Manual Correction of Voxel Misclassifications in Mesiotemporal Structures Does Not Alter Brain–Behavioral Results in an Episodic Memory Task
Abstract
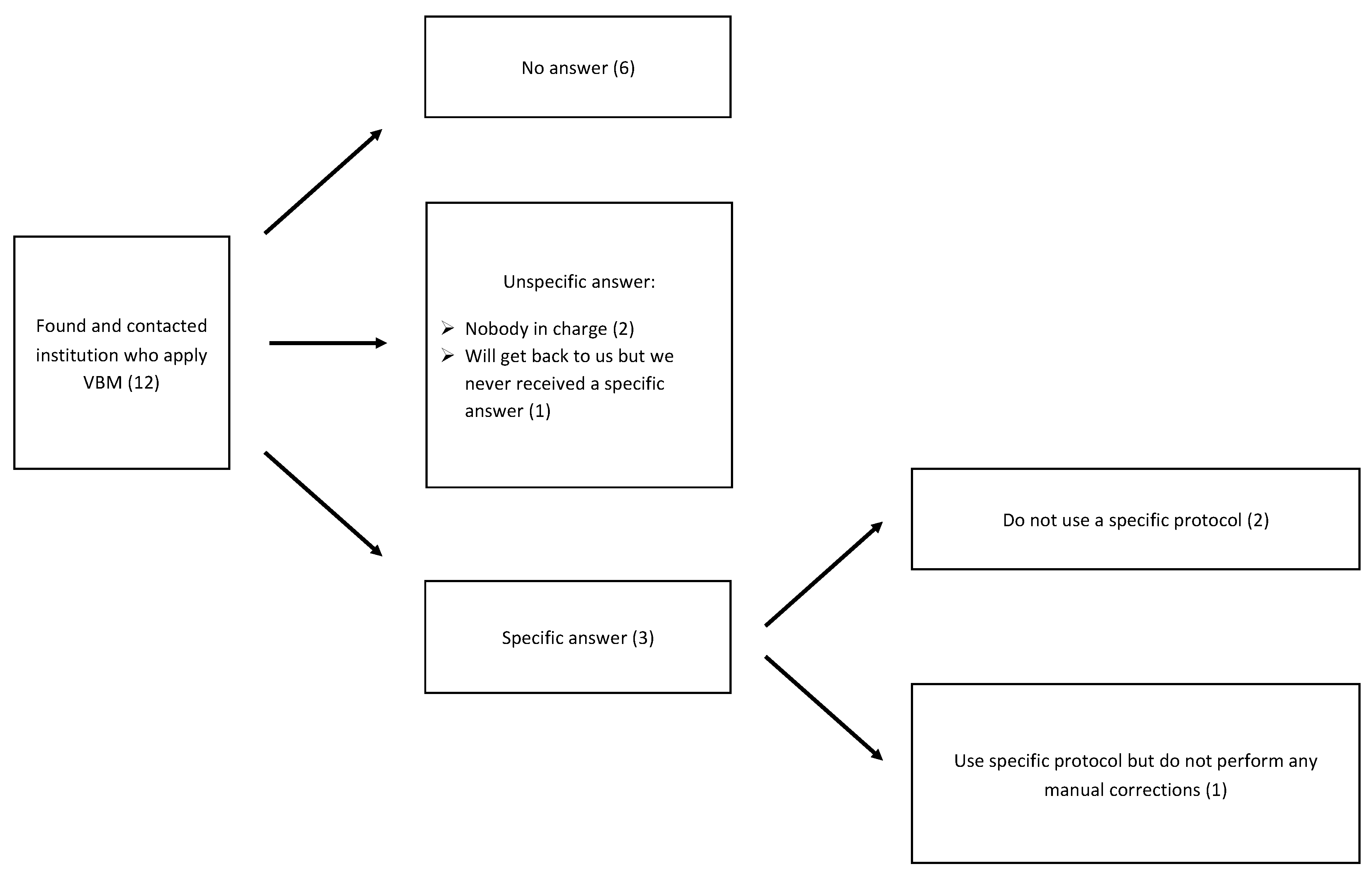
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Measures
2.2.1. MRI Acquisition
2.2.2. Preprocessing of Structural MRI
2.3. California Verbal Learning Task
2.4. Statistical Analyses
3. Results
3.1. Behavioral Data
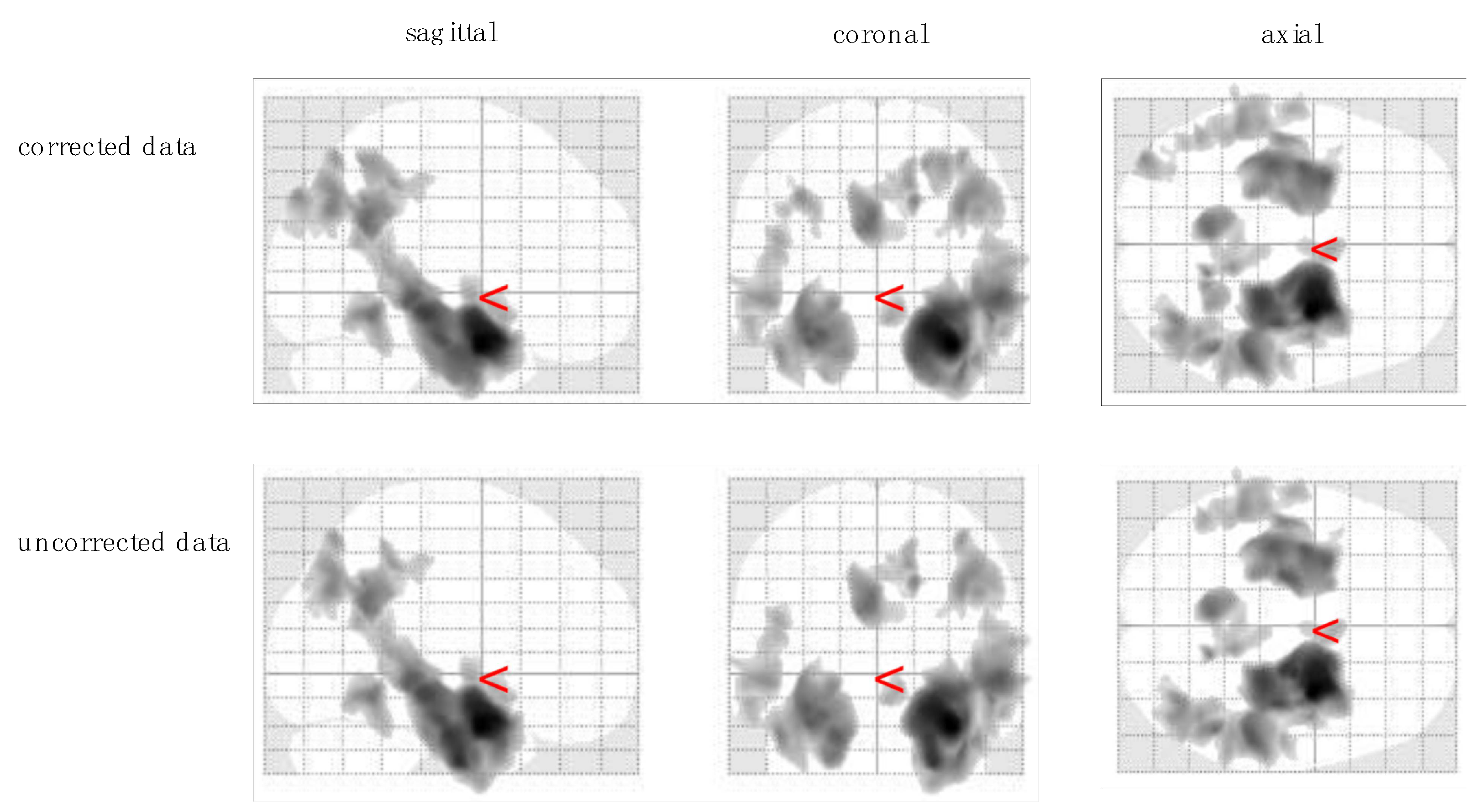
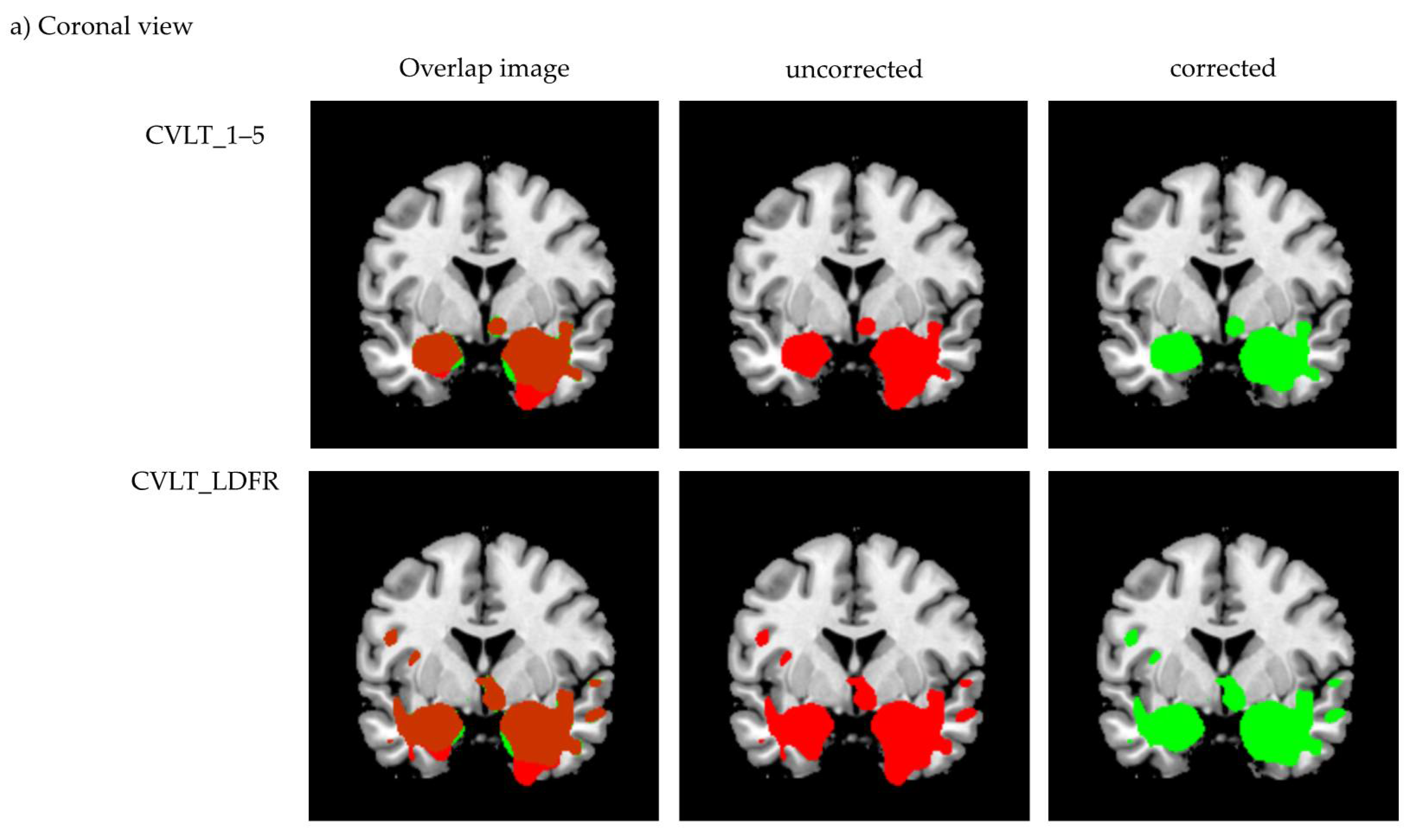
3.2. Voxel-Based Morphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paola, M.; Luders, E.; Di Iulio, F.; Cherubini, A.; Passafiume, D.; Thompson, P.M.; Caltagirone, C.; Toga, A.W.; Spalletta, G. Callosal atrophy in mild cognitive impairment and Alzheimer’s disease: Different effects in different stages. NeuroImage 2010, 49, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Krumm, S.; Kivisaari, S.L.; Probst, A.; Monsch, A.U.; Reinhardt, J.; Ulmer, S.; Stippich, C.; Kressig, R.W.; Taylor, K.I. Cortical thinning of parahippocampal subregions in very early Alzheimer’s disease. Neurobiol. Aging 2016, 38, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Feldkamp, H.; Hoppstädter, M.; King, A.V.; Frölich, L.; Wessa, M.; Flor, H. Using Voxel-Based Morphometry to Examine the Relationship between Regional Brain Volumes and Memory Performance in Amnestic Mild Cognitive Impairment. Front. Behav. Neurosci. 2013, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitter, D.; Roche, A.; Maréchal, B.; Ribes, D.; Abdulkadir, A.; Bach-Cuadra, M.; Daducci, A.; Granziera, C.; Klöppel, G.; Maeder, P.; et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2014, 7, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Delis, D.C.; Massman, P.J.; Butters, N.; Salmon, D.P.; Cermak, L.S.; Kramer, J.H. Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the assessment of memory disorders. Psychol. Assess. 1991, 3, 19–26. [Google Scholar] [CrossRef]
