A Decade of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis: Does the Outcome Improve?
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Definitions and Outcome Measurements
2.3. Statistical Analyses
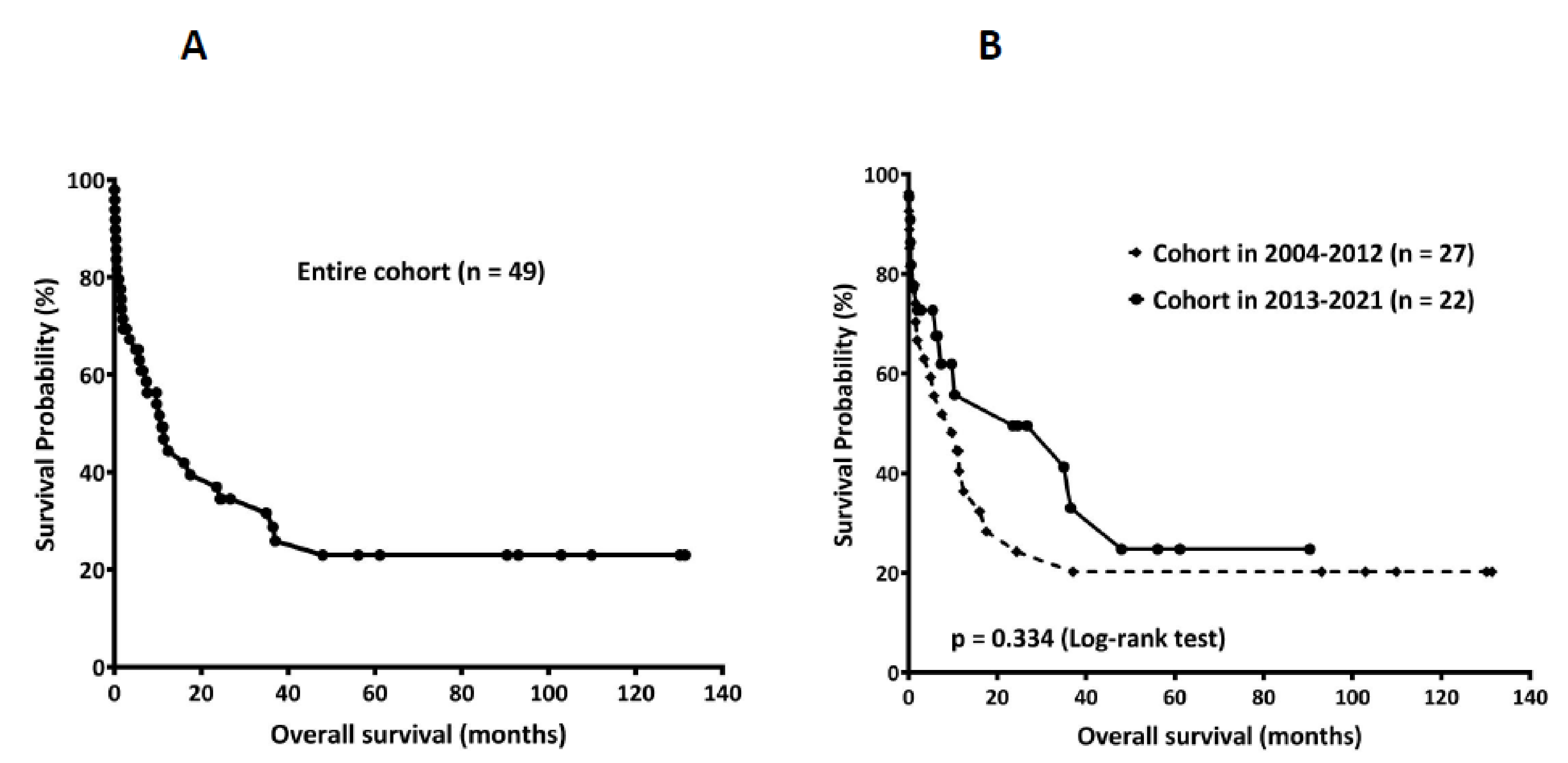
3. Results
3.1. Clinical Characteristic Comparisons among Patients Diagnosed in 2004–2012 and 2013–2021
3.2. Treatment and Outcome Comparison
3.3. Overall Survival Comparison
3.4. Prognostic Factors for Lymphoma-Associated HLH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bhatt, N.S.; Oshrine, B.; An Talano, J. Hemophagocytic lymphohistiocytosis in adults. Leuk. Lymphoma 2019, 60, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gholam, C.; Grigoriadou, S.; Gilmour, K.C.; Gaspar, H.B. Familial haemophagocytic lymphohistiocytosis: Advances in the genetic basis, diagnosis and management. Clin. Exp. Immunol. 2011, 163, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Lopez-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Machaczka, M.; Vaktnas, J.; Klimkowska, M.; Hagglund, H. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: A retrospective population-based analysis from a single center. Leuk. Lymphoma 2011, 52, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.T.; Wang, C.Y.; Yang, Y.; Wang, R.C.; Chang, K.H.; Hwang, W.L.; Teng, C.L. Lymphoma-associated hemophagocytic lymphohistiocytosis: Experience in adults from a single institution. Ann. Hematol. 2013, 92, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henter, J.I.; Horne, A.; Arico, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lister, T.A.; Crowther, D.; Sutcliffe, S.B.; Glatstein, E.; Canellos, G.P.; Young, R.C.; Rosenberg, S.A.; Coltman, C.A.; Tubiana, M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J. Clin. Oncol. 1989, 7, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, J.; Li, T.; Gu, J.; Zeng, C.; Xiao, M.; Zhang, W.; Li, Q.; Zhou, J.; Zhou, X. Clinical Characteristics of Hemophagocytic Lymphohistiocytosis Associated with Non-Hodgkin B-Cell Lymphoma: A Multicenter Retrospective Study. Clin. Lymphoma Myeloma Leuk. 2021, 21, e198–e205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kan, Y.; Meeks, J.K.; Ma, D.; Yang, J. 18F-FDG PET/CT for identifying the potential causes and extent of secondary hemophagocytic lymphohistiocytosis. Diagn. Interv. Radiol. 2016, 22, 471–475. [Google Scholar] [CrossRef] [PubMed]
