Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Preparation and Analysis of SF and Serum Proteins
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, A. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.C.; Ritvo, S.E.; Ferguson, M.K.; Clarke, H.; Seltzer, Z.; Katz, J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. J. Pain Res. 2015, 8, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Hootman, J.M.; Sitler, M.R.; Harris, K.; Cattano, N.M. Is Participation in Certain Sports Associated With Knee Osteoarthritis? A Systematic Review. J. Athl. Train. 2017, 52, 497–506. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of hip and knee osteoarthritis A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- US Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): U.S. City Average by Expenditure Category. Available online: https://www.bls.gov/news.release/cpi.t01.htm (accessed on 1 April 2020).
- Meehan, R.T.; Amigues, I.A.; Knight, V. Precision medicine for rheumatoid arthritis: The right drug for the right patient-companion Diagnostics. Diagnostics 2021, 11, 1362. [Google Scholar] [CrossRef]
- Cretu, D.; Diamandis, E.P.; Chandran, V. Delineating the synovial fluid proteome: Recent advancements and ongoing chal-lenges in biomarker research. Crit. Rev. Clin. Lab. Sci. 2013, 50, 51–63. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, T.A.; Szukiewicz, D. The Role of inflammatory and anti-inflammatory cytokines in the path-ogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2014, 2, 202–220. [Google Scholar] [CrossRef]
- Mobasheri, A.; Bay-Jensen, A.C.; van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis year in review 2016: Biomarkers (bi-ochemical markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar] [CrossRef]
- Bay-Jenson, A.C.; Thudium, C.S.; Mobasheri, A. Development and use of biochemical markers in osteoarthritis: Current up-date. Curr. Opin. Rheumatol. 2018, 30, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.J.; Martelpelletier, J.; Christiansen, C.F.; Brandi, M.L.; Bruyere, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Republished: Value of biomarkers in osteoarthritis: Current status and perspectives. Postgrad. Med. J. 2014, 90, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Galasso, O.; Falcone, D.; Southworth, S.; Greco, M.; Ventura, V.; Romualdi, P.; Corigliano, A.; Terracciano, R.; Savino, R.; et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthr. Cartil. 2013, 21, 1400–1408. [Google Scholar] [CrossRef]
- Bay-Jensen, A.; Reker, D.; Kjelgaard-Petersen, C.; Mobasheri, A.; Karsdal, M.; Ladel, C.; Henrotin, Y.; Thudium, C. Osteoarthritis year in review 2015: Soluble biomarkers and the BIPED criteria. Osteoarthr. Cartil. 2016, 24, 9–20. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Scuderi, G.J.; Cuellar, V.G.; Golish, S.R.; Yeomans, D.C. Diagnostic utility of cytokine biomarkers in the evalua-tion of acute knee pain. J. Bone Jt. Surg. 2009, 91, 2313–2320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing Immune-Mediated Inflammatory Diseases through Signature Cytokine Hubs. N. Engl. J. Med. 2021, 385, 628–639. [Google Scholar] [CrossRef]
- Meehan, R.; Wilson, C.; Hoffman, E.; Altimier, L.; Kaessner, M.; Regan, E.A. Ultrasound measurement of knee synovial fluid during external pneumatic compression. J. Orthop. Res. 2019, 37, 601–608. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 13 November 2020).
