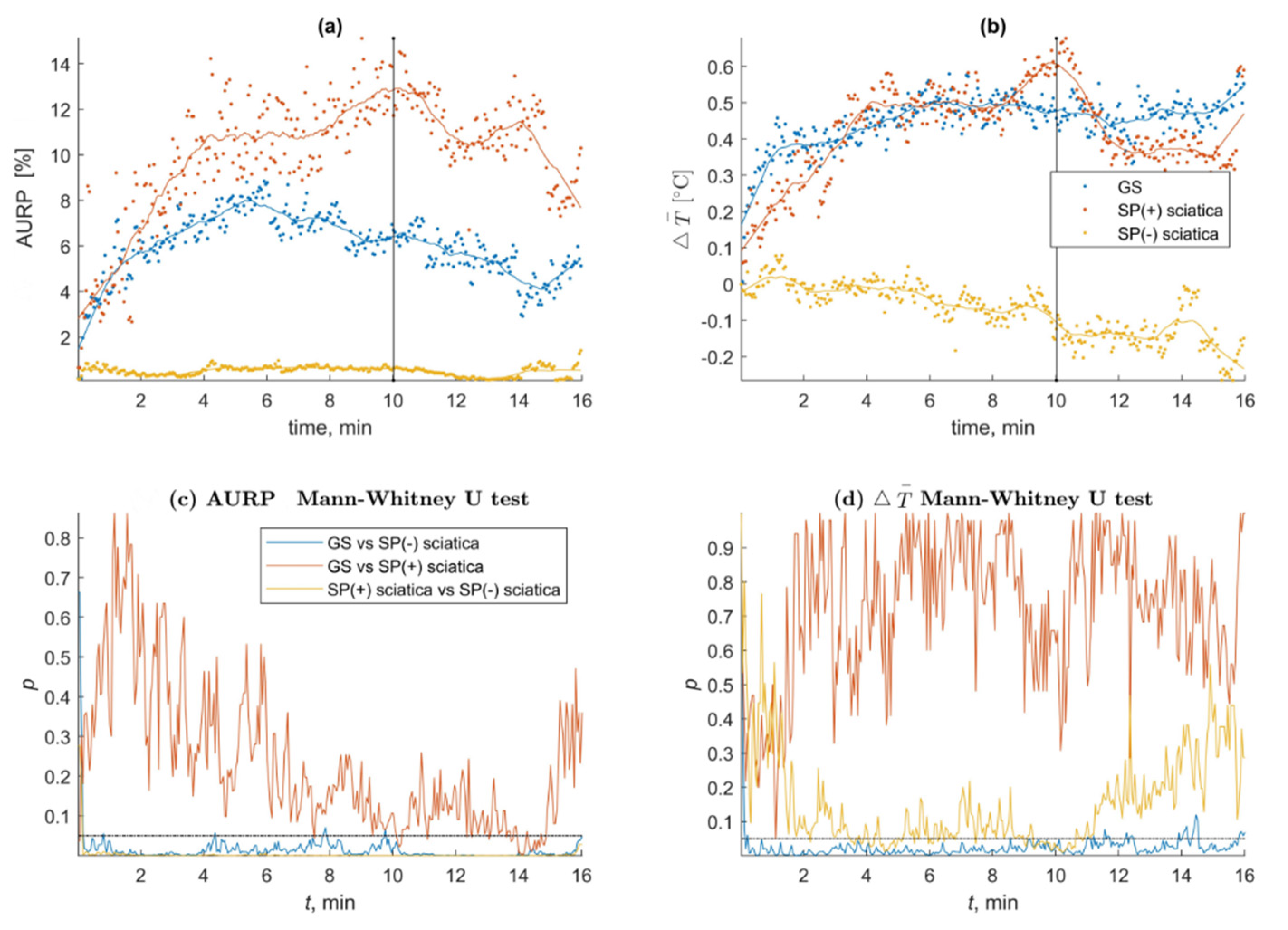
| SP | Phases of the SP Test | Group | Average (SD) | 1Q/Median/3Q | Out of 320 SP(+) n = (%) | Significance of Difference between Groups |
|---|---|---|---|---|---|---|
| Δ₸° | the stimulation phase (10′) | GS | 0.5 (0.6) | 0.0/0.3/1.0 | GS vs. SP(−) Sciatica 195 (97.5) GS vs. SP(+) Sciatica 1 (0.5) SP(+) Sciatica vs. SP(−) Sciatica 52 (26.0) | GS vs. SP(−) Sciatica 0.031 * GS vs. SP(+) Sciatica 0.285 SP(+) Sciatica vs. SP(−) Sciatica 0.039 * |
| SP(−) Sciatica | −0.1 (0.7) | −0.7/−0.1/0.4 | ||||
| SP(+) Sciatica | 0.6 (0.7) | 0.3/0.5/0.9 | ||||
| the observation phase (16′) | GS | 0.6 (0.7) | 0.3/0.7/1.0 | GS vs. SP(−) Sciatica 95 (79.2) GS vs. SP(+) Sciatica 0 (0.0) SP(+) Sciatica vs. SP(−) Sciatica 14 (11.7) | GS vs. SP(−) Sciatica 0.026 * GS vs. SP(+) Sciatica 0.500 SP(+) Sciatica vs. SP(−) Sciatica 0.216 | |
| SP(−) Sciatica | −0.2 (0.9) | −0.7/−0.2/0.5 | ||||
| SP(+) Sciatica | 0.6 (0.8) | 0.2/0.6/1.0 | ||||
| AURPT0 | the stimulation phase (10′) | GS | 6.7 (11.4) | 0.3/2.9/7.4 | GS vs. SP(−) Sciatica 192 (96.0) GS vs. SP(+) Sciatica 2 (1.0) SP(+) Sciatica vs. SP(−) Sciatica | GS vs. SP(−) Sciatica 0.019 * GS vs. SP(+) Sciatica 0.040 * SP(+) Sciatica vs. SP(−) Sciatica 0.001* |
| SP(−) Sciatica | 0.6 (1.1) | 0.1/0.3/1.2 | ||||
| SP(+) Sciatica | 11.7 (10.5) | 1.9/10.8/22.0 | ||||
| the observation phase (16′) | GS | 6.6 (8.7) | 0.6/4.2/12.2 | GS vs. SP(−) Sciatica 120 (100.0) GS vs. SP(+) Sciatica 27 (22.5) SP(+) Sciatica vs. SP(−) Sciatica 120 (100.0) | GS vs. SP(−) Sciatica 0.047 * GS vs. SP(+) Sciatica 0.158 SP(+) Sciatica vs. SP(−) Sciatica 0.034 * | |
