Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair
Abstract
:1. Introduction
2. Methods
2.1. Data Collection and Definitions
2.2. Statistical Analysis
2.3. Missing Data
2.4. Results
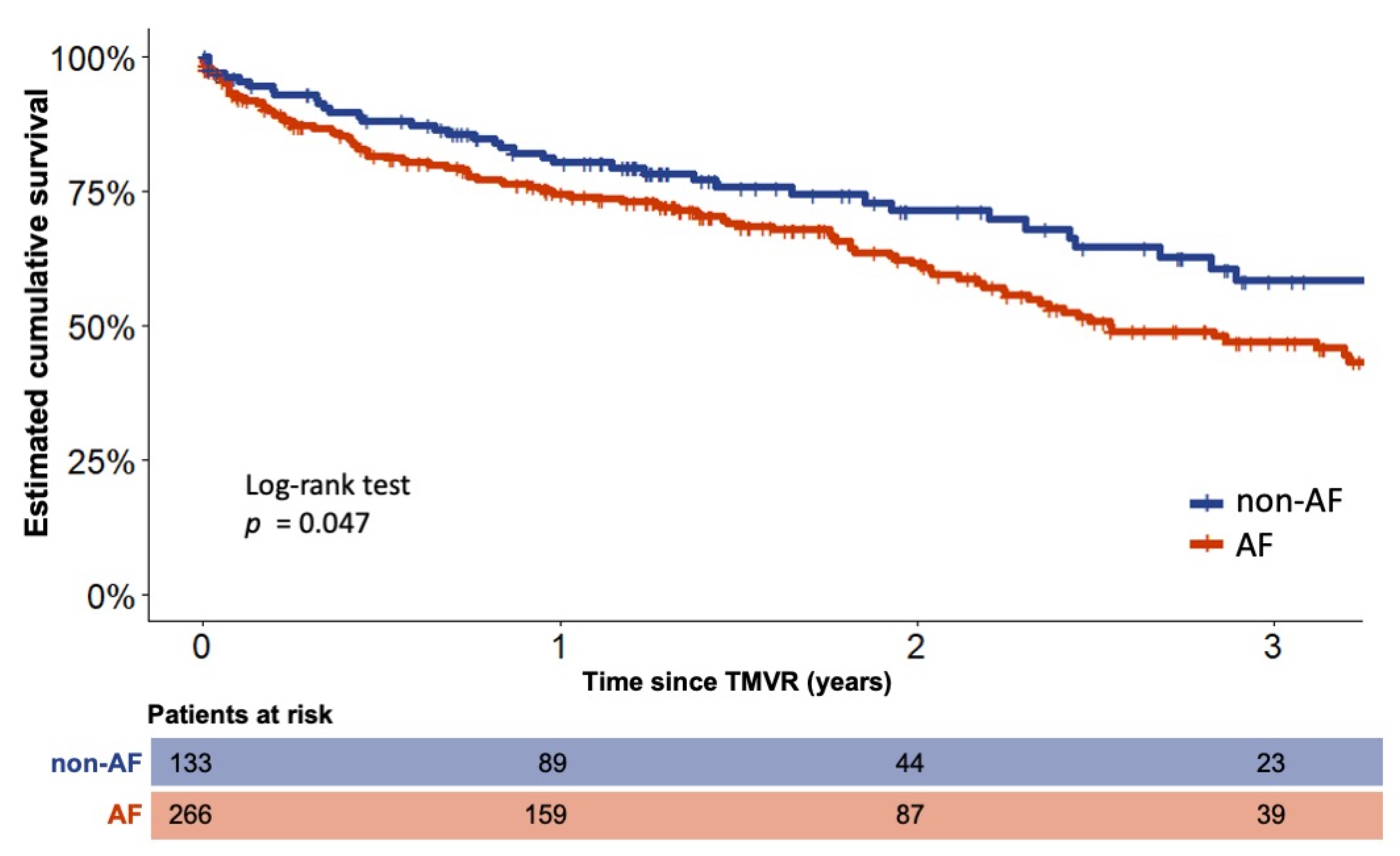
3. Long-Term Outcome of AF and Non-AF Patients
4. Long-Term Outcome of Different AF Types and AF Treatment Strategies
5. Use of Amiodarone within the Entire Cohort and Overlapping Indications
6. Discussion
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, R.A.; Vahanian, A.; Eleid, M.F.; Mack, M.J. Mitral valve disease—Current management and future challenges. Lancet 2016, 387, 1324–1334. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Waechter, C.; Ausbuettel, F.; Chatzis, G.; Fischer, D.; Nef, H.; Barth, S.; Halbfaß, P.; Deneke, T.; Kerber, S.; Divchev, D.; et al. Analysis of Atrial Fibrillation Treatment Regimes in a Multicenter Cohort of Transcatheter Edge-to-Edge Mitral Valve Repair Patients. J. Interv. Cardiol. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Velu, J.F.; Kortlandt, F.A.; Hendriks, T.; Schurer, R.A.J.; Van Boven, A.J.; Koch, K.T.; Vis, M.M.; Henriques, J.P.; Piek, J.; Branden, B.J.L.V.D.; et al. Comparison of Outcome After Percutaneous Mitral Valve Repair With the MitraClip in Patients With Versus Without Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 2035–2040. [Google Scholar] [CrossRef] [Green Version]
- Keßler, M.; Pott, A.; Mammadova, E.; Seeger, J.; Wöhrle, J.; Rottbauer, W.; Markovic, S. Atrial Fibrillation Predicts Long-Term Outcome after Transcatheter Edge-to-Edge Mitral Valve Repair by MitraClip Implantation. Biomolecules 2018, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.M.; Kassis, N.; Gad, M.M.; Abdelfattah, O.; Ahuja, K.R.; Farwati, M.; Isogai, T.; Bazarbashi, N.; Shekhar, S.; Kapadia, S.R. Impact of atrial fibrillation on outcomes following MitraClip: A contemporary population-based analysis. Catheter. Cardiovasc. Interv. 2021, 97, 1252–1256. [Google Scholar] [CrossRef]
- Arora, S.; Vemulapalli, S.; Stebbins, A.; Ramm, C.J.; Kosinski, A.S.; Sorajja, P.; Piccini, J.P.; Cavender, M.A.; Vavalle, J.P. The Prevalence and Impact of Atrial Fibrillation on 1-Year Outcomes in Patients Undergoing Transcathe-ter Mitral Valve Repair Results From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. JACC Cardiovasc. Interv. 2019, 12, 569–578. [Google Scholar] [CrossRef]