- Berenbaum, F.; Griffin, T.M.; Liu-Bryan, R. Review: Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol. 2017, 69, 9–21. [Google Scholar] [CrossRef]
- Watt, F.; Paterson, E.; Freidin, A.; Kenny, M.; Judge, A.; Saklatvala, J.; Williams, A.; Vincent, T. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis Rheumatol. 2016, 68, 2129–2140. [Google Scholar] [CrossRef]
- Catterall, J.B.; Stabler, T.V.; Flannery, C.R.; Kraus, V.B. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Ther. 2010, 12, R229. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability, inflammatory mediators of potential clin-ical relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef]
- Moradi, B.; Rosshirt, N.; Tripel, E.; Kirsch, J.; Barié, A.; Zeifang, F.; Gotterbarm, T.; Hagmann, S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015, 180, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Englund, M.; Struglics, A.; Lohmander, L.S. Inerleukin -6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr. Cartil. 2015, 23, 1906–1914. [Google Scholar] [CrossRef]
- Sauerschnig, M.; Stolberg-Stolberg, J.; Schulze, A.; Salzmann, G.M.; Perka, C.; Dynybil, C.J. Diverse expression of selected cyto-kines and proteinases in synovial fluid obtained from osteoarthritic and healthy human knee joints. Eur. J. Med. Res. 2014, 19, 65–70. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Q.; Chen, J.; Wang, H.; Wu, W.; Zha, Z. Expression and significance of MMPs in synovial fluid, serum and PBMNC culture supernatant stimulated by LPS in osteoarthritis patients with or without diabetes. Exp. Clin. Endocrinol. Diabetes 2019, 127, 195–202. [Google Scholar] [PubMed]
- Hyldahl, R.D.; Evans, A.; Kwon, S.; Ridge, S.T.; Robinson, E.; Hopkins, J.T.; Seeley, M.K. Running decreases knee in-tra-articular cytokine and cartilage oligomeric matrix concentrations: A pilot study. Eur. J. Appl. Physiol. 2016, 116, 2305–2314. [Google Scholar]
- McCabe, P.; Parkes, M.J.; Maricar, N.; Hutchinson, C.E.; Freemont, T.; O’Neill, T.W.; Felson, D. Brief Report: Synovial Fluid White Blood Cell Count in Knee Osteoarthritis: Association With Structural Findings and Treatment Response. Arthritis Rheumatol. 2017, 69, 103–107. [Google Scholar] [CrossRef]
