Sphinkeeper Procedure for Treating Severe Faecal Incontinence—A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Outcome Measurements
2.3. Statistical Analysis
2.4. Study Registration
3. Results
3.1. Patient Characteristics
3.2. Surgical Outcome
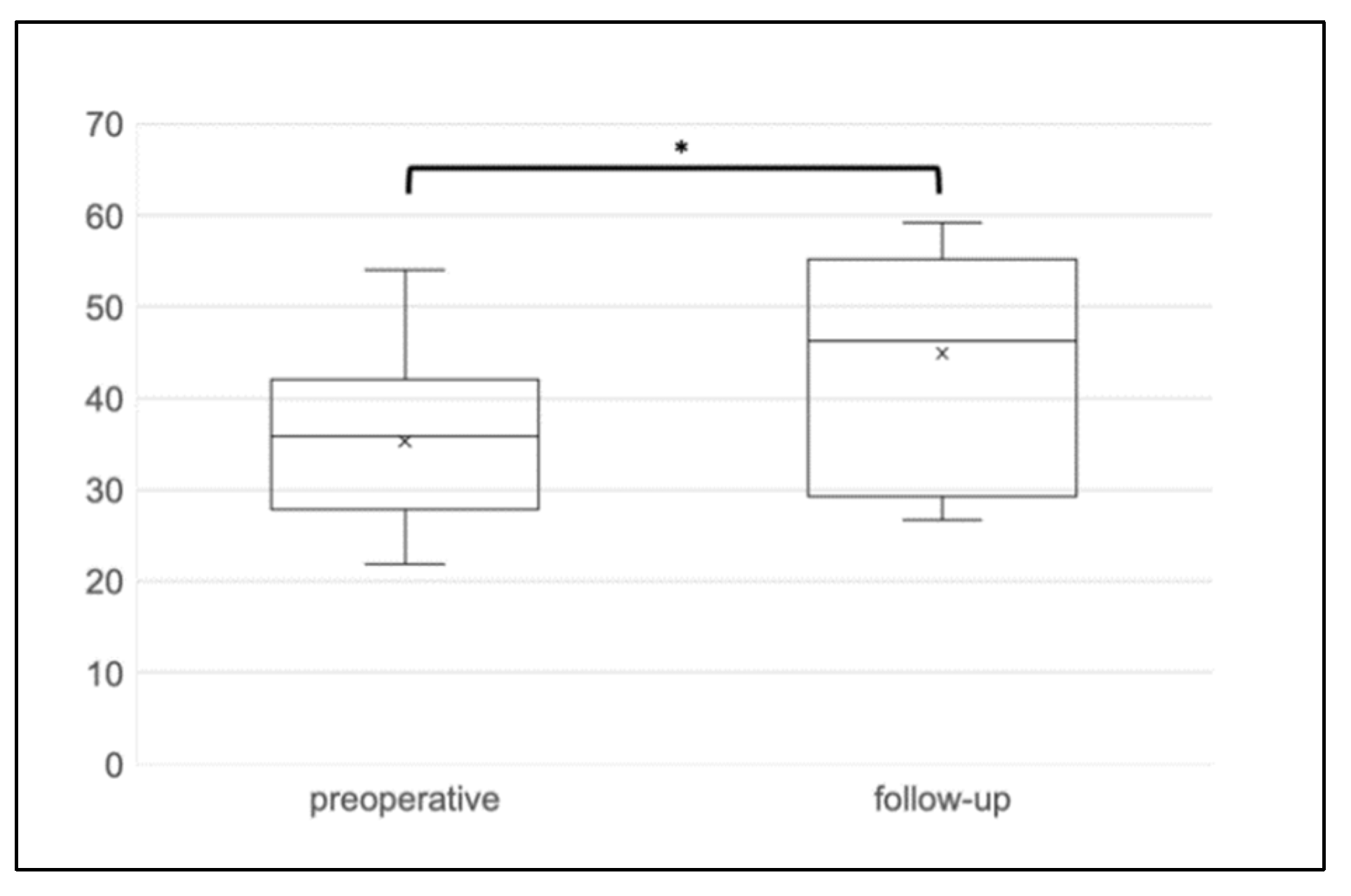
3.3. Functional Outcome
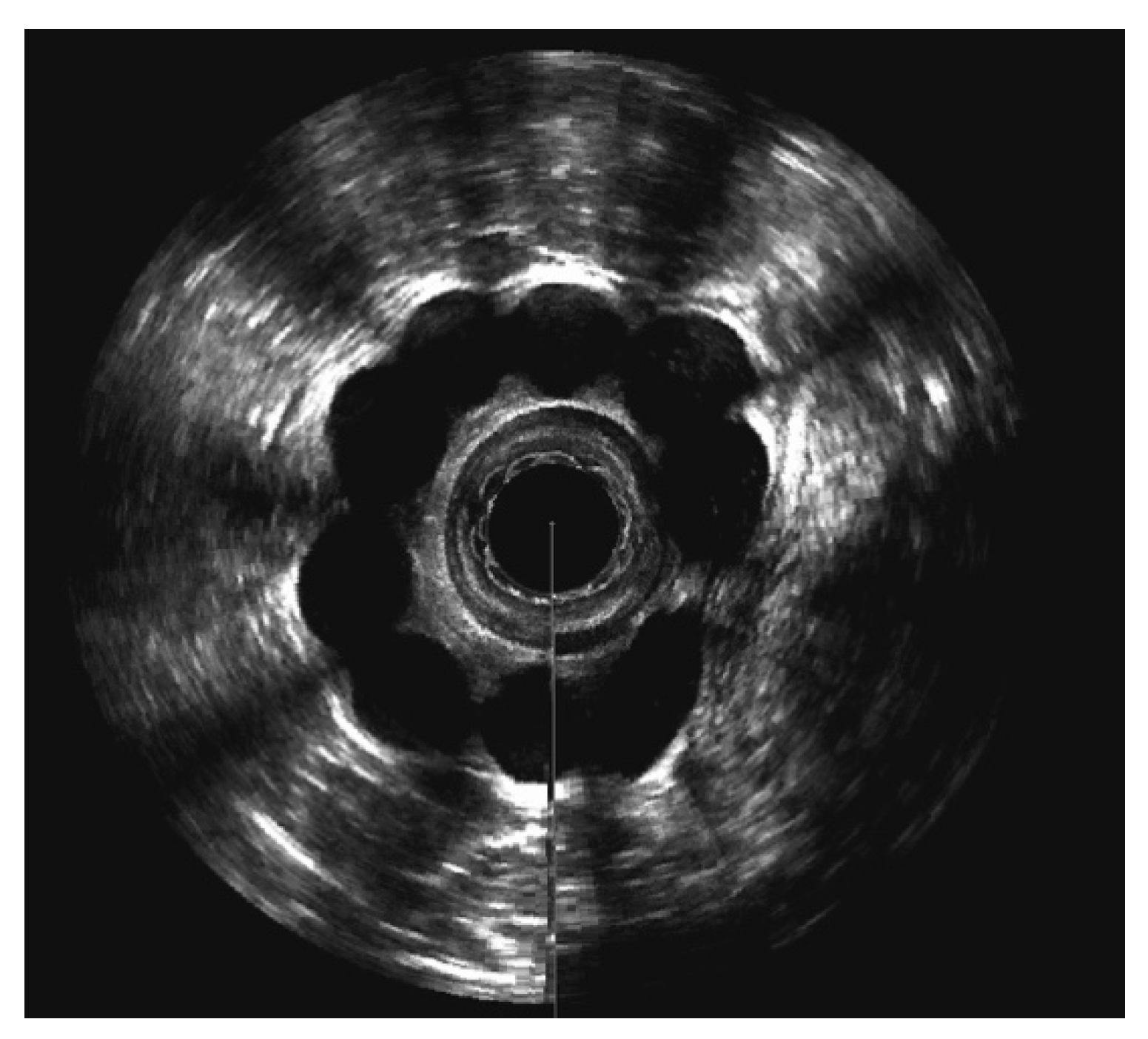
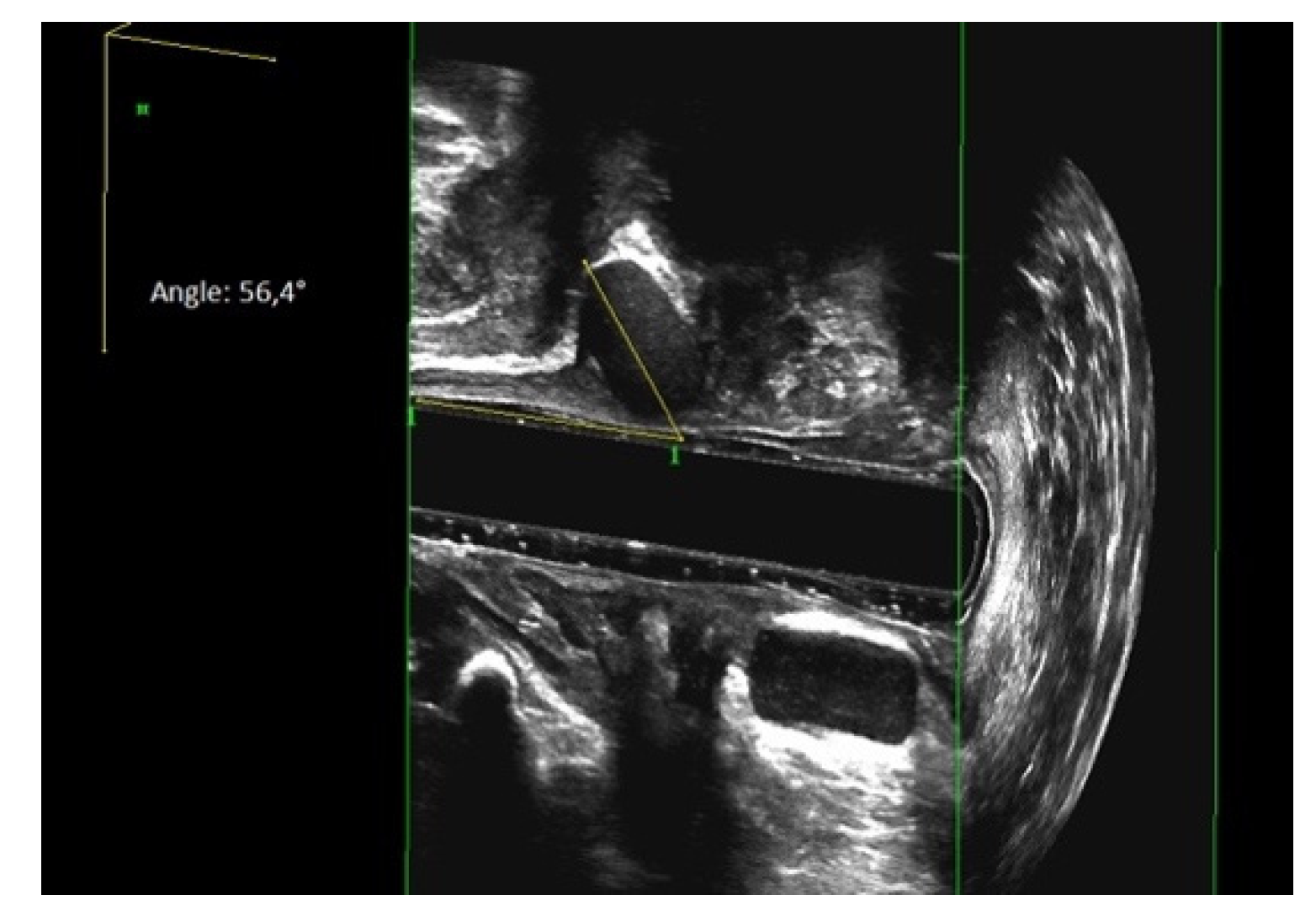
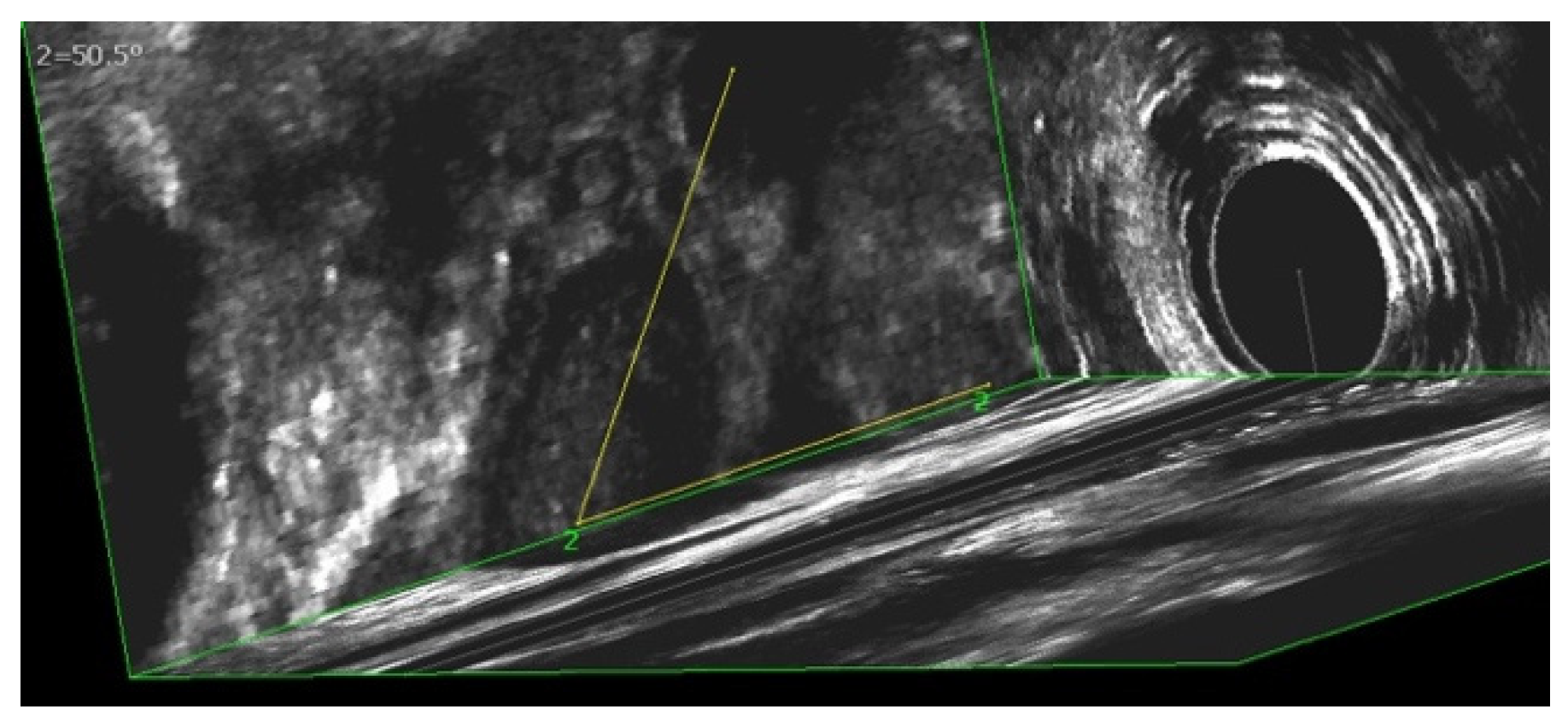
3.4. Evaluation of Sphinkeeper Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, K.S.; Sivakumaran, Y.; Nassar, N.; Gladman, M.A. Fecal Incontinence: Community Prevalence and Associated Factors—A Systematic Review. Dis. Colon Rectum 2015, 58, 1194–1209. [Google Scholar] [CrossRef] [PubMed]
- Damon, H.; Schott, A.M.; Barth, X.; Faucheron, J.L.; Abramowitz, L.; Siproudhis, L.; Fayard, M.O.; Colin, C.; Valancogne, G.; Bonniaud, V.; et al. Clinical characteristics and quality of life in a cohort of 621 patients with faecal incontinence. Int. J. Colorectal Dis. 2008, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yuan, L.; Marshall, R.J.; Merrie, A.E.; Bissett, I.P. Systematic review of the prevalence