- Halas, M.; Kruse, J.; McCarthy, P.M. Concomitant treatment of atrial fibrillation during mitral valve surgery. J. Cardiovasc. Electrophysiol. 2021, 32, 2873–2878. [Google Scholar] [CrossRef]
- Badhwar, V.; Rankin, J.S.; Damiano, R.J.; Gillinov, A.M.; Bakaeen, F.G.; Edgerton, J.R.; Philpott, J.M.; McCarthy, P.M.; Bolling, S.F.; Roberts, H.G.; et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2017, 103, 329–341. [Google Scholar] [CrossRef]
- Ad, N.; Damiano, R.J.; Badhwar, V.; Calkins, H.; La Meir, M.; Nitta, T.; Doll, N.; Holmes, S.D.; Weinstein, A.; Gillinov, M. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 153, 1330–1354. [Google Scholar] [CrossRef] [Green Version]
- Barth, S.; Hautmann, M.B.; Kerber, S.; Gietzen, F.; Reents, W.; Zacher, M.; Halbfass, P.; Griese, D.P.; Schieffer, B.; Hamm, K. Left ventricular ejection fraction of <20%: Too bad for MitraClip©? Catheter. Cardiovasc. Interv. 2017, 90, 1038–1045. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 4194. [Google Scholar] [CrossRef]
- Sun, F.; Liu, H.; Zhang, Q.; Lu, F.; Zhan, H.; Zhou, J. Impact of atrial fibrillation on outcomes of patients treated by transcatheter mitral valve repair: A systematic review and meta-analysis. Medicine 2020, 99, e22195. [Google Scholar] [CrossRef]
- Shah, S.; Raj, V.; Abdelghany, M.; Mena-Hurtado, C.; Riaz, S.; Patel, S.; Wiener, H.; Chaudhuri, D. Impact of atrial fibrillation on the outcomes of transcatheter mitral valve repair using MitraClip: A systematic review and meta-analysis. Heart Fail. Rev. 2020, 26, 1–13. [Google Scholar] [CrossRef]
- Grigioni, F.; Benfari, G.; Vanoverschelde, J.-L.; Tribouilloy, C.; Avierinos, J.-F.; Bursi, F.; Suri, R.M.; Guerra, F.; Pasquet, A.; Rusinaru, D.; et al. Long-Term Implications of Atrial Fibrillation in Patients With Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 264–274. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Chen, S.; Pürerfellner, H.; Meyer, C.; Acou, W.-J.; Schratter, A.; Ling, Z.; Liu, S.; Yin, Y.; Martinek, M.; Kiuchi, M.G.; et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: A stratified pooled analysis of randomized data. Eur. Heart J. 2020, 41, 2863–2873. [Google Scholar] [CrossRef]
- Saglietto, A.; De Ponti, R.; Di Biase, L.; Matta, M.; Gaita, F.; Romero, J.; De Ferrari, G.M.; Anselmino, M. Impact of atrial fibrillation catheter ablation on mortality, stroke, and heart failure hospitalizations: A meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1040–1047. [Google Scholar] [CrossRef]
