How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients?
Abstract
:1. Introduction
2. Epidemiology
3. Pathophysiology, Diagnosis and Clinical Presentation
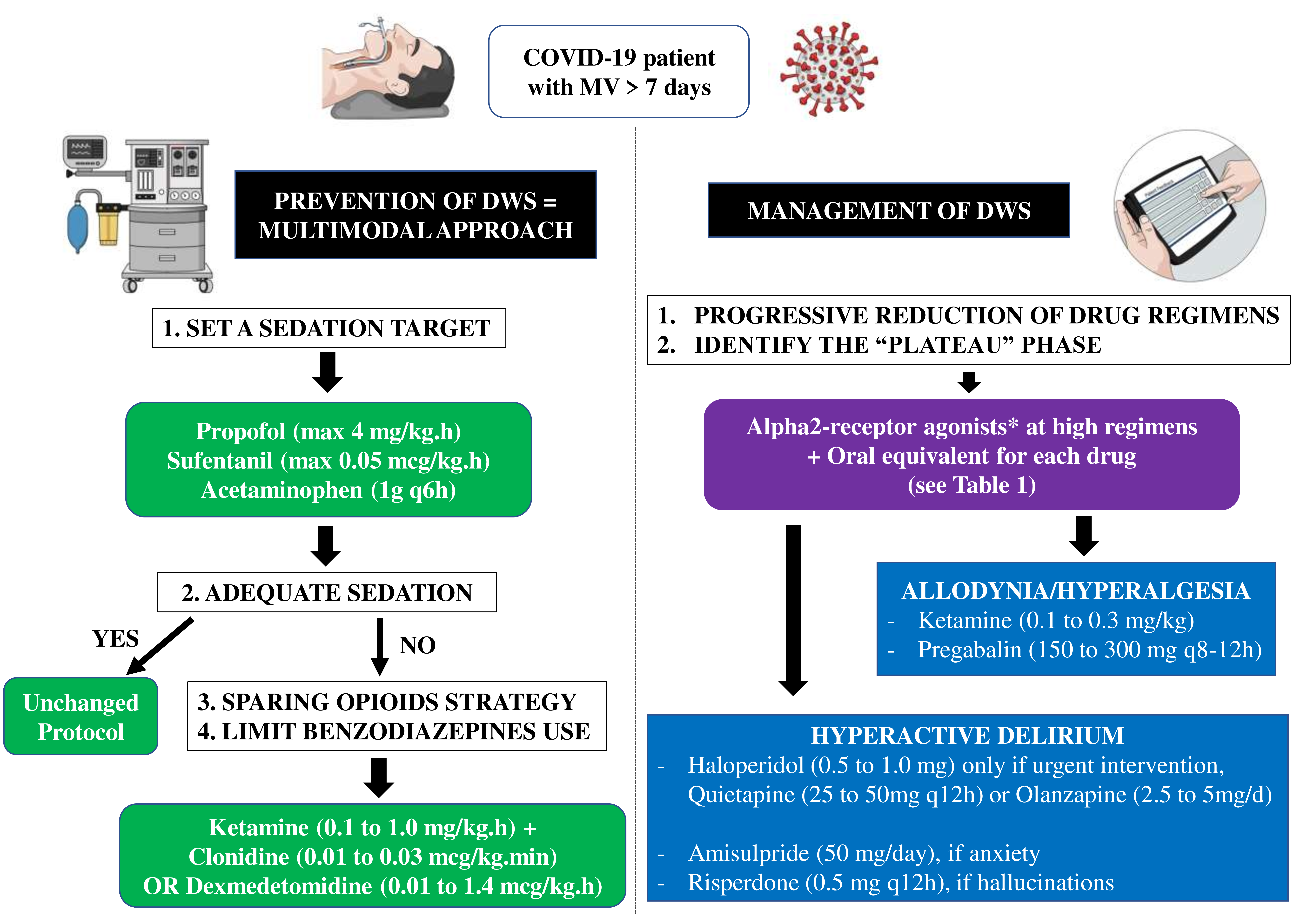
4. How to Manage Sedation and Analgesia Withdrawal Syndrome in COVID-19 Patients?
4.1. Development of an Analgo-Sedation Plan
4.2. Weaning Plan
4.3. Treatment of DWS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apigo, M.; Schechtman, J.; Dhliwayo, N.; Al Tameemi, M.; Gazmuri, R.J. Development of a work of breathing scale and monitoring need of intubation in COVID-19 pneumonia. Crit. Care 2020, 24, 477. [Google Scholar] [CrossRef]
- Grasselli, G.; Cattaneo, E.; Scaravilli, V. Ventilation of coronavirus disease 2019 patients. Curr. Opin. Crit. Care 2020, 27, 6–12. [Google Scholar] [CrossRef]
- Adams, C.D.; Altshuler, J.; Barlow, B.L.; Dixit, D.; Droege, C.A.; Effendi, M.K.; Heavner, M.S.; Johnston, J.P.; Kiskaddon, A.L.; Lemieux, D.G.; et al. Analgesia and Sedation Strategies in Mechanically Ventilated Adults with COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 1180–1191. [Google Scholar] [CrossRef]
- Tapaskar, N.; Hidalgo, D.C.; Koo, G.; Shingada, K.; Rao, S.; Rodriguez, R.; Alcantar, D.; Barrera, D.E.; Lee, R.; Rameshkumar, N.; et al. Sedation Usage in COVID-19 Acute Respiratory Distress Syndrome: A Multicenter Study. Ann. Pharmacother. 2021. [Google Scholar] [CrossRef]
- Kapp, C.M.; Zaeh, S.; Niedermeyer, S.; Punjabi, N.M.; Siddharthan, T.; Damarla, M. The Use of Analgesia and Sedation in Mechanically Ventilated Patients with COVID-19 Acute Respiratory Distress Syndrome. Anesthesia Analg. 2020, 131, e198–e200. [Google Scholar] [CrossRef]
- Chanques, G.; Constantin, J.-M.; Devlin, J.W.; Ely, E.W.; Fraser, G.L.; Gélinas, C.; Girard, T.D.; Guérin, C.; Jabaudon, M.; Jaber, S.; et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020, 46, 2342–2356. [Google Scholar] [CrossRef]
- Arroyo-Novoa, C.M.; I Figueroa-Ramos, M.; Puntillo, K.A. Opioid and Benzodiazepine Iatrogenic Withdrawal Syndrome in Patients in the Intensive Care Unit. AACN Adv. Crit. Care 2019, 30, 353–364. [Google Scholar] [CrossRef]
- Dokken, M.; Rustøen, T.; Diep, L.M.; Fagermoen, F.E.; Huse, R.I.; Rosland, G.A.; Egerod, I.; Bentsen, G.K. Iatrogenic withdrawal syndrome frequently occurs in paediatric intensive care without algorithm for tapering of analgosedation. Acta Anaesthesiol. Scand. 2021, 65, 928–935. [Google Scholar] [CrossRef]
- Ista, E.; van Dijk, M.; Gamel, C.; Tibboel, D.; de Hoog, M. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: A first evaluation*. Crit. Care Med. 2008, 36, 2427–2432. [Google Scholar] [CrossRef] [Green Version]
