Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pulmonary Function Measurements
2.3. Statistical Analysis
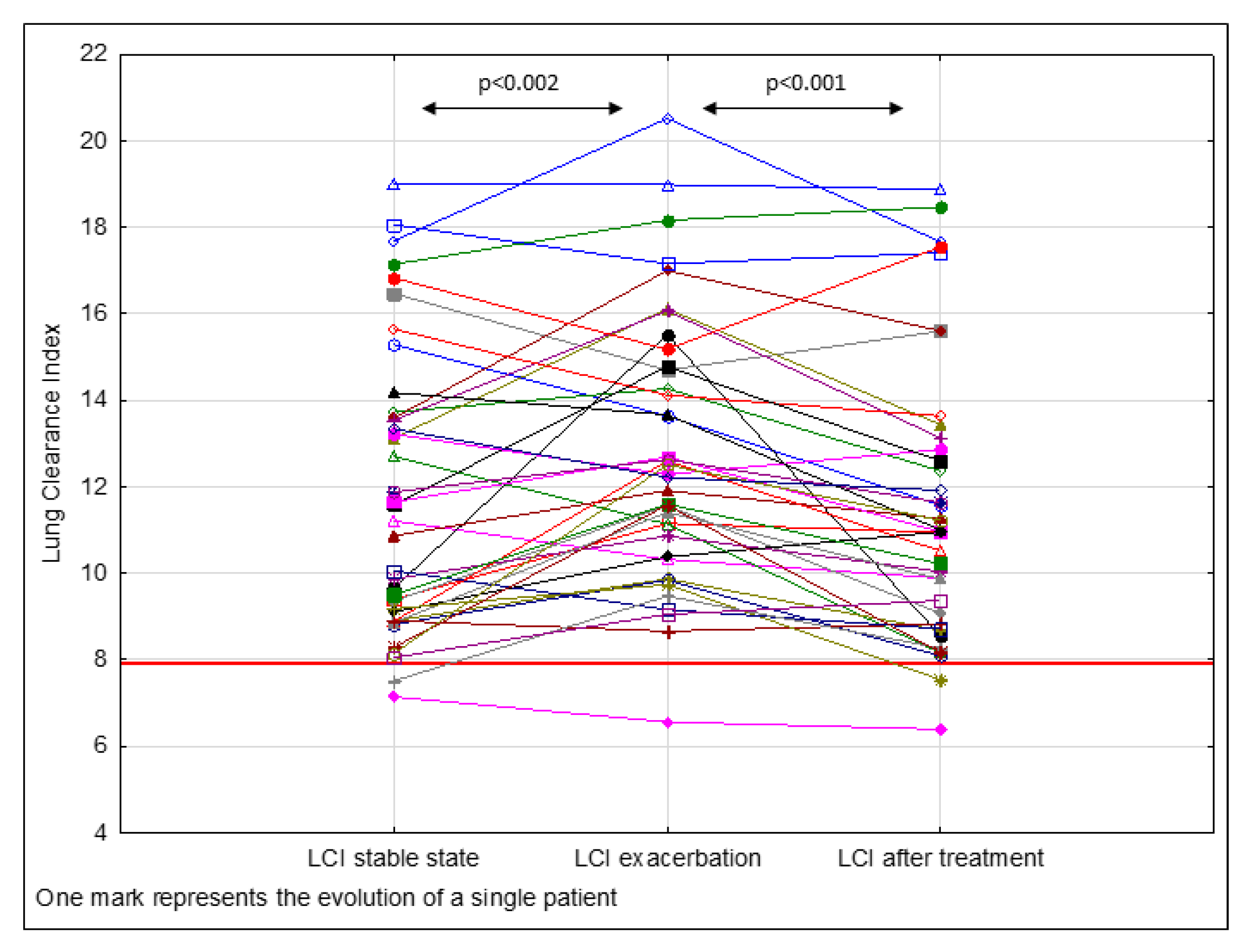
3. Results
3.1. Subject Characteristics
3.2. Pulmonary Function Measurements
3.2.1. Change in LCI and FEV1 at the Beginning of PEx
3.2.2. Pulmonary Function Response to PEx Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- De Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.; Stanojevic, S.; Atenafu, E.G.; Lu, A.; Yau, Y.; Tullis, E.; Ratjen, F. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur. Respir. J. 2012, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Horsley, A.; Siddiqui, S. Putting lung function and physiology into perspective: Cystic fibrosis in adults. Respirology 2015, 20, 33–45. [Google Scholar] [CrossRef]
- Gustafsson, P.M.; De Jong, P.A.; Tiddens, H.A.; Lindblad, A. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 2008, 63, 129–134. [Google Scholar] [CrossRef]
- Stanojevic, S.; Davis, S.D.; Perrem, L.; Shaw, M.; Retsch-Bogart, G.; Davis, M.; Jensen, R.; Clem, C.C.; Isaac, S.M.; Guido, J.; et al. Determinants of lung disease progression measured by lung clearance index in children with cystic fibrosis. Eur. Respir. J. 2021, 58. [Google Scholar] [CrossRef] [PubMed]
- Perrem, L.; Stanojevic, S.; Shaw, M.; Jensen, R.; McDonald, N.; Isaac, S.M.; Davis, M.; Clem, C.; Guido, J.; Jara, S.; et al. Lung Clearance Index to Track Acute Respiratory Events in School-age Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 977–986. [Google Scholar] [CrossRef]
- Frauchiger, B.S.; Binggeli, S.; Yammine, S.; Spycher, B.; Krüger, L.; Ramsey, K.A.; Latzin, P. Longitudinal Course of Clinical Lung Clearance Index in Children with Cystic Fibrosis. Eur. Respir. J. 2021, 58, 2002686. [Google Scholar] [CrossRef]
- Rayment, J.H.; Stanojevic, S.; Davis, S.D.; Retsch-Bogart, G.; Ratjen, F. Lung clearance index to monitor treatment response in pulmonary exacerbations in preschool children with cystic fibrosis. Thorax 2018, 73, 451–458. [Google Scholar] [CrossRef]
- Singer, F.; Kieninger, E.; Abbas, C.; Yammine, S.; Fuchs, O.; Proietti, E.; Regamey, N.; Casaulta, C.; Frey, U.; Latzin, P. Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr. Pulmonol. 2013, 48, 739–746. [Google Scholar] [CrossRef]
- Horsley, A.R.; Gustafsson, P.M.; Macleod, K.A.; Saunders, C.; Greening, A.P.; Porteous, D.J.; Davies, J.C.; Cunningham, S.; Alton, E.W.; Innes, J.A. Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax 2008, 63, 135–140. [Google Scholar] [CrossRef]
- Horsley, A.R.; Davies, J.C.; Gray, R.D.; Macleod, K.A.; Donovan, J.; Aziz, Z.A.; Bell, N.J.; Rainer, M.; Mt-Isa, S.; Voase, N.; et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax 2013, 68, 532–539. [Google Scholar] [CrossRef]