- Rolle, N.A.; Jan, I.; Sibbitt, W.L., Jr.; Band, P.A.; Haseler, L.J.; Hayward, W.A.; Muruganandam, M.; Emil, N.S.; Fangtham, M.; Bankhurst, A.D. Extractable synovial fluid in inflammatory and non-inflammatory arthritis of the knee. Clin. Rheumatol. 2019, 38, 2255–2263. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Celis, R.; Hernández, M.V.; Ruiz-Esquide, V.; Ramírez, J.; Haro, I.; Cañete, J.D.; Sanmartí, R. Differences in synovial fluid cytokine levels but not in synovial tissue cell infiltrate between anti-citrullinated peptide/protein antibody-positive and negative rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R182. [Google Scholar] [CrossRef]
- Wright, H.; Bucknall, R.C.; Moots, R.J.; Edwards, S.W. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology 2011, 51, 451–459. [Google Scholar] [CrossRef]
- Osiri, M.; Wongpiyabovorn, J.; Sattayasomboon, Y.; Thammacharoenrach, N. Inflammatory cytokine levels, disease activity, and function of patients with rheumatoid arthritis treated with combined conventional disease-modifying antirheumatic drugs or biologics. Clin. Rheumatol. 2016, 35, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Connolly, S.E.; Maldonado, M.A.; Schiff, M. Brief Report: Estimating Disease Activity Using Multi-Biomarker Disease Activity Scores in Rheumatoid Arthritis Patients Treated With Abatacept or Adalimumab. Arthritis Rheumatol. 2016, 68, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.; Pitzalis, C. Stratified medicine in rheumatoid arthritis—The MATURA programme. Rheumatology 2017, 56, 1247–1250. [Google Scholar] [CrossRef]
- Knight, V.; Long, T.; Meng, Q.H.; Linden, M.A.; Roads, D.D. Variability in the Laboratory Measurement of Cytokines: A Lon-gitudinal Summary of a College of American Pathologists Proficiency Testing Survey. Arch. Pathol. Lab. Med. 2020, 144, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.H.; Dong, J.; Yuan, X.H.; Dong, Z.N.; Tian, Y.P. Clinical evaluation of the levels of 12 cytokines in serum/plasma under vari-ous storage conditions using evidence biochip arrays. Mol. Med. Rep. 2013, 7, 775–780. [Google Scholar] [CrossRef] [PubMed]
- De Jager, W.; Bourcier, K.; Rijkers, G.T.; Prakken, B.J.; Seyfert-Margolis, V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Block, J.; Berkoff, D.J.; Miller, L. Clinical utility of ultrasound guidance for intra-articular knee injections: A review. Clin. Interv. Aging 2012, 7, 89–95. [Google Scholar] [CrossRef]