of faecal incontinence. Br. J. Surg. 2016, 103, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Ratto, C.; Parello, A.; Donisi, L.; Litta, F.; De Simone, V.; Spazzafumo, L.; Giordano, P. Novel bulking agent for faecal incontinence. Br. J. Surg. 2011, 98, 1644–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossi, U.; Brusciano, L.; Tolone, S.; Del Genio, G.; Di Tanna, G.L.; Gambardella, C.; Docimo, L. Implantable Agents for Fecal Incontinence: An Age-Matched Retrospective Cohort Analysis of GateKeeper versus SphinKeeper. Surg. Innov. 2020, 27, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Ratto, C.; Donisi, L.; Litta, F.; Campennì, P.; Parello, A. Implantation of SphinKeeper(TM): A new artificial anal sphincter. Tech. Coloproctol. 2016, 20, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litta, F.; Parello, A.; De Simone, V.; Campennì, P.; Orefice, R.; Marra, A.A.; Goglia, M.; Moroni, R.; Ratto, C. Efficacy of Sphinkeeper™ implant in treating faecal incontinence. Br. J. Surg. 2020, 107, 484–488. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.; Lisi, G.; Milito, G.; Campanelli, M.; Clementi, I. Sphinkeeper™ for faecal incontinence: A preliminary report. Colorectal Dis. 2020, 22, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, C.A.; Leeuwenburgh, M.; Orlando, A.; Corr, A.; Scott, S.M.; Murphy, J.; Knowles, C.H.; Vaizey, C.J.; Giordano, P. Initial experience with SphinKeeper™ intersphincteric implants for faecal incontinence in the UK: A two-centre retrospective clinical audit. Colorectal Dis. 2020, 22, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Vaizey, C.J.; Carapeti, E.; Cahill, J.A.; Kamm, M.A. Prospective comparison of faecal incontinence grading systems. Gut 1999, 44, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Jorge, J.M.; Wexner, S.D. Etiology and management of fecal incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef]
- Rome Foundation. Guidelines—Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J. Gastrointest. Liver Dis. 2006, 15, 307–312. [Google Scholar]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 170, 1453–1457. [Google Scholar] [CrossRef]