- Zhao, Y.; Krupadev, V.; Dagher, L.; Mahnkopf, C.; Sohns, C.; Sehner, S.; Suling, A.; Sanders, P.; Boersma, L.; Schunkert, H.; et al. Pharmacological rhythm versus rate control in patients with atrial fibrillation and heart failure: The CASTLE-AF trial. J. Interv. Card. Electrophysiol. 2021, 61, 609–615. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef]
- Shariff, N.; Desai, R.V.; Patel, K.; Ahmed, M.I.; Fonarow, G.; Rich, M.W.; Aban, I.B.; Banach, M.; Love, T.E.; White, M.; et al. Rate-control versus Rhythm-control Strategies and Outcomes in Septuagenarians with Atrial Fibrillation. Am. J. Med. 2013, 126, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Ruzieh, M.; Mandrola, J.; Dyer, A.-M.; Chinchilli, V.M.; Naccarelli, G.V.; Foy, A.J. A Multimorbidity-Based, Risk-Stratified Reanalysis of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Trial. Drugs Aging 2020, 37, 839–844. [Google Scholar] [CrossRef]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef]
- Li, W.; Tiwari, N. Amiodarone use in elderly atrial fibrillation patients with preserved ejection fraction is associated with increased short-term mortality. Eur. Heart J. 2020, 41, 1. [Google Scholar] [CrossRef]

| Before Propensity Score Matching | After Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|---|
| Total n = 506 | Non-AF n = 133 | AF n = 373 | p-Value | Non-AF n = 133 | AF n = 266 | p-Value | |
| Age (years) | 78.1 ± 7.8 | 77.4 ± 9.1 | 78.4 ± 7.3 | 0.3 | 77.4 ± 9.1 | 77.8 ± 7.3 | 0.6 |
| Male sex | 62.9% | 60.9% | 63.5% | 0.6 | 60.9% | 63.2% | 0.6 |
| EuroSCORE II (Q1; Q3) | 19.97% (10.7; 33.6) | 19.11% (12.1; 34.0) | 19.98% (4.7; 13.6) | 0.7 | 19.1% (12.1; 34.0) | 20.0% (10.7; 33.4) | 1 |
| STS-Risk-Score (Q1; Q3) | 7.4% (4.7; 13.4) | 7.3% (4.2; 12.8) | 7.6% (4.7; 13.6) | 0.6 | 7.3% (4.2; 12.8) | 7.7% (4.8; 15.2) | 0.3 |
| NYHA class I NYHA class II NYHA class III NYHA class IV | 0.2% 4.7% 71.0% 24.1% | 0% 5.3% 73.7% 21.0% | 0.3% 4.6% 70.0% 25.2% | 0.7 | 0.0% 5.3% 73.7% 21.1% | 0.4% 2.6% 69.2% 27.8% | 0.2 |
| COPD | 20.6% | 22.6% | 19.8% | 0.5 | 22.6% | 21.1% | 0.7 |
| Coronary artery disease | 67.0% | 77.4% | 63.3% | 0.002 | 77.4% | 77.1% | 0.9 |
| Prior CABG surgery | 26.7% | 35.3% | 23.6% | 0.008 | 35.3% | 28.2% | 0.2 |