| SP(−) Sciatica | 1.5 (4.3) | 0.0/0.1/0.4 | ||||
| SP(+) Sciatica | 10.4 (8.9) | 6.5/12.2/15.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorupska, E.; Dybek, T.; Rychlik, M.; Jokiel, M.; Zawadziński, J.; Dobrakowski, P. Amplified Vasodilatation within the Referred Pain Zone of Trigger Points Is Characteristic of Gluteal Syndrome—A Type of Nociplastic Pain Mimicking Sciatica. J. Clin. Med. 2021, 10, 5146. https://doi.org/10.3390/jcm10215146
Skorupska E, Dybek T, Rychlik M, Jokiel M, Zawadziński J, Dobrakowski P. Amplified Vasodilatation within the Referred Pain Zone of Trigger Points Is Characteristic of Gluteal Syndrome—A Type of Nociplastic Pain Mimicking Sciatica. Journal of Clinical Medicine. 2021; 10(21):5146. https://doi.org/10.3390/jcm10215146
Chicago/Turabian StyleSkorupska, Elzbieta, Tomasz Dybek, Michał Rychlik, Marta Jokiel, Jarosław Zawadziński, and Paweł Dobrakowski. 2021. "Amplified Vasodilatation within the Referred Pain Zone of Trigger Points Is Characteristic of Gluteal Syndrome—A Type of Nociplastic Pain Mimicking Sciatica" Journal of Clinical Medicine 10, no. 21: 5146. https://doi.org/10.3390/jcm10215146
APA StyleSkorupska, E., Dybek, T., Rychlik, M., Jokiel, M., Zawadziński, J., & Dobrakowski, P. (2021). Amplified Vasodilatation within the Referred Pain Zone of Trigger Points Is Characteristic of Gluteal Syndrome—A Type of Nociplastic Pain Mimicking Sciatica. Journal of Clinical Medicine, 10(21), 5146. https://doi.org/10.3390/jcm10215146






