Serum Calretinin as a Biomarker in Malignant Mesothelioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Serum Calretinin
2.3. Measurement of Serum Soluble Mesothelin-Related Peptides
2.4. Statistical Analysis
3. Results
3.1. Association of Clinical Parameters with Calretinin Levels
3.2. Association of Asbestos Exposure with Calretinin Levels
3.3. Association of MM Clinical Parameters with Calretinin Levels
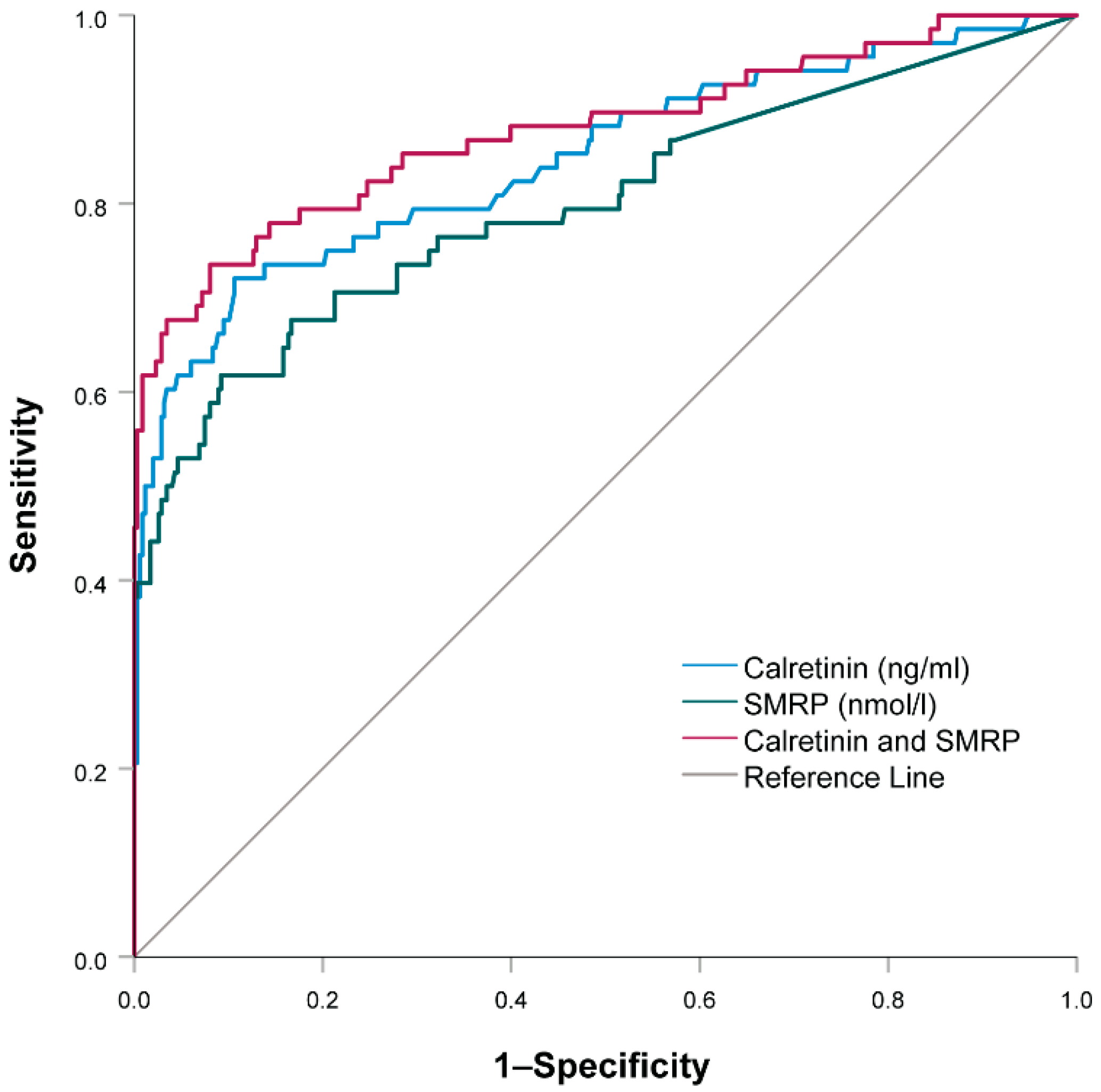
3.4. Combination of Soluble Mesothelin-Related Peptides and Calretinin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stayner, L.; Welch, L.S.; Lemen, R. The Worldwide Pandemic of Asbestos-Related Diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Landrigan, P.J.; Ramazzini, C. The Global Health Dimensions of Asbestos and Asbestos-Related Diseases. Ann. Glob. Health 2016, 82, 209–213. [Google Scholar] [CrossRef]
- Visonà, S.D.; Villani, S.; Manzoni, F.; Chen, Y.; Ardissino, G.; Russo, F.; Moretti, M.; Javan, G.T.; Osculati, A. Impact of asbestos on public health: A retrospective study on a series of subjects with occupational and non-occupational exposure to asbestos during the activity of Fibronit plant (Broni, Italy). J. Public Health Res. 2018, 7, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovac, V. Mezoteliomi. Onkologija 2012, 16, 64–68. [Google Scholar]
- Chapman, S.J.; Cookson, W.O.; Musk, A.W.; Lee, Y.C.G. Benign asbestos pleural diseases. Curr. Opin. Pulm. Med. 2003, 9, 266–271. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, S.; Pan, H.; Li, S.; He, J. Does size matter?—A population-based analysis of malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020, 9, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Kovac, V.; Zwitter, M.; Žagar, T. Improved survival after introduction of chemotherapy for malignant pleural mesothelioma in Slovenia: Population-based survey of 444 patients. Radiol. Oncol. 2012, 46, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Johnen, G.; Gawrych, K.; Raiko, I.; Casjens, S.; Pesch, B.; Weber, D.G.; Taeger, D.; Lehnert, M.; Kollmeier, J.; Bauer, T.; et al. Calretinin as a blood-based biomarker for mesothelioma. BMC Cancer 2017, 17, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapel, D.B.; Schulte, J.J.; Husain, A.N.; Krausz, T. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl. Lung Cancer Res. 2020, 9, S3–S27. [Google Scholar] [CrossRef]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Porcel, J.M. Biomarkers in the diagnosis of pleural diseases: A 2018 update. Ther. Adv. Respir. Dis. 2018, 12, 1753466618808660. [Google Scholar] [CrossRef]
- Broeckx, G.; Pauwels, P. Malignant peritoneal mesothelioma: A review. Transl. Lung Cancer Res. 2018, 7, 537–542. [Google Scholar] [CrossRef]