- La Rosee, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellapandian, D.; Das, R.; Zelley, K.; Wiener, S.J.; Zhao, H.; Teachey, D.T.; Nichols, K.E.; Group, E.-H.R.S. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br. J. Haematol. 2013, 162, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Cui, M.; Fu, X.; Han, L.; Zhang, L.; Li, L.; Li, X.; Sun, Z.; Wu, J.; Zhang, X.; et al. Lymphoma associated hemophagocytic syndrome: A single-center retrospective study. Oncol. Lett. 2018, 16, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wang, Y.; Wang, J.; Wang, Z. The DEP regimen is superior to the HLH-1994 regimen as first-line therapy for lymphoma-associated haemophagocytic lymphohistiocytosis. Leuk. Lymphoma 2021, 62, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.A.; Kapoor, P.; Letendre, L.; Kumar, S.; Wolanskyj, A.P. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin. Proc. 2014, 89, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, J.; Wu, Z.Q.; Goyal, H.; Xu, H.G. A novel prognostic model for adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J. Rare Dis. 2020, 15, 215. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 56) | Year of Diagnosis 2004–2012 (n = 30) | Year of Diagnosis 2013–2021 (n = 26) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age, median (range, years) | 57.5 | (21–83) | 51 | (21–77) | 62 | (27–83) | 0.068 ‡ |
| Gender, n (%) | 0.818 † | ||||||
| Male | 26 | (46.4%) | 13 | (43.3%) | 13 | (50.0%) | |
| Female | 30 | (53.6%) | 17 | (56.7%) | 13 | (50.0%) | |
| Stage, n (%) | 1.000 # | ||||||
| Stage 1 & 2 | 4 | (7.1%) | 2 | (6.7%) | 2 | (7.7%) | |
| Stage 3 & 4 | 52 | (92.9%) | 28 | (93.3%) | 24 | (92.3%) | |
| Cytopenia (≥2 lines), n (%) | 0.481 # | ||||||
| No | 9 | (16.1%) | 6 | (20.0%) | 3 | (11.5%) | |
| Yes | 47 | (83.9%) | 24 | (80.0%) | 23 | (88.5%) | |
| Triglyceride, median (range, mg/dL) | 215.5 | (69–998) | 189 | (69–998) | 280 | (129–511) | 0.095 ‡ |
| Fibrinogen, median (range, mg/dL) | 219.8 | (60–738) | 232 | (60–738) | 189.5 | (81.7–575) | 0.424 ‡ |
| Ferritin, median (range, ng/mL) | 5152.9 | (500–59,384) | 4994.5 | (500–34,755) | 5250.9 | (1185–59,384) | 0.304 ‡ |
| LDH, median (range, IU/L) | 1205 | (196–7271) | 941 | (196–7271) | 1587 | (227–6777) | 0.058 ‡ |
| DIC, n (%) | 0.935 † | ||||||
| No | 38 | (67.9%) | 21 | (70.0%) | 17 | (65.4%) | |
| Yes | 18 | (32.1%) | 9 | (30.0%) | 9 | (34.6%) | |