- Trenti, L.; Biondo, S.; Noguerales, F.; Nomdedeu, J.; Coret, A.; Scherer, R.; Fraccalvieri, D.; Frago, R.; Kreisler, E. Outcomes of GatekeeperTMprosthesis implantation for the treatment of fecal incontinence: A multicenter observational study. Tech. Coloproctol. 2017, 21, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Vaizey, C.J.; Kamm, M.A. Pilot study of two new injectable bulking agents for the treatment of faecal incontinence. Colorectal Dis. 2008, 10, 268–272. [Google Scholar] [CrossRef] [PubMed]


| n = 11 | |
|---|---|
| Demographics | |
| Age [years], median (range) | 75 (46–89) |
| Female sex, n (%) | 9 (81.8) |
| BMI [kg/m2], median (range) | 27 (21–30) |
| Clinical history, n (%) | |
| ASA classification I/II/III/IV | 0/6/5/0 |
| History of smoking | 3 (27.3) |
| Childbirth, n (%) | 7 (77.8) |
| Perineal tear [grade], median (range) | 0 (0–4) |
| Previous pelvic floor surgery, n (%) | 7 (63.6) |
| sphincteroplasty surgery, n (%) | 1 (9.1) |
| rectopexy, n (%) | 4 (36.3) |
| sacral nerve stimulation, n (%) | 5 (45.4) |
| EAUS—internal sphincter defect [grade], median (range) | 67.5 (38–121) |
| Stool parameters | |
| FI form | |
| Active FI, n (%) | 6 (54.5) |
| Passive FI, n (%) | 2 (18.2) |
| Mixed FI, n (%) | 3 (27.3) |
| Baseline n = 11 | Follow-Up n = 11 | |