- Moulin, C.J.; James, N.; Freeman, J.E.; Jones, R.W. Deficient Acquisition and Consolidation: Intertrial Free Recall Performance in Alzheimer’s Disease and Mild Cognitive Impairment. J. Clin. Exp. Neuropsychol. 2004, 26, 1–10. [Google Scholar] [CrossRef]
- Traykov, L.; Rigaud, A.-S.; Cesaro, P.; Boller, F. Le déficit neuropsychologique dans la maladie d’Alzheimer débutante. L’Encephale 2007, 33, 310–316. [Google Scholar] [CrossRef]
- Leube, D.T.; Weis, S.; Freymann, K.; Erb, M.; Jessen, F.; Heun, R.; Grodd, W.; Kircher, T.T. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer’s disease—A VBM study. Int. J. Geriatr. Psychiatry 2008, 23, 1114–1118. [Google Scholar] [CrossRef]
- Di Paola, M.; Macaluso, E.; Carlesimo, G.A.; Tomaiuolo, F.; Worsley, K.J.; Fadda, L.; Caltagirone, C. Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy. J. Neurol. 2007, 254, 774–781. [Google Scholar] [CrossRef]
- Hirni, D.I.; Kivisaari, S.L.; Krumm, S.; Monsch, A.U.; Berres, M.; Oeksuez, F.; Reinhardt, J.; Ulmer, S.; Kressig, R.W.; Stippich, C.; et al. Neuropsychological Markers of Medial Perirhinal and Entorhinal Cortex Functioning are Impaired Twelve Years Preceding Diagnosis of Alzheimer’s Dementia. J. Alzheimer’s Dis. 2016, 52, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, F.; Feyen, L.; Freymann, K.; Tepest, R.; Maier, W.; Heun, R.; Schild, H.-H.; Scheef, L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging 2006, 27, 1751–1756. [Google Scholar] [CrossRef]
- Beck, I.R.; Gagneux-Zurbriggen, A.; Berres, M.; Taylor, K.I.; Monsch, A.U. Comparison of Verbal Episodic Memory Measures: Consortium to Establish a Registry for Alzheimer’s Disease—Neuropsychological Assessment Battery (CERAD-NAB) versus California Verbal Learning Test (CVLT). Arch. Clin. Neuropsychol. 2012, 27, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A.; Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test: Adult Version; The Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Greenaway, M.C.; Lacritz, L.H.; Binegar, D.; Weiner, M.; Lipton, A.; Cullum, C.M. Patterns of Verbal Memory Performance in Mild Cognitive Impairment, Alzheimer Disease, and Normal Aging. Cogn. Behav. Neurol. 2006, 19, 79–84. [Google Scholar] [CrossRef]