| Jaundice, n (%) | 0.235 † | ||||||
| No | 23 | (41.1%) | 15 | (50.0%) | 8 | (30.8%) | |
| Yes | 33 | (58.9%) | 15 | (50.0%) | 18 | (69.2%) | |
| ECOG performance status, n (%) | 0.890 † | ||||||
| 1–2 | 35 | (62.5%) | 18 | (60.0%) | 17 | (65.4%) | |
| 3–4 | 21 | (37.5%) | 12 | (40.0%) | 9 | (34.6%) | |
| IPI, n (%) | 0.954 † | ||||||
| 0–3 | 25 | (44.6%) | 14 | (46.7%) | 11 | (42.3%) | |
| 4–5 | 31 | (55.4%) | 16 | (53.3%) | 15 | (57.7%) | |
| HLH onset, n (%) | 0.544 † | ||||||
| With lymphoma | 40 | (71.4%) | 21 | (70.0%) | 19 | (73.1%) | |
| Before lymphoma | 7 | (12.5%) | 5 | (16.7%) | 2 | (7.7%) | |
| After lymphoma | 9 | (16.1%) | 4 | (13.3%) | 5 | (19.2%) | |
| EBER, n (%) | 0.021 † | ||||||
| Positive | 14 | (25.0%) | 6 | (20.0%) | 8 | (30.8%) | |
| Negative | 14 | (25.0%) | 4 | (13.3%) | 10 | (38.5%) | |
| Unknown | 28 | (50.0%) | 20 | (66.7%) | 8 | (30.8%) | |
| Lymphoma subtype, n (%) | 0.427 † | ||||||
| Diffuse large B-cell lymphoma | 29 | (51.8%) | 13 | (43.3%) | 16 | (61.5%) | |
| Peripheral T-cell lymphoma | 20 | (35.7%) | 13 | (43.3%) | 7 | (26.9%) | |
| Angioimmunoblastic T-cell lymphoma | 4 | (7.1%) | 3 | (10.0%) | 1 | (3.8%) | |
| Anaplastic large cell lymphoma | 1 | (1.8%) | 0 | (0.0%) | 1 | (3.8%) | |
| NK/T-cell lymphoma | 2 | (3.6%) | 1 | (3.3%) | 1 | (3.8%) | |
| Total | Year of Diagnosis 2004–2012 | Year of Diagnosis 2013–2021 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Treatment of B-cell lymphoma (n = 29) | |||||||
| Without rituximab | 8 | (27.6%) | 5 | (38.5%) | 3 | (18.8%) | 0.406 |
| With rituximab | 21 | (72.4%) | 8 | (61.5%) | 13 | (81.2%) | |
| Treatment of B-cell lymphoma (n = 29) | |||||||
| Best supportive care | 4 | (13.8%) | 1 | (7.7%) | 3 | (18.8%) | 0.606 |
| Intent-to-cure chemotherapy | 25 | (86.2%) | 12 | (92.3%) | 13 | (81.2%) | |
| Treatment of T-cell lymphoma (n = 27) | |||||||
| Best supportive care | 8 | (29.6%) | 4 | (23.5%) | 4 | (40.0%) | 0.415 |
| Intent-to-cure chemotherapy | 19 | (70.4%) | 13 | (76.5%) | 6 | (60.0%) | |
| Total (n = 56) | Diagnosis in 2004–2012 (n = 30) | Diagnosis in 2013–2021 (n = 26) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Response *, n (%) | 0.137 † | ||||||
| CR | 21 | (55.3%) | 11 | (50.0%) | 10 | (62.5%) | |
| PR | 4 | (10.5%) | 1 | (4.5%) | 3 | (18.8%) | |
| PD | 13 | (34.2%) | 10 | (45.5%) | 3 | (18.8%) | |
| Death, n (%) | 0.353 † | ||||||