- Bruno, F.; Baratti, D.; Martinetti, A.; Morelli, D.; Sottotetti, E.; Bonini, C.; Guaglio, M.; Kusamura, S.; Deraco, M. Mesothelin and osteopontin as circulating markers of diffuse malignant peritoneal mesothelioma: A preliminary study. Eur. J. Surg. Oncol. 2018, 44, 792–798. [Google Scholar] [CrossRef]
- Bonotti, A.; Simonini, S.; Pantani, E.; Giusti, L.; Donadio, E.; Mazzoni, M.R.; Chella, A.; Marconi, L.; Ambrosino, N.; Lucchi, M.; et al. Serum Mesothelin, Osteopontin and Vimentin: Useful Markers for Clinical Monitoring of Malignant Pleural Mesothelioma. Int. J. Biol. Markers 2017, 32, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Cristaudo, A.; Bonotti, A.; Guglielmi, G.; Fallahi, P.; Foddis, R. Serum mesothelin and other biomarkers: What have we learned in the last decade? J. Thorac. Dis. 2018, 10, S353–S359. [Google Scholar] [CrossRef] [Green Version]
- Hollevoet, K.; Reitsma, J.B.; Creaney, J.; Grigoriu, B.D.; Robinson, B.W.; Scherpereel, A.; Cristaudo, A.; Pass, H.; Nackaerts, K.; Portal, J.A.R.; et al. Serum Mesothelin for Diagnosing Malignant Pleural Mesothelioma: An Individual Patient Data Meta-Analysis. J. Clin. Oncol. 2012, 30, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, W.; Pecze, L.; Rodriguez, J.W.; Steinauer, M.; Schwaller, B. Regulation of calretinin in malignant mesothelioma is mediated by septin 7 binding to the CALB2 promoter. BMC Cancer 2018, 18, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wörthmüller, J.; Blum, W.; Pecze, L.; Salicio, V.; Schwaller, B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget 2018, 9, 36256–36272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Schwaller, B. Overexpression or absence of calretinin in mouse primary mesothelial cells inversely affects proliferation and cell migration. Respir. Res. 2015, 16, 153. [Google Scholar] [CrossRef] [Green Version]
- Blum, W.; Schwaller, B. Calretinin is essential for mesothelioma cell growth/survival in vitro: A potential new target for malignant mesothelioma therapy? Int. J. Cancer 2013, 133, 2077–2088. [Google Scholar] [CrossRef] [Green Version]
- Lehnert, M.; Weber, D.G.; Taeger, D.; Raiko, I.; Kollmeier, J.; Stephan-Falkenau, S.; Brüning, T.; Johnen, G.; Brik, A.; Burek, K.; et al. Determinants of plasma calretinin in patients with malignant pleural mesothelioma. BMC Res. Notes 2020, 13, 359. [Google Scholar] [CrossRef]
- Raiko, I.; Sander, I.; Weber, D.G.; Raulf-Heimsoth, M.; Gillissen, A.; Kollmeier, J.; Scherpereel, A.; Bruning, T.; Johnen, G. Development of an enzyme-linked immunosorbent assay for the detection of human calretinin in plasma and serum of meso-thelioma patients. BMC Cancer 2010, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Madrid, G.; Pesch, B.; Calderón-Aranda, E.S.; Burek, K.; Jiménez-Ramírez, C.; Juárez-Pérez, C.A.; Ochoa-Vázquez, M.D.; Torre-Bouscoulet, L.; Acosta-Saavedra, L.C.; Sada-Ovalle, I.; et al. Biomarkers for predicting malignant pleural mesothelioma in a mexican population. Int. J. Med. Sci. 2018, 15, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Ramírez, C.; Casjens, S.; Juárez-Pérez, C.A.; Raiko, I.; Del Razo, L.M.; Taeger, D.; Calderón-Aranda, E.S.; Rihs, H.