- Kent, L.; Reix, P.; Innes, J.A.; Zielen, S.; Le Bourgeois, M.; Braggion, C.; Lever, S.; Arets, H.G.; Brownlee, K.; Bradley, J.M.; et al. Lung clearance index: Evidence for use in clinical trials in cystic fibrosis. J. Cyst. Fibros. 2014, 13, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Jensen, R.; Robinson, P.D.; Stanojevic, S.; Klingel, M.; Short, C.; Davies, J.C.; Ratjen, F. Integrating the multiple breath washout test into international multicentre trials. J. Cyst. Fibros. 2020, 19, 602–607. [Google Scholar] [CrossRef]
- Ratjen, F.; Klingel, M.; Black, P.; Powers, M.R.; Grasemann, H.; Solomon, M.; Sagel, S.D.; Donaldson, S.H.; Rowe, S.M.; Rosenfeld, M. Changes in lung clearance index in preschool-aged patients with cystic fibrosis treated with ivacaftor (GOAL): A clinical trial. Am. J. Respir. Crit. Care Med. 2018, 198, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, N.; Stanojevic, S.; Amin, R.; Aurora, P.; Davies, J.; Elborn, J.S.; Horsley, A.; Latzin, P.; O’Neill, K.; Robinson, P.; et al. Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur. Respir. J. 2015, 46, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Bayfield, K.; Irving, S.; Short, C.; Bush, A.; Davies, J.C. Developments in multiple breath washout testing in children with cystic fibrosis. Curr. Med. Res. Opin. 2017, 33, 613–620. [Google Scholar] [CrossRef]
- Fretzayas, A.; Douros, K.; Moustaki, M.; Loukou, I. Applications of lung clearance index in monitoring children with cystic fibrosis. World J. Clin. Pediatr. 2019, 8, 15–22. [Google Scholar] [CrossRef]
- Anagnostopoulou, P.; Latzin, P.; Jensen, R.; Stahl, M.; Harper, A.; Yammine, S.; Usemann, J.; Foong, R.E.; Spycher, B.; Hall, G.L.; et al. Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur. Respir. J. 2020, 55, 1901302. [Google Scholar] [CrossRef]
- Yammine, S.; Bigler, A.; Casaulta, C.; Singer, F.; Latzin, P. Reasons for heterogeneous change in LCI in children with cystic fibrosis after antibiotic treatment. Thorax 2014, 69, 183. [Google Scholar] [CrossRef][Green Version]
- Vermeulen, F.; Proesmans, M.; Boon, M.; Havermans, T.; De Boeck, K. Lung clearance index predicts pulmonary exacerbations in young patients with cystic fibrosis. Thorax 2014, 69, 39–45. [Google Scholar] [CrossRef]
- Oude Engberink, E.; Ratjen, F.; Davis, S.D.; Retsch-Bogart, G.; Amin, R.; Stanojevic, S. Inter-test reproducibility of the lung clearance index measured by multiple breath washout. Eur. Respir. J. 2017, 50, 1700433. [Google Scholar] [CrossRef]
- Sanders, D.B.; Bittner, R.C.; Rosenfeld, M.; Hoffman, L.R.; Redding, G.J.; Goss, C.H. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am. J. Respir. Crit. Care Med. 2010, 182, 627–632. [Google Scholar] [CrossRef] [PubMed]
- VanDevanter, D.R.; O'Riordan, M.A.; Blumer, J.L.; Konstan, M.W. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir. Res. 2010, 11, 137. [Google Scholar] [CrossRef]