- Stormorken, A. Tolerance and Withdrawal in Critically Ill Children. In Sedation and Analgesia for the Pediatric Intensivist: A Clinical Guid; Kamat, P.P., Berkenbosch, J.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–151. [Google Scholar]
- Cammarano, W.B.; Pittet, J.-F.; Weitz, S.; Schlobohm, R.M.; Marks, J.D. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit. Care Med. 1998, 26, 676–684. [Google Scholar] [CrossRef]
- Brown, C.; Albrecht, R.; Pettit, H.; McFadden, T.; Schermer, C. Opioid and benzodiazepine withdrawal syndrome in adult burn patients. Am. Surg. 2000, 66, 367–370, Discussion 370–371. [Google Scholar]
- Korak-Leiter, M.; Likar, R.; Oher, M.; Trampitsch, E.; Ziervogel, G.; Levy, J.V.; Freye, E.C. Withdrawal following sufentanil/propofol and sufentanil/midazolam. Sedation in surgical ICU patients: Correlation with central nervous parameters and endogenous opioids. Intensive Care Med. 2005, 31, 380–387. [Google Scholar] [CrossRef]
- Wang, P.P.; Huang, E.; Feng, X.; Bray, C.; Perreault, M.M.; Rico, P.; Bellemare, P.; Murgo, P.; Gélinas, C.; Lecavalier, A.; et al. Opioid-associated iatrogenic withdrawal in critically ill adult patients: A multicenter pro-spective observational study. Ann. Intensive Care 2017, 7, 88. [Google Scholar] [CrossRef]
- Girard, T.D.; Pandharipande, P.P.; Ely, E.W. Delirium in the intensive care unit. Crit. Care 2008, 12 (Suppl. 3), S3. [Google Scholar] [CrossRef] [Green Version]
- Kotfis, K.; Marra, A.; Ely, E.W. ICU delirium―A diagnostic and therapeutic challenge in the intensive care unit. Anestezjol. Intensywna Ter. 2018, 50, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Petriceks, A.H.; Burke, H.; Stern, T.A. Weaning from Exogenous Sedation in the Era of COVID-19 Infection: Recommendations for Sedation and Its Discontinuation. Prim. Care Companion CNS Disord. 2020, 22, 20f02686. [Google Scholar] [CrossRef]
- Bouajram, R.H.; Bhatt, K.; Croci, R.; Baumgartner, L.; Puntillo, K.; Ramsay, J.; Thompson, A. Incidence of Dexmedetomidine Withdrawal in Adult Critically Ill Patients: A Pilot Study. Crit. Care Explor. 2019, 1, e0035. [Google Scholar] [CrossRef]
- Kosten, T.R.; O’Connor, P.G. Management of Drug and Alcohol Withdrawal. N. Engl. J. Med. 2003, 348, 1786–1795. [Google Scholar] [CrossRef] [Green Version]
- Mirski, M.A.; Ledroux, S.N.; Lewin, J.J.; Thompson, C.B.; Mirski, K.T.; Griswold, M. Validity and reliability of an intuitive conscious sedation scoring tool: The nursing instrument for the communication of sedation*. Crit. Care Med. 2010, 38, 1674–1684. [Google Scholar] [CrossRef]
- Witenko, C.J.; Littlefield, A.J.; Abedian, S.; An, A.; Barie, P.S.; Berger, K. The Safety of Continuous Infusion Propofol in Mechanically Ventilated Adults with Coronavirus Disease 2019. Ann. Pharmacother. 2021, 10600280211017316. [Google Scholar] [CrossRef]