|---|---|---|
| Faecal Incontinence symptoms | ||
| Incontinence to solid stool [daily episodes], n (%) | 8 (72.7) | 1 (9.1) |
| Incontinence to fluid stool [daily episodes], n (%) | 8 (72.7) | 1 (9.1) |
| Incontinence to gas [daily episodes], n (%) | 7 (63.6) | 2 (18.2) |
| Lifestyle alteration [daily], n (%) | 8 (72.7) | 2 (18.2) |
| Wears pad [daily], n (%) | 11 (100) | 8 (72.7) |
| Obstructed defecation symptoms | ||
| Feeling of blockade in the rectum, n (%) | 4 (36.4) | 3 (27.3) |
| Feeling of incomplete defecation, n (%) | 4 (36.4) | 2 (18.2) |
| Strong squeezing in defecation, n (%) | 2 (18.2) | 3 (27.3) |
| Emptying effort >1 per day, n (%) | 5 (45.5) | 3 (27.2) |
| Preoperative | Follow-Up | p | |
|---|---|---|---|
| Anorectal manometry | |||
| Resting pressure [mmHg], median (range) | 15 (9–48) | 16 (11–29) | 0.593 |
| Squeezing pressure [mmHg], median (range) | 39 (33–90) | 40 (21–85) | 1.000 |
| Straining pressure [mmHg], median (range) | 19 (12–42) | 34.5 (17–89) | 0.109 |
| Anorectal sensation | |||
| first anorectal sensation [ml], median (range) | 60 (10–60) | 22.5 (10–60) | 0.180 |
| rectal tenesmus [ml], median (range) | 140 (140–120) | 45 (30–120) | 0.109 |