- Bhavsar, T.B.; Sibbitt, W.L.; Band, P.A.; Cabacungan, R.J.; Moore, T.S.; Salayandia, L.C.; Fields, R.A.; Kettwich, S.K.; Roldan, L.P.; Emil, N.S.; et al. Improvement in diagnostic and therapeutic arthrocentesis via constant compression. Clin. Rheumatol. 2017, 37, 2251–2259. [Google Scholar] [CrossRef]
- Aigner, T.; Sachse, A.; Gebhard, P.M.; Roach, H.I. Osteoarthritis: Pathobiology—Targets and ways for therapeutic intervention. Adv. Drug Deliv. Rev. 2006, 58, 128–149. [Google Scholar] [CrossRef] [PubMed]
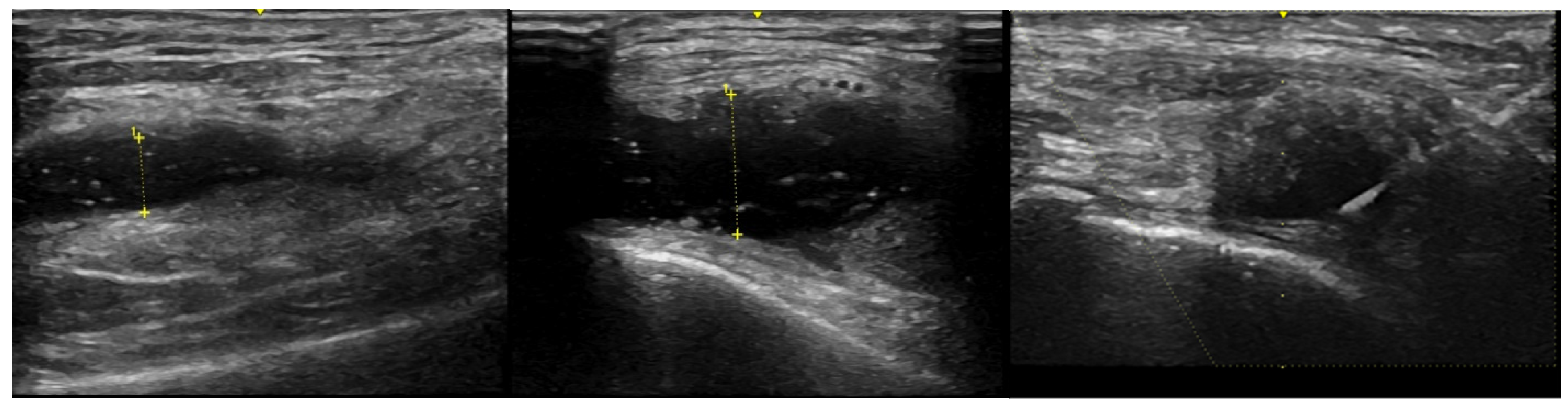


| RA Active | RA Controlled | OA | Normal | |
|---|---|---|---|---|
| Number of Subjects | 20 | 7 | 21 | 3 |
| Age Range (years) | 33–75 | 49–78 | 39–88 | 44–68 |
| Mean Age | 55 | 63 | 63 | 57 |
| Gender (F vs. M) | 15(75%)/5(25%) | 7(100%)/0(0%) | 11(52%)/10(48%) | 2(66%)/1(33%) |
| BMI | ||||
| Range | 20–35 | 17–37 | 23–41 | 21–24 |
| Mean | 26 | 27 | 30 | 22 |
| Number on Prednisone, DMARD or Biologic | 11 (58%) | 6 (86%) | 1 (5%) | 0 |
| Prednisone | 6 (32%) | 1 (14%) | 0 | 0 |
| Infliximab | 3 (16%) | 0 | 0 | 0 |
| MTX | 2 (11%) | 2 (29%) | 0 | 0 |
| HCQ | 3 (16%) | 3 (43%) | 1 | 0 |
| Rituximab | 2 (11%) | 1 (14%) | 0 | 0 |
| Tocilizumab | 1 (5%) | 1 (14%) | 0 | 0 |
| Sulfasalazine | 1 (5%) | 0 | 0 | 0 |
| Etanercept | 1 (5%) | 0 | 0 | 0 |
| Adalimumab | 1 (5%) | 0 | 0 | 0 |
| Golimumab | 0 | 1 (14%) | 0 | 0 |
| +RF > 14 | 7 (35%) | 6 (86%) | ND | ND |
| +CCP > 20 | 10 (50%) | 6 (86%) | ND | ND |
| SF WBC (cells/mm3) | ||||
| Range | 331–65,000 | 8–270 | 0–260 | 0 |
| Mean | 9620 | 85 | 131 | 0 |
| Normal | OA | RA Controlled | RA Active | |||||
|---|---|---|---|---|---|---|---|---|
| Protein | Mean +/− SD | Range | Mean +/− SD | Range | Mean +/− SD | Range | Mean +/− SD | Range |
| IGFBP-3 | 172,185 +/− 146,424 | 72,675–340,318 | 161,179 +/− 113,010 | 8501–400,152 | 256,352 +/− 82,938 | 158,808–388,506 | 247,003 +/− 115,594 | 55,900–441,896 |
| MMP-2 | 135,886 +/− 106,161 | 46,625–253,279 | 104,914 +/− 86,836 | 40,305–370,590 | 273,587 +/− 107,471 | 66,885–400,806 | 251,110 +/− 191,950 | 66,885–833,826 |
| MMP-3 | 102,994 +/− 135,473 | 11,821–258,664 | 67,820 +/− 46,784 | 5671–172,394 | 89,966 +/− 41,527 | 56,800–140,274 | 147,129 +/− 110,500 | 56,800–443,737 |
| MMP-1 | 1259 +/− 994 | 370–2331 | 6300 +/− 8107 | 36–24,804 | 60,084 +/− 60,225 | 3041–145,083 | 29,601 +/− 30,809 | 16,569–115,877 |
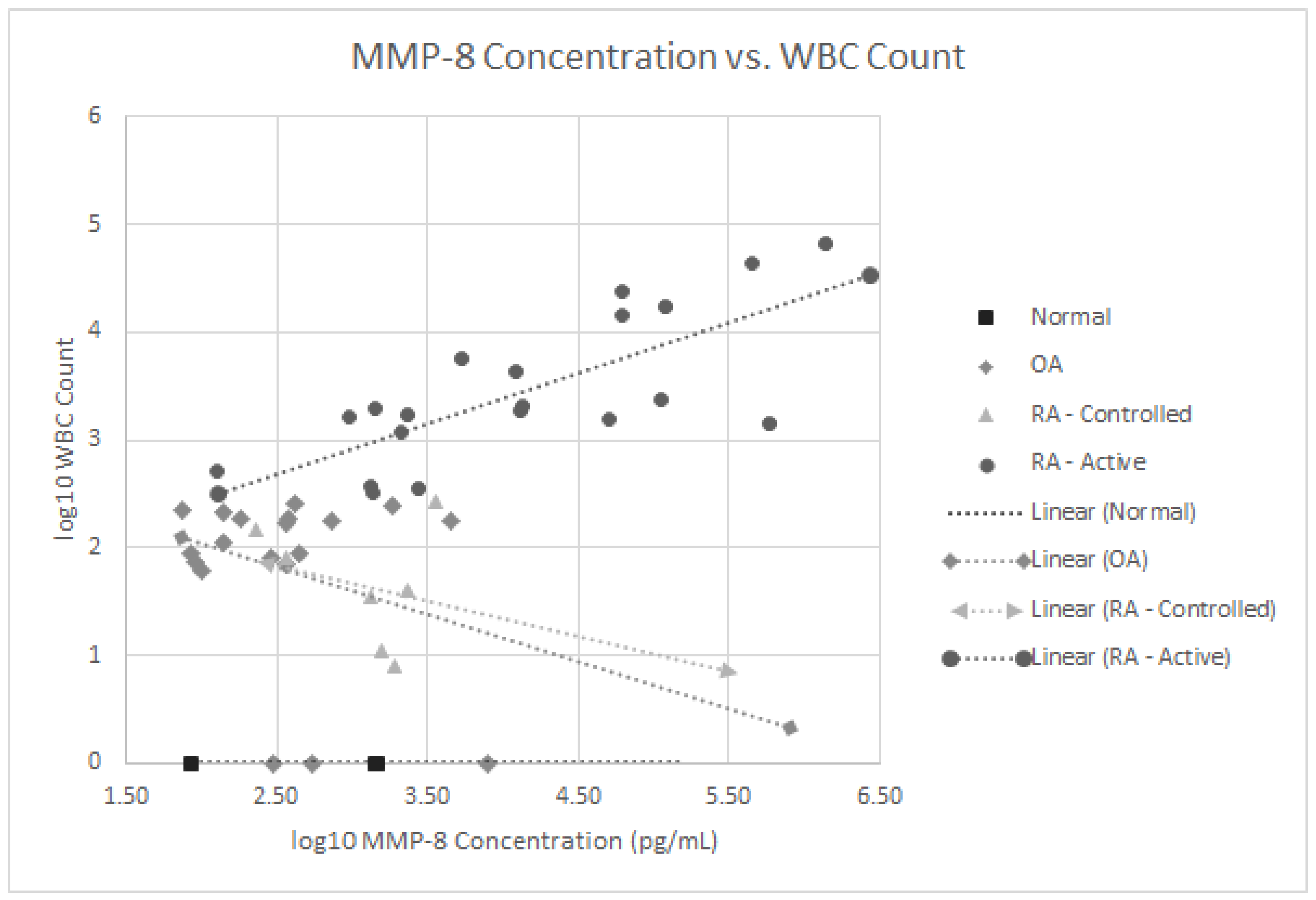