- Spinelli, E.; Mauri, T.; Beitler, J.R.; Pesenti, A.; Brodie, D. Respiratory drive in the acute respiratory distress syndrome: Pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020, 46, 606–618. [Google Scholar] [CrossRef]
- Moitra, V.K.; Patel, M.K.; Darrah, D.; Moitra, A.; Wunsch, H. Low-Dose Ketamine in Chronic Critical Illness. J. Intensive Care Med. 2015, 31, 216–220. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Z.; Xin, Y.C.; Cai, Y.; Chen, Y.; Pan, S.M. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Gowing, L.; Farrell, M.; Ali, R.; White, J.M. Alpha₂-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2016, 2016, CD002024. [Google Scholar] [CrossRef]
- Karol, M.D.; Maze, M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 261–269. [Google Scholar] [CrossRef]
- Bhatt, K.; Quan, A.T.; Baumgartner, L.; Jia, S.; Croci, R.; Puntillo, K.; Ramsay, J.; Bouajram, R.H. Effects of a Clonidine Taper on Dexmedetomidine Use and Withdrawal in Adult Critically Ill Patients—A Pilot Study. Crit. Care Explor. 2020, 2, e0245. [Google Scholar] [CrossRef]
- Kukoyi, A.; Coker, S.; Lewis, L.; Nierenberg, D. Two cases of acute dexmedetomidine withdrawal syndrome following prolonged infusion in the intensive care unit: Report of cases and review of the literature. Hum. Exp. Toxicol. 2013, 32, 107–110. [Google Scholar] [CrossRef]
- Srivastava, A.B.; Mariani, J.J.; Levin, F.R. New directions in the treatment of opioid withdrawal. Lancet 2020, 395, 1938–1948. [Google Scholar] [CrossRef]
- Gowing, L.; Ali, R.; White, J.M. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2009, CD002025. [Google Scholar] [CrossRef]
- Sigmon, S.C.; Bisaga, A.; Nunes, E.; O’Connor, P.G.; Kosten, T.; Woody, G. Opioid Detoxification and Naltrexone Induction Strategies: Recommendations for Clinical Practice. Am. J. Drug Alcohol Abus. 2012, 38, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Siddappa, R.; Fletcher, J.E.; Heard, A.M.; Kielma, D.; Cimino, M.; Heard, C.M. Methadone dosage for prevention of opioid withdrawal in children. Pediatr. Anesthesia 2003, 13, 805–810. [Google Scholar] [CrossRef]
- Martyn, J.A.J.; Mao, J.; Bittner, E.A. Opioid Tolerance in Critical Illness. N. Engl. J. Med. 2019, 380, 365–378. [Google Scholar] [CrossRef]
- Colvin, L.A.; Fallon, M.T. Opioid-induced hyperalgesia: A clinical challenge. Br. J. Anaesth. 2010, 104, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, A.; Vennat, B.; Llorca, P.-M.; Eschalier, A. Benzodiazepine dependence: Focus on withdrawal syndrome. Ann. Pharm. Fr. 2009, 67, 408–413. [Google Scholar] [CrossRef]