- Hirata, Y.; Matsuda, H.; Nemoto, K.; Ohnishi, T.; Hirao, K.; Yamashita, F.; Asada, T.; Iwabuchi, S.; Samejima, H. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci. Lett. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Kawachi, T.; Ishii, K.; Sakamoto, S.; Sasaki, M.; Mori, T.; Yamashita, F.; Matsuda, H.; Mori, E. Comparison of the diagnostic performance of FDG-PET and VBM-MRI in very mild Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Yu, J.-T.; Liu, Y.; Yin, R.-H.; Wang, H.-F.; Wang, J.; Tan, L.; Radua, J.; Tan, L. Voxel-based meta-analysis of grey matter changes in Alzheimer’s disease. Transl. Neurodegener. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chételat, G.; Landeau, B.; Eustache, F.; Mézenge, F.; Viader, F.; de la Sayette, V.; Desgranges, B.; Baron, J.-C. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: A longitudinal MRI study. NeuroImage 2005, 27, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K. Voxel-Based Morphometry—The Methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [Green Version]
- Kurth, F.; Luders, E.; Gaser, C. Voxel-based morphometry. In Brain Mapping; Elsevier: Amsterdam, The Netherlands, 2015; pp. 345–349. ISBN 978-0-12-397316-0. [Google Scholar]
- Scarpazza, C.; Tognin, S.; Frisciata, S.; Sartori, G.; Mechelli, A. False positive rates in Voxel-based Morphometry studies of the human brain: Should we be worried? Neurosci. Biobehav. Rev. 2015, 52, 49–55. [Google Scholar] [CrossRef]
- Ashburner, J. SPM: A History. NeuroImage 2012, 62, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, J.; Friston, K. Why Voxel-Based Morphometry Should Be Used. NeuroImage 2001, 14, 1238–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmondab, C.H.; Ashburner, J.; Khadema, F.; Connelly, A.; Gadian, D.; Friston, K. Distributional Assumptions in Voxel-Based Morphometry. NeuroImage 2002, 17, 1027–1030. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viviani, R.; Beschoner, P.; Ehrhard, K.; Schmitz, B.; Thöne, J. Non-normality and transformations of random fields, with an application to voxel-based morphometry. NeuroImage 2007, 35, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L. Voxel-Based Morphometry: An Automated Technique for Assessing Structural Changes in the Brain. J. Neurosci. 2009, 29, 9661–9664. [Google Scholar] [CrossRef] [Green Version]
- Despotović, I.; Goossens, B.; Philips, W. MRI Segmentation of the Human Brain: Challenges, Methods, and Applications. Comput. Math. Methods Med. 2015, 2015, 450341. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Callaert, D.V.; Eribbens, A.; Maes, F.; Swinnen, S.P.; Ewenderoth, N. Assessing age-related gray matter decline with voxel-based morphometry depends significantly on segmentation and normalization procedures. Front. Aging Neurosci. 2014, 6, 124. [Google Scholar] [CrossRef]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.-C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 2009, 46, 786–802. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Mizumura, S.; Nemoto, K.; Yamashita, F.; Imabayashi, E.; Sato, N.; Asada, T. Automatic Voxel-Based Morphometry of Structural MRI by SPM8 plus Diffeomorphic Anatomic Registration Through Exponentiated Lie Algebra Improves the Diagnosis of Probable Alzheimer Disease. Am. J. Neuroradiol. 2012, 33, 1109–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blatter, D.D.; Bigler, E.D.; Gale, S.D.; Johnson, S.C.; Anderson, C.V.; Burnett, B.M.; Parker, N.; Kurth, S.; Horn, S.D. Quantitative volumetric analysis of brain MR: Normative database spanning 5 decades of life. Am. J. Neuroradiol. 1995, 16, 241–251. [Google Scholar] [PubMed]