| Prior PCI | 55.1% | 62.4% | 52.5% | 0.049 | 62.4% | 60.2% | 0.7 |
| Diabetes mellitus | 33.4% | 35.3% | 32.7% | 0.6 | 35.3% | 35.7% | 0.9 |
| Art. Hypertension | 80.0% | 79.7% | 80.2% | 0.9 | 79.7% | 79.7% | 1 |
| Prior Stroke | 10.9% | 11.3% | 10.7% | 0.9 | 11.3% | 9.8% | 0.7 |
| Pre-existing ICD | 26.9% | 26.3% | 27.1% | 0.9 | 26.3% | 29.7% | 0.5 |
| Pre-existing CRT | 11.7% | 11.3% | 11.8% | 0.9 | 11.3% | 13.5% | 0.5 |
| GFR > 60 mL/Min GFR 30–59 mL/Min GFR < 30 mL/Min | 25.5% 55.3% 19.0% | 30.1% 56.6% 19.3% | 23.9% 51.9% 18% | 0.5 | 30.1% 51.9% 18% | 25.9% 55.6% 18.4% | 0.7 |
| NT-pro BNP (ng/L) (Q1; Q3) | 2945 (1089; 5989) | 2960 (1129; 7000) | 2935 (1085; 5671) | 0.4 | 2960 (1129; 7000) | 3031 (1056; 5994) | 0.5 |
| LV function > 45% LV function 30–44% LV function < 30% | 38.7% 34.6% 26.7% | 30.1% 38.3% 31.6% | 41.8% 33.2% 24.9% | 0.06 | 30.1% 38.3% 31.6% | 38% 35% 27.1% | 0.3 |
| TR grade III | 18.8% | 11.3% | 21.4% | 0.01 | 11.3% | 10.5% | 0.8 |
| Degenerative MR etiology Functional MR etiology Combined MR etiology | 27.5% 64.6% 7.9% | 27.1% 65.4% 7.5% | 27.6% 64.3% 8.0% | 0.9 | 27.1% 65.4% 7.5% | 25.6% 66.9% 7.5% | 0.9 |
| Procedural characteristics | |||||||
| Mean procedure duration (min) | 108.2 ± 63.1 | 104.0 ± 53.0 | 109.6 ± 66.3 | 0.3 | 104 ± 53 | 110 ± 67 | 0.4 |
| Postinterventional no MR Postinterventional MR grade I Postinterventional MR grade II Postinterventional MR grade III | 24.7% 62.9% 12.0% 0.4% | 23.3% 62.4% 13.5% 0.8% | 25.2% 63.0% 11.5% 0.3% | 0.6 | 23.3% 62.4% 13.5% 0.8% | 25.2% 62.4% 12.4% 0.0% | 0.6 |
| 1 Clip implanted 2 Clips implanted 3 Clips implanted 4 Clips implanted | 37.9% 52.8% 9.1% 0.2% | 36.8% 51.9% 11.3% 0.0% | 36.3% 53.1% 8.3% 0.3% | 0.7 | 36.8% 51.9% 11.3% 0.0% | 42.1% 50.4% 7.1% 0.4% | 0.4 |
| Length of hospital stay (days) (Q1; Q3) | 7 (5; 10) | 7 (4; 9) | 7 (5; 10) | 0.2 | 7 (4; 9) | 7 (5; 10) | 0.5 |
| MACCE | 4.4% | 5.3% | 3.5% | 0.4 | 5.3% | 4.1% | 0.6 |
| In-hospital death from any cause | 4.2% | 4.5% | 4.0% | 0.8 | 4.5% | 4.5% | 1 |
| Heart Failure and anti-arrhythmic medication | |||||||
| ACE-/AT1 Inhibitors | 74.1% | 72.9% | 74.5% | 0.7 | 72.9% | 75.2% | 0.6 |
| ARN Inhibitor | 7.9% | 7.5% | 8.0% | 0.8 | 7.5% | 7.9% | 1 |
| Beta Blockers | 87.9% | 87.2% | 88.2% | 0.7 | 87.2% | 90.2% | 0.4 |
| Loop diuretics | 89.5% | 87.2% | 90.3% | 0.3 | 87.2% | 90.2% | 0.4 |
| Thiazid diuretics | 21.3% | 21.8% | 21.1% | 0.9 | 21.8% | 19.5% | 0.6 |