| No | 15 | (26.8%) | 6 | (20.0%) | 9 | (34.6%) | |
| Yes | 41 | (73.2%) | 24 | (80.0%) | 17 | (65.4%) | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p Value | HR | (95% CI) | p Value | |
| Age, years | ||||||
| ≤60 | 1.00 | |||||
| >60 | 1.40 | (0.72–2.75) | 0.324 | |||
| Gender | ||||||
| Male | 1.00 | |||||
| Female | 0.90 | (0.45–1.79) | 0.757 | |||
| Lymphoma subtype | ||||||
| B-cell lymphoma | 1.00 | |||||
| T-cell lymphoma | 1.17 | (0.60–2.29) | 0.650 | |||
| ECOG Performance status | ||||||
| 1–2 | 1.00 | 1.00 | ||||
| 3–4 | 4.19 | (2.08–8.42) | <0.001 | 5.38 | (2.49–11.61) | <0.001 |
| Stage | ||||||
| Stage 1, 2 | 1.00 | |||||
| Stage 3, 4 | 1.50 | (0.36–6.26) | 0.580 | |||
| Ferritin, ng/mL | ||||||
| <3000 | 1.00 | |||||
| ≥3000 | 1.44 | (0.68–3.07) | 0.339 | |||
| LDH, IU/L | ||||||
| <1000 | 1.00 | |||||
| ≥1000 | 1.17 | (0.60–2.31) | 0.645 | |||
| DIC | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.06 | (1.01–4.19) | 0.046 | 2.08 | (0.99–4.40) | 0.054 |
| Jaundice | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.78 | (1.34–5.76) | 0.006 | 2.91 | (1.37–6.18) | 0.006 |
| IPI | ||||||
| 0–3 | 1.00 | |||||
| 4–5 | 1.74 | (0.87–3.48) | 0.118 | |||
| Year of diagnosis | ||||||
| 2013–2021 | 1.00 | |||||
| 2004–2012 | 1.41 | (0.70–2.81) | 0.336 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Shih, Y.-H.; Chen, T.-C.; Chou, C.-W.; Hsu, C.-Y.; Teng, C.-L.J. A Decade of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis: Does the Outcome Improve? J. Clin. Med. 2021, 10, 5114. https://doi.org/10.3390/jcm10215114
Lin C-H, Shih Y-H, Chen T-C, Chou C-W, Hsu C-Y, Teng C-LJ. A Decade of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis: Does the Outcome Improve? Journal of Clinical Medicine. 2021; 10(21):5114. https://doi.org/10.3390/jcm10215114
Chicago/Turabian StyleLin, Cheng-Hsien, Yu-Hsuan Shih, Tsung-Chih Chen, Cheng-Wei Chou, Chiann-Yi Hsu, and Chieh-Lin Jerry Teng. 2021. "A Decade of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis: Does the Outcome Improve?" Journal of Clinical Medicine 10, no. 21: 5114. https://doi.org/10.3390/jcm10215114
APA StyleLin, C.-H., Shih, Y.-H., Chen, T.-C., Chou, C.-W., Hsu, C.-Y., & Teng, C.-L. J. (2021). A Decade of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis: Does the Outcome Improve? Journal of Clinical Medicine, 10(21), 5114. https://doi.org/10.3390/jcm10215114