- Mu, Q.; Xie, J.; Wen, Z.; Weng, Y.; Shuyun, Z. A Quantitative MR Study of the Hippocampal Formation, the Amygdala, and the Temporal Horn of the Lateral Ventricle in Healthy Subjects 40 to 90 Years of Age. Am. J. Neuroradiol. 1999, 20, 207–211. [Google Scholar]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef]
- Resnick, S.M.; Lamar, M.; Driscoll, I. Vulnerability of the Orbitofrontal Cortex to Age-Associated Structural and Functional Brain Changes. Ann. N. Y. Acad. Sci. 2007, 1121, 562–575. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Press: Washington, DC, USA, 1994. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Backman, L.J.; Albert, M.S.; Almkvist, O.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Kivisaari, S.; Tyler, L.; Monsch, A.U.; Taylor, K.I. Medial perirhinal cortex disambiguates confusable objects. Brain 2012, 135, 3757–3769. [Google Scholar] [CrossRef] [Green Version]
- Berres, M.; Monsch, A.U.; Bernasconi, F.; Thalmann, B.; Stähelin, H.B. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer’s disease. Stud. Health Technol. Inform. 2000, 77, 195–199. [Google Scholar] [CrossRef]
- Berres, M.; Zehnder, A.; Bläsi, S.; Monsch, A.U. Evaluation of diagnostic scores with adjustment for covariates. Stat. Med. 2007, 27, 1777–1790. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Borghi, J.; Van Gulick, A.E. Data management and sharing in neuroimaging: Practices and perceptions of MRI researchers. PLoS ONE 2018, 13, e0200562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carp, J. On the Plurality of (Methodological) Worlds: Estimating the Analytic Flexibility of fMRI Experiments. Front. Behav. Neurosci. 2012, 6, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poldrack, R.A.; Fletcher, P.; Henson, R.N.; Worsley, K.J.; Brett, M.; Nichols, T. Guidelines for reporting an fMRI study. NeuroImage 2008, 40, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieve, S.M.; Clark, C.R.; Williams, L.M.; Peduto, A.J.; Gordon, E. Preservation of limbic and paralimbic structures in aging. Hum. Brain Mapp. 2005, 25, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kalpouzos, G.; Chételat, G.; Baron, J.