| Aldosteron antagonists | 48.0% | 47.4% | 48.3% | 0.9 | 47.4% | 48.5% | 0.9 |
| Ivabradin | 1.4% | 3.8% | 0.5% | 0.015 | 3.8% | 0.8% | 0.04 |
| Digitalis | 7.7% | 0.0% | 10.5% | <0.0001 | 0.0% | 10.2% | <0.0001 |
| Amiodarone | 18.2% | 6.0% | 22.5% | <0.0001 | 6.0% | 22.9% | <0.0001 |
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Rhythm-Control n = 161 | Rate-Control n = 212 | p-Value | Rhythm-Control n = 161 | Rate-Control n = 161 | p-Value | |
| Age (years) | 76.8 ± 8.3 | 79.5 ± 6.3 | <0.0001 | 76.8 ± 8.3 | 78.4 ± 6.4 | 0.06 |
| Male sex | 62.1% | 64.6% | 0.6 | 62.1% | 62.1% | 1 |
| EuroSCORE II (Q1; Q3) | 20.0% (11.6; 36.2) | 19.1% (9.7; 32.5) | 0.3 | 20.0% (11.6; 36.2) | 17.5% (9.3; 32.9) | 0.2 |
| STS-Risk-Score (Q1; Q3) | 7.2% (4.3; 13.0) | 7.6% (5.0; 14.3) | 0.3 | 7.2% (4.3; 13.0) | 6.8% (4.4; 13.4) | 0.9 |
| NYHA class I NYHA class II NYHA class III NYHA class IV | 0.0% 5.6% 71.4% 23.0% | 0.5% 3.8% 68.9% 26.9% | 0.6 | 0.0% 5.6% 71.4% 23% | 0.6% 5% 64.6% 29.8% | 0.4 |
| COPD | 18.6% | 20.8% | 0.6 | 18.6% | 18.6% | 1 |
| Coronary artery disease | 63.4% | 63.2% | 1 | 63.4% | 64.4% | 0.8 |
| Prior CABG surgery | 25.5% | 22.2% | 0.5 | 25.5% | 24.8% | 0.9 |
| Prior PCI | 55.3% | 50.5% | 0.4 | 55.3% | 49.1% | 0.3 |
| Diabetes mellitus | 28.0% | 36.3% | 0.09 | 28% | 33.5% | 0.3 |
| Art. Hypertension | 78.9% | 81.1% | 0.6 | 78.9% | 79.5% | 0.9 |
| Prior Stroke | 8.1% | 12.7% | 0.1 | 8.1% | 14.1% | 0.1 |
| Pre-existing ICD | 32.3% | 23.1% | 0.05 | 32.3% | 27.6% | 0.3 |
| Pre-existing CRT | 13.6% | 10.4% | 0.3 | 13.7% | 9.9% | 0.6 |
| GFR > 60 mL/Min GFR 30–59 mL/Min GFR < 30 mL/Min | 19.3% 58.4% 21.7% | 27.4% 55.2% 17.5% | 0.15 | 19.3% 58.4% 21.7% | 28.6% 50.9% 20.5% | 0.14 |
| NT-pro BNP (ng/L) (Q1; Q3) | 2915 (1055; 5528) | 2948 (1109; 5696) | 0.7 | 2915 (1055; 5528) | 2935 (1094; 5601) | 0.2 |
| LV function > 45% LV function 30–44% LV function < 30% | 38.5% 35.4% 26.1% | 44.3% 31.6% 24.1% | 0.5 | 38.5% 35.4% 26.1% | 44.7% 30.4% 24.8% | 0.5 |
| TR grade III | 14.3% | 26.9% | 0.003 | 14.3% | 13.7% | 0.9 |
| Degenerative MR etiology Functional MR etiology Combined MR etiology | 28.0% 65.2% 6.8% | 27.4% 63.7% 9.0% | 0.8 | 28% 65.2% 6.8% | 25.5% 65.2% 9.3% | 0.7 |
| Procedural characteristics | ||||||
| Mean procedure duration (min) | 115.2 ± 68 | 105.4 ± 64.7 | 0.2 | 115 ± 68 | 106 ± 67 | 0.2 |