- Collaco, J.M.; Green, D.M.; Cutting, G.R.; Naughton, K.M.; Mogayzel, P.J. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am. J. Respir. Crit. Care Med. 2010, 182, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Cooper, P.; Van Asperen, P.; Fitzgerald, D.; Selvadurai, H. Using index of ventilation to assess response to treatment for acute pulmonary exacerbation in children with cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 733–742. [Google Scholar] [CrossRef]
- Vanderhelst, E.; De Meirleir, L.; Schuermans, D.; Malfroot, A.; Vincken, W.; Verbanck, S. Evidence of an acinar response following treatment for exacerbation in adult patients with cystic fibrosis. Respiration 2014, 87, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Welsh, L.; Nesci, C.; Tran, H.; Tomai, M.; Ranganathan, S. Lung clearance index during hospital admission in school-age children with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 687–691. [Google Scholar] [CrossRef][Green Version]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181S, S4–S15.el. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.J.; Borowitz, D.S.; Christiansen, D.H.; Morris, E.M.; Nash, M.L.; Ramsey, B.W.; Rosenstein, B.J.; Smith, A.L.; Wohl, M.E. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N. Engl. J. Med. 1994, 331, 637–642. [Google Scholar] [CrossRef]
- Jensen, R.; Stanojevic, S.; Klingel, M.; Pizarro, M.E.; Hall, G.L.; Ramsey, K.; Foong, R.; Saunders, C.; Robinson, P.D.; Webster, H.; et al. A Systematic Approach to Multiple Breath Nitrogen Washout Test Quality. PLoS ONE 2016, 11, e0157523. [Google Scholar] [CrossRef]
- Robinson, P.D.; Latzin, P.; Verbanck, S.; Hall, G.L.; Horsley, A.; Gappa, M.; Thamrin, C.; Arets, H.G.; Aurora, P.; Fuchs, S.I.; et al. Consensus statement for inert gas washout measurement using multiple-and single-breath tests. Eur. Respir. J. 2013, 41, 507–522. [Google Scholar] [CrossRef]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H.; et al. An official American Thoracic Society/European Respiratory Society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Zapletal, A. Lung Function in Children and Adolescents: Methods, Reference Values/A. Zapletal; Samanek, M., Paul, T., Eds.; Karger: Basel, Switzerland; New York, NY, USA, 1987. [Google Scholar]
- Waters, V.; Ratjen, F. Pulmonary Exacerbations in Children with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S200–S206. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Bittner, R.C.; Rosenfeld, M.; Redding, G.J.; Goss, C.H. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr. Pulmonol. 2011, 46, 393–400. [Google Scholar] [CrossRef]
- Stenbit, A.E.; Flume, P.A. Pulmonary exacerbations in cystic fibrosis. Curr. Opin. Pulm. Med. 2011, 17, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.G.; Elkin, E.P.; Pasta, D.J.; Jacobs, J.R.; Konstan, M.W.; Morgan, W.J.; Wagener, J.S. Year-to-year changes in lung function in individuals with cystic fibrosis. J. Cyst. Fibros. 2010, 9, 250–256. [Google Scholar] [CrossRef]