-C.; Landeau, B.; Mevel, K.; Godeau, C.; Barré, L.; Constans, J.-M.; Viader, F.; Eustache, F.; et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol. Aging 2009, 30, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terribilli, D.; Schaufelberger, M.S.; Souza-Duran, F.L.; Zanetti, M.V.; Curiati, P.K.; Menezes, P.; Scazufca, M.; Amaro, E.; Leite, C.C.; Busatto, G.F. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol. Aging 2011, 32, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K. Computing average shaped tissue probability templates. NeuroImage 2009, 45, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H. Voxel-based Morphometry of Brain MRI in Normal Aging and Alzheimer’s Disease. Aging Dis. 2012, 4, 29–37. [Google Scholar] [PubMed]


| NC (N = 63) | AD (N = 63) | z | p-Value | |
|---|---|---|---|---|
| Gender (m/f) | 38/25 | 27/36 | 1.78 | 0.05 |
| Mean ± SD | Mean ± SD | t | p-value | |
| Age (years) | 74.54 ± 6.53 | 75.67 ± 7.92 | −0.87 | 0.39 |
| Education (years) | 12.73 ± 2.55 | 12.90 ± 3.04 | −0.34 | 0.73 |
| MMSE score | 29.25 ± 0.92 | 27.51 ± 2.04 | 6.20 | <0.0001 |
| TIV (cm3) | 1474.23 ± 137.97 | 1480.96 ± 131.61 | −0.28 | 0.78 |
| Anatomical Region | Side | MNI Coordinates Peak Voxel 1 | Cluster Size (kE) | p (FWE-corr) 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Corrected | Uncorrected | Corrected | Uncorrected | |||
| (a) | hippocampus * | right | 32 (33) | −3 | −24 | 55,074 | 57,166 | <0.001 | <0.001 |
| hippocampus * | left | −33 | −7 | −22 | 26,067 | 27,664 | 4.59 × 10−12 | 1.28 × 10−12 | |
| precuneus | left | −5 | −55 | 29 | 12,527 | 9555 | 3.91 × 10−7 | 7.36 × 10−6 | |
| inferior temporal gyrus | right | 50 | −52 | −12 | 3978 | 3850 | 0.006 | 0.007 | |
| middle occipital gyrus | right | 37 | −71 | 34 | 9136 | 8816 | 1.20 × 10−5 | 1.62 × 10−5 | |
| middle occipital gyrus * | left | −37 | −87 | 38 | 2930 | 0.028 | |||