| Postinterventional no MR Postinterventional MR grade I Postinterventional MR grade II Postinterventional MR grade III | 24.8% 64.6% 10.6% 0.0% | 25.5% 61.8% 12.3% 0.5% | 0.9 | 24.8% 64.6% 10.6% 0.0% | 26.1% 60.2% 13.7% 0.0% | 0.6 |
| Length of hospital stay (days) (Q1; Q3) | 7 (6; 11) | 7 (4; 10) | 0.3 | 7 (6; 11) | 7 (4; 10) | 0.3 |
| MACCE | 5.6% | 1.9% | 0.053 | 5.6% | 1.9% | 0.08 |
| In-hospital death from any cause | 5.0% | 3.3% | 0.4 | 5% | 3.7% | 0.6 |
| Heart Failure and anti-arrhythmic medication | ||||||
| ACE-/AT1 Inhibitors | 76.7% | 74.6% | 0.6 | 75.8% | 73.9% | 0.6 |
| ARN Inhibitor | 7.5% | 8.6% | 0.7 | 7.5% | 8.1% | 0.8 |
| Beta Blockers | 91.8% | 87.1% | 0.2 | 90.7% | 85.7% | 0.2 |
| Loop diuretics | 91.8% | 91.4% | 0.9 | 90.7% | 88.2% | 0.5 |
| Thiazid diuretics | 18.2% | 23.9% | 0.2 | 18% | 24.2% | 0.4 |
| Aldosteron antagonists | 48.4% | 49.3% | 0.9 | 47.8% | 47.8% | 1 |
| Ivabradin | 1.3% | 0.0% | 0.2 | 1.2% | 0.0% | 0.5 |
| Digitalis | 4.4% | 15.3% | <0.001 | 4.3% | 17.4% | <0.0001 |
| Amiodarone | 47.2% | 3.8% | <0.0001 | 47.2% | 5.0% | <0.0001 |
| Oral Anticoagulation | 92.5% | 90.1% | 0.4 | 92.5% | 89.4% | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waechter, C.; Ausbuettel, F.; Chatzis, G.; Cheko, J.; Fischer, D.; Nef, H.; Barth, S.; Halbfass, P.; Deneke, T.; Kerber, S.; et al. Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. J. Clin. Med. 2021, 10, 5044. https://doi.org/10.3390/jcm10215044
Waechter C, Ausbuettel F, Chatzis G, Cheko J, Fischer D, Nef H, Barth S, Halbfass P, Deneke T, Kerber S, et al. Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. Journal of Clinical Medicine. 2021; 10(21):5044. https://doi.org/10.3390/jcm10215044
Chicago/Turabian StyleWaechter, Christian, Felix Ausbuettel, Georgios Chatzis, Juan Cheko, Dieter Fischer, Holger Nef, Sebastian Barth, Philipp Halbfass, Thomas Deneke, Sebastian Kerber, and et al. 2021. "Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair" Journal of Clinical Medicine 10, no. 21: 5044. https://doi.org/10.3390/jcm10215044
APA StyleWaechter, C., Ausbuettel, F., Chatzis, G., Cheko, J., Fischer, D., Nef, H., Barth, S., Halbfass, P., Deneke, T., Kerber, S., Kikec, J., Mueller, H.-H., Divchev, D., Schieffer, B., & Luesebrink, U. (2021). Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. Journal of Clinical Medicine, 10(21), 5044. https://doi.org/10.3390/jcm10215044