-P.; Acosta-Saavedra, L.C.; Weber, D.G.; et al. Mesothelin, Calretinin, and Megakaryocyte Potentiating Factor as Biomarkers of Malignant Pleural Mesothelioma. Lung 2019, 197, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, A. Asbestos, asbestosis, and cancer: The helsinki criteria for diagnosis and attribution. Scand. J. Work. Environ. Health 1997, 23, 311–316. [Google Scholar] [CrossRef]
- American Thoracic, S. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 2004, 170, 691–715. [Google Scholar]
- Fikfak, M.D.; Kriebel, D.; Quinn, M.M.; Eisen, E.A.; Wegman, D.H. A Case Control Study of Lung Cancer and Exposure to Chrysotile and Amphibole at a Slovenian Asbestos-Cement Plant. Ann. Occup. Hyg. 2007, 51, 261–268. [Google Scholar] [CrossRef]
- Franko, A.; Dodic-Fikfak, M.; Arnerić, N.; Dolzan, V. Glutathione s-transferases gstm1 and gstt1 polymorphisms and asbestosis. J. Occup. Environ. Med. 2007, 49, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Goricar, K.; Kovac, V.; Dodic-Fikfak, M.; Dolzan, V.; Franko, A. Evaluation of soluble mesothelin-related peptides and MSLN genetic variability in asbestos-related diseases. Radiol. Oncol. 2020, 54, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnen, G.; MoMar Study Group; Burek, K.; Raiko, I.; Wichert, K.; Pesch, B.; Weber, D.G.; Lehnert, M.; Casjens, S.; Hagemeyer, O.; et al. Prediagnostic detection of mesothelioma by circulating calretinin and mesothelin—A case-control comparison nested into a prospective cohort of asbestos-exposed workers. Sci. Rep. 2018, 8, 14321. [Google Scholar] [CrossRef]
- Chirieac, L.R.; Pinkus, G.S.; Pinkus, J.L.; Godleski, J.; Sugarbaker, D.J.; Corson, J.M. The immunohistochemical characterization of sarcomatoid malignant mesothelioma of the pleura. Am. J. Cancer Res. 2011, 1, 14–24. [Google Scholar] [PubMed]
- Tandon, R.T.; Jimenez-Cortez, Y.; Taub, R.; Borczuk, A.C. Immunohistochemistry in peritoneal mesothelioma: A single-center experience of 244 cases. Arch. Pathol. Lab. Med. 2018, 142, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Pass, H.V.M.; Cengel, K.; Vachani, A.; Carbone, M.; Yang, H.; Johnen, G.; Goparaju, C. Blood and fbln3: Be careful how and what you collect. In The International Mesothelioma Interest Group Programme Book; MS07.08; International Mesothelioma Interest Group; 2021. Available online: https://imig2021.org/wp-content/uploads/2021/05/imig2021_Virtual_programmebook.pdf (accessed on 19 October 2021).