| middle temporal gyrus | left | −53 | −38 | −5 | 9726 | 9385 | 6.42 × 10−6 | 8.81 × 10−6 | |
| (b) | hippocampus * | right | 32 | −3 | −24 | 79,229 | 81,115 | <0.001 | <0.001 |
| amygdala | left | −27 (−28) | 4 | −20 | 55,166 | 56,963 | <0.001 | <0.001 | |
| inferior parietal gyrus | right | 26 | −52 | 54 | 25,172 | 24,521 | 8.57 × 10−12 | 1.29 × 10−11 | |
| precuneus | left | −4 | −54 | 30 | 11,400 | 11,158 | 1.16 × 10−6 | 1.38 × 10−6 | |
| inferior frontal gyrus, pars opercularis | left | −51 | 9 | 25 | 2694 | 2589 | 0.040 | 0.046 | |
| medial frontal gyrus, pars orbitalis | left | −5 | 63 | −1 | 10,499 | 10,347 | 2.82 × 10−6 | 3.14 × 10−6 | |
| inferior parietal gyrus | left | −24 | −66 | 44 | 6687 | 1.82 × 10−4 | |||
| middle occipital gyrus | left | −30 | −89 | 22 | 6434 | 2.38 × 10−4 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, F.; Reinhardt, J.; Stippich, C.; Krumm, S. Manual Correction of Voxel Misclassifications in Mesiotemporal Structures Does Not Alter Brain–Behavioral Results in an Episodic Memory Task. J. Clin. Med. 2021, 10, 4869. https://doi.org/10.3390/jcm10214869
Hartmann F, Reinhardt J, Stippich C, Krumm S. Manual Correction of Voxel Misclassifications in Mesiotemporal Structures Does Not Alter Brain–Behavioral Results in an Episodic Memory Task. Journal of Clinical Medicine. 2021; 10(21):4869. https://doi.org/10.3390/jcm10214869
Chicago/Turabian StyleHartmann, Francina, Julia Reinhardt, Christoph Stippich, and Sabine Krumm. 2021. "Manual Correction of Voxel Misclassifications in Mesiotemporal Structures Does Not Alter Brain–Behavioral Results in an Episodic Memory Task" Journal of Clinical Medicine 10, no. 21: 4869. https://doi.org/10.3390/jcm10214869
APA StyleHartmann, F., Reinhardt, J., Stippich, C., & Krumm, S. (2021). Manual Correction of Voxel Misclassifications in Mesiotemporal Structures Does Not Alter Brain–Behavioral Results in an Episodic Memory Task. Journal of Clinical Medicine, 10(21), 4869. https://doi.org/10.3390/jcm10214869