- Fuchs, S.I.; Eder, J.; Ellemunter, H.; Gappa, M. Lung clearance index: Normal values, repeatability, and reproducibility in healthy children and adolescents. Pediatr. Pulmonol. 2009, 44, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Ellemunter, H.; Eder, J.; Fuchs, S.; Gappa, M.; Steinkamp, G. Long-term improvement of lung clearance index in patients with mild cystic fibrosis lung disease: Does hypertonic saline play a role? J. Cyst. Fibros. 2016, 15, 123–126. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Characteristics | |
|---|---|
| Patients, n (%) | 40 (100%) |
| Females, n (%) | 26 (65%) |
| Age at admission (years) | 12.7 ± 2.86 |
| Height (meters) | 1.55 ± 0.14 |
| Height z-score | 0.10 ± 1.09 |
| Weight (kg) | 45.06 ± 11.46 |
| Weight z-score | −0.15 ± 0.97 |
| BMI | 18.29 ± 2.12 |
| BMI z-score | −0.21 ± 0.77 |
| CFTR genotype | |
| F508del/F508del, n (%) | 14 (35%) |
| F508del/other, n (%) | 19 (47.5%) |
| other/other, n (%) | 7 (17.5%) |
| CF comorbidities | |
| Pancreatic insufficiency, n (%) | 35 (87.5%) |
| CFRD, n (%) | 7 (18%) |
| ABPA, n (%) | 1 (2.5%) |
| Microbiology | |
| P. aeruginosa, n (%) | 23 (57.5) |
| -chronic, n (%) | 12 (52) |
| -intermittent, n (%) | 6 (26) |
| -first time, n (%) | 5 (22) |
| MSSA, n (%) | 35 (87.5) |
| MRSA, n (%) | 1 (2.5) |
| H. influenzae, n (%) | 5 (12.5) |
| S. maltophilia, n (%) | 3 (7.5) |
| B. cepacia complex, n (%) | 0 |
| A. fumigatus, n (%) | 6 (15) |
| Parameter Average (SD) | Baseline | P Baseline vs. Admission to Hospital | At Admission to Hospital | P Exacerbation vs. after Treatment | After PEX Treatment |
|---|---|---|---|---|---|
| FVC %pred | 97.80 ± 12.41 | <0.001 | 92.98 ± 13.02 | <0.001 | 98.68 ± 13.26 |
| FVC z-score | −0.20 ± 1.07 | <0.001 | −0.61 ± 1.11 | <0.001 | −0.12 ± 1.13 |
| FEV1%pred | 88.70 ± 14.26 | <0.001 | 79.48 ± 13.19 | <0.001 | 90.53 ± 15.44 |
| FEV1 z-score | −0.95 ± 1.18 | <0.001 | −1.62 ± 1.12 | <0.001 | −0.79 ± 1.28 |
| LCI | 11.65 ± 3.34 | <0.002 | 12.70 ± 3.10 | <0.001 | 11.40 ± 3.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walicka-Serzysko, K.; Postek, M.; Milczewska, J.; Sands, D. Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation. J. Clin. Med. 2021, 10, 4884. https://doi.org/10.3390/jcm10214884
Walicka-Serzysko K, Postek M, Milczewska J, Sands D. Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation. Journal of Clinical Medicine. 2021; 10(21):4884. https://doi.org/10.3390/jcm10214884
Chicago/Turabian StyleWalicka-Serzysko, Katarzyna, Magdalena Postek, Justyna Milczewska, and Dorota Sands. 2021. "Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation" Journal of Clinical Medicine 10, no. 21: 4884. https://doi.org/10.3390/jcm10214884
APA StyleWalicka-Serzysko, K., Postek, M., Milczewska, J., & Sands, D. (2021). Lung Clearance Index in Children with Cystic Fibrosis during Pulmonary Exacerbation. Journal of Clinical Medicine, 10(21), 4884. https://doi.org/10.3390/jcm10214884