| MMP-8 | 999 +/− 790 | 86–1478 | 1000 +/− 1977 | 74–7978 | 1619 +/− 1162 | 232–3605 | 141,947 +/− 325,035 | 127–1,358,400 |
| IGFBP-4 | 320 +/− 183 | 215–531 | 825 +/− 1292 | 78–5384 | 1983 +/− 2727 | 0–7988 | 657 +/− 618 | 0–2167 |
| IL-4 | 294 +/− 268 | 6–536 | 119 +/− 154 | 0–563 | 95 +/− 77 | 0–194 | 129 +/− 156 | 0–618 |
| IL-1ra | 241 +/− 131 | 122–381 | 527 +/− 581 | 60–2254 | 681 +/− 599 | 200–1727 | 5021 +/− 5032 | 0–19800 |
| IL-18 | 97 +/− 104 | 25–216 | 103 +/− 87 | 0–302 | 75 +/− 76 | 0–186 | 745 +/− 2545 | 0–11,486 |
| G-CSF | 23 +/− 2 | 20–24 | 20 +/− 8 | 0–27 | 15 +/− 5 | 10–24 | 20 +/− 29 | 0–128 |
| IL-2 | 14 +/− 20 | 2–37 | 29 +/− 74 | 2–327 | 988 +/− 2128 | 37–4794 | 429 +/− 694 | 1–2320 |
| IL-15 | 11 +/− 5 | 7–16 | 12 +/− 10 | 0–40 | 14 +/− 11 | 0–22 | 10 +/− 12 | 0–40 |
| IL-7 | 6 +/− 5 | 1–11 | 8 +/− 16 | 1–59 | 22 +/− 18 | 6–47 | 23 +/− 19 | 1–47 |
| IL-1b | 6 +/− 10 | 0–18 | 5 +/− 7 | 0–18 | 15 +/− 6 | 5–18 | 33 +/− 62 | 0–263 |
| IL-6 | 3 +/− 3 | 1–6 | 36 +/− 39 | 0–135 | 225 +/− 221 | 0–550 | 1662 +/− 2763 | 0–11190 |
| TNF-α | 1 +/− 1 | 1–2 | 2 +/− 3 | 0–8 | 4 +/− 3 | 1–8 | 34 +/− 43 | 0–176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meehan, R.T.; Regan, E.A.; Hoffman, E.D.; Wolf, M.L.; Gill, M.T.; Crooks, J.L.; Parmar, P.J.; Scheuring, R.A.; Hill, J.C.; Pacheco, K.A.; et al. Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients. J. Clin. Med. 2021, 10, 5027. https://doi.org/10.3390/jcm10215027
Meehan RT, Regan EA, Hoffman ED, Wolf ML, Gill MT, Crooks JL, Parmar PJ, Scheuring RA, Hill JC, Pacheco KA, et al. Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients. Journal of Clinical Medicine. 2021; 10(21):5027. https://doi.org/10.3390/jcm10215027
Chicago/Turabian StyleMeehan, Richard T., Elizabeth A. Regan, Eric D. Hoffman, Molly L. Wolf, Mary T. Gill, James L. Crooks, Prashant J. Parmar, Richard A. Scheuring, John C. Hill, Karin A. Pacheco, and et al. 2021. "Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients" Journal of Clinical Medicine 10, no. 21: 5027. https://doi.org/10.3390/jcm10215027
APA StyleMeehan, R. T., Regan, E. A., Hoffman, E. D., Wolf, M. L., Gill, M. T., Crooks, J. L., Parmar, P. J., Scheuring, R. A., Hill, J. C., Pacheco, K. A., & Knight, V. (2021). Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients. Journal of Clinical Medicine, 10(21), 5027. https://doi.org/10.3390/jcm10215027