- Van Der Vossen, A.C.; Van Nuland, M.; Ista, E.G.; de Wildt, S.; Hanff, L.M. Oral lorazepam can be substituted for intravenous midazolam when weaning paediatric intensive care patients off sedation. Acta Paediatr. 2018, 107, 1594–1600. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Huang, Y.; Lu, Y.; Chen, J.; Jiang, H. Repeated administration of ketamine can induce hippocampal neurodegeneration and long-term cognitive impairment via the ROS/HIF-1α pathway in developing rats. Cell Physiol. Biochem. 2014, 33, 1715–1732. [Google Scholar] [CrossRef]
- Liu, K.; Nakamura, K.; Katsukawa, H.; Elhadi, M.; Nydahl, P.; Ely, E.W.; Kudchadkar, S.R.; Takahashi, K.; Inoue, S.; Lefor, A.K.; et al. ABCDEF Bundle and Supportive ICU Practices for Patients with Coronavirus Disease 2019 Infection: An International Point Prevalence Study. Crit. Care Explor. 2021, 3, e0353. [Google Scholar] [CrossRef]
- Gardner, D.M.; Baldessarini, R.J.; Waraich, P. Modern antipsychotic drugs: A critical overview. Can. Med. Assoc. J. 2005, 172, 1703–1711. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, T.L.; Masand, P.S. The Role of Atypical Antipsychotics in the Treatment of Delirium. J. Psychosom. Res. 2002, 43, 171–174. [Google Scholar] [CrossRef]
| Drug | Symptoms | Specific Treatment |
|---|---|---|
| General symptoms | Anxiety, agitation, tremor, tachycardia and hypertension | Neuroleptics Alpha-2-agonists |
| Opioids | Mydriasis, nausea/vomiting, abdominal cramps, diarrhea, tachypnea, hot flushes/chills, sweating and pain | Buprenorphine Methadone Pregabalin (if hyperalgesia) |
| Benzodiazepines | Fever and seizures | Lorazepam |
| Ketamine | Hallucinations, nightmares, depersonalization and general distress | Benzodiazepines Alpha-2-agonists |
| Dexmedetomidine | Delirium, hypertension, tachycardia and agitation | Clonidine |
| Propofol | Not described | No specific treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ego, A.; Halenarova, K.; Creteur, J.; Taccone, F.S. How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients? J. Clin. Med. 2021, 10, 4917. https://doi.org/10.3390/jcm10214917
Ego A, Halenarova K, Creteur J, Taccone FS. How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients? Journal of Clinical Medicine. 2021; 10(21):4917. https://doi.org/10.3390/jcm10214917
Chicago/Turabian StyleEgo, Amédée, Katarina Halenarova, Jacques Creteur, and Fabio Silvio Taccone. 2021. "How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients?" Journal of Clinical Medicine 10, no. 21: 4917. https://doi.org/10.3390/jcm10214917
APA StyleEgo, A., Halenarova, K., Creteur, J., & Taccone, F. S. (2021). How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients? Journal of Clinical Medicine, 10(21), 4917. https://doi.org/10.3390/jcm10214917