- Casjens, S.; Johnen, G.; Raiko, I.; Pesch, B.; Taeger, D.; Töpfer, C.; Schonefeld, S.; Moebus, S.; Jöckel, K.-H.; Brüning, T.; et al. Re-evaluation of potential predictors of calretinin and mesothelin in a population-based cohort study using assays for the routine application in clinical medicine. BMJ Open 2021, 11, e039079. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Ji, H.; Chen, M.; Robinson, B.W.S.; Dick, I.M.; Creaney, J.; Simpson, R. Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci. Rep. 2016, 6, 32643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.O.; Choi, D.-Y.; Choi, D.-S.; Kim, H.J.; Kang, J.W.; Jung, J.H.; Lee, J.H.; Kim, J.; Freeman, M.R.; Lee, K.Y.; et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics 2013, 13, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.; Weber, D.G.; Johnen, G.; Raiko, I.; Taeger, D.; Meinig, C.; Moebus, S.; Jöckel, K.-H.; Brüning, T.; Pesch, B. Assessment of potential predictors of calretinin and mesothelin to improve the diagnostic performance to detect malignant mesothelioma: Results from a population-based cohort study. BMJ Open 2017, 7, e017104. [Google Scholar] [CrossRef]



| Characteristic, Category/Unit | No Disease (N = 73) | Pleural Plaques (N = 195) | Asbestosis (N = 117) | MM (N = 164) | p |
|---|---|---|---|---|---|
| Gender | |||||
| Male, N (%) | 52 (71.2) | 134 (68.7) | 91 (77.8) | 118 (72.0) | 0.392 a |
| Female, N (%) | 21 (28.8) | 61 (31.3) | 26 (22.2) | 46 (28.0) | |
| Age | |||||
| Years, Median (25–75%) | 53.4 (47.7–60.7) | 55.1 (48.8–64) | 59.7 (51.1–66.6) | 66.5 (60–73) | <0.001 b |
| Smoking | |||||
| No, N (%) | 38 (52.1) | 99 (50.8) | 56 (47.9) | 97 (61.0) [5] | 0.125 a |
| Yes, N (%) | 35 (47.9) | 96 (49.2) | 61 (52.1) | 62 (39.0) |
| Characteristic | Category | N (%) | Calretinin Level (ng/mL) Median (25–75%) | p |
|---|---|---|---|---|
| Histological type | Epithelioid | 117 (71.3) | 0.67 (0.30–1.66) | 0.001 sarcomatoid vs. epithelioid: p = 0.001 sarcomatoid vs. biphasic: p = 0.057 |
| Biphasic | 20 (12.2) | 0.51 (0.20–1.17) | ||
| Sarcomatoid | 13 (7.9) | 0.17 (0.13–0.23) | ||
| Not determined | 14 (8.5) | 0.44 (0.20–0.65) a | ||
| Stage | 1 | 8 (4.9) [1] b | 0.36 (0.17–0.93) | 0.794 |
| 2 | 37 (22.7) | 0.44 (0.26–1.02) | ||
| 3 | 48 (29.4) | 0.56 (0.22–1.43) | ||
| 4 | 50 (30.7) | 0.50 (0.21–1.70) | ||
| Location | Pleural MM | 144 (87.9 | 0.48 (0.22–1.27) | 0.065 |
| Peritoneal MM | 20 (12.2) | 1.00 (0.40–2.41) | ||
| ECOG | 0 | 8 (4.9) [1] | 0.32 (0.08–1.08) | 0.191 |
| 1 | 81 (49.7) | 0.48 (0.21–1.17) | ||
| 2 | 64 (39.3) | 0.68 (0.24–1.67) | ||
| 3 | 10 (6.1) | 0.50 (0.39–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupanc, C.; Franko, A.; Štrbac, D.; Dodič Fikfak, M.; Kovač, V.; Dolžan, V.; Goričar, K. Serum Calretinin as a Biomarker in Malignant Mesothelioma. J. Clin. Med. 2021, 10, 4875. https://doi.org/10.3390/jcm10214875
Zupanc C, Franko A, Štrbac D, Dodič Fikfak M, Kovač V, Dolžan V, Goričar K. Serum Calretinin as a Biomarker in Malignant Mesothelioma. Journal of Clinical Medicine. 2021; 10(21):4875. https://doi.org/10.3390/jcm10214875
Chicago/Turabian StyleZupanc, Cita, Alenka Franko, Danijela Štrbac, Metoda Dodič Fikfak, Viljem Kovač, Vita Dolžan, and Katja Goričar. 2021. "Serum Calretinin as a Biomarker in Malignant Mesothelioma" Journal of Clinical Medicine 10, no. 21: 4875. https://doi.org/10.3390/jcm10214875
APA StyleZupanc, C., Franko, A., Štrbac, D., Dodič Fikfak, M., Kovač, V., Dolžan, V., & Goričar, K. (2021). Serum Calretinin as a Biomarker in Malignant Mesothelioma. Journal of Clinical Medicine, 10(21), 4875. https://doi.org/10.3390/jcm10214875