| maximal tolerated volume [ml], median (range) | 220 (150–250) | 70 (40–135) | 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawoud, C.; Bender, L.; Widmann, K.M.; Harpain, F.; Riss, S. Sphinkeeper Procedure for Treating Severe Faecal Incontinence—A Prospective Cohort Study. J. Clin. Med. 2021, 10, 4965. https://doi.org/10.3390/jcm10214965
Dawoud C, Bender L, Widmann KM, Harpain F, Riss S. Sphinkeeper Procedure for Treating Severe Faecal Incontinence—A Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(21):4965. https://doi.org/10.3390/jcm10214965
Chicago/Turabian StyleDawoud, Christopher, Leonhard Bender, Kerstin Melanie Widmann, Felix Harpain, and Stefan Riss. 2021. "Sphinkeeper Procedure for Treating Severe Faecal Incontinence—A Prospective Cohort Study" Journal of Clinical Medicine 10, no. 21: 4965. https://doi.org/10.3390/jcm10214965
APA StyleDawoud, C., Bender, L., Widmann, K. M., Harpain, F., & Riss, S. (2021). Sphinkeeper Procedure for Treating Severe Faecal Incontinence—A Prospective Cohort Study. Journal of Clinical Medicine, 10(21), 4965. https://doi.org/10.3390/jcm10214965






