Sentinel Lymph Node Staging with Indocyanine Green for Patients with Cervical Cancer: The Safety and Feasibility of Open Approach Using SPY-PHI Technique
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Rustum, N.R.; Angioli, R.; Bailey, A.E.; Broach, V.; Buda, A.; Coriddi, M.R.; Dayan, J.H.; Frumovitz, M.; Kim, Y.M.; Kimmig, R.; et al. IGCS Intraoperative Technology Taskforce. Update on near infrared imaging technology: Beyond white light and the naked eye, indocyanine green and near infrared technology in the treatment of gynecologic cancers. Int. J. Gynecol. Cancer 2020, 30, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 10 (Suppl. S2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Crivellaro, C.; Signorelli, M.; Guerra, L.; De Ponti, E.; Buda, A.; Dolci, C.; Pirovano, C.; Todde, S.; Fruscio, R.; Messa, C. 18F-FDG PET/CT can predict nodal metastases but not recurrence in early stage uterine cervical cancer. Gynecol. Oncol. 2012, 127, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.O.; Halpenny, D.; Johnston, C.; Sheehy, N.; Keogan, M. 18F-FDG-PET/CT is of limited value in primary staging of early stage cervical cancer. Abdom. Imaging 2015, 40, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph node metastasis in patients with clinical early-stage cervical cancer: Detection with integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Bipat, S.; Glas, A.S.; van der Velden, J.; Zwinderman, A.H.; Bossuyt, P.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol. Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef]
- Fischerova, D.; Cibula, D.; Stenhova, H.; Vondrichova, H.; Calda, P.; Zikan, M.; Freitag, P.; Slama, J.; Dundr, P.; Belacek, J. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Havrilesky, L.J.; Kulasingam, S.L.; Matchar, D.B.; Myers, E.R. FDG-PET for management of cervical and ovarian cancer. Gynecol. Oncol. 2005, 97, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, G.F.; Erdemoglu, E.; Lichtenberg, P.; Muallem, M.Z.; Richter, R.; Abu-Rustum, N.R.; Plante, M.; Lécuru, F.; Greggi, S.; Monk, B.J.; et al. A GCIG international survey: Clinical practice patterns of sentinel lymph node biopsies in cervical cancer. Arch. Gynecol. Obstet. 2019, 300, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, G.; Crivellaro, C.; De Ponti, E.; Bussi, B.; Papadia, A.; Zapardiel, I.; Vizza, E.; Elisei, F.; Diestro, M.D.; Locatelli, L.; et al. Indocyanine green versus radiotracer with or without blue dye for sentinel lymph node mapping in stage >IB1 cervical cancer (>2 cm). J. Minim. Invasive Gynecol. 2017, 24, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, N.; Luigi, P.A.; Ferrandina, G.; Zannoni, G.F.; Carbone, M.V.; Fedele, C.; Teodorico, E.; Gallotta, V.; Gueli Alletti, S.; Chiantera, V.; et al. Sentinel lymph node mapping with indocyanine green in cervical cancer patients undergoing open radical hysterectomy: A single-institution series. J. Cancer Res. Clin. Oncol. 2021, 147, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Crane, L.M.; Themelis, G.; Pleijhuis, R.G.; Harlaar, N.J.; Sarantopoulos, A.; Arts, H.J.; van der Zee, A.G.; Vasilis, N.; van Dam, G.M. Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: A novel concept. Mol. Imaging Biol. 2011, 13, 1043–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, N.; Oi, H.; Yoshida, S.; Shigetomi, H.; Kanayama, S.; Kobayashi, H. The usefulness of photodynamic eye for sentinel lymph node identification in patients with cervical cancer. Tumori J. 2010, 96, 936–940. [Google Scholar] [CrossRef]
- Buda, A.; Dell’Anna, T.; Vecchione, F.; Verri, D.; Di Martino, G.; Milani, R. Near-infrared sentinel lymph node mapping with indocyanine green using the VITOM II ICG exoscope for open surgery for gynecologic malignancies. J. Minim. Invasive Gynecol. 2016, 23, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Marin, S.; De Santiago, J.; Zapardiel, I. Utility of laparoscopic indocyanine green-guided sentinel node biopsy in open cervical cancer surgery. Int. J. Gynecol. Cancer 2016, 26, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; van der Vorst, J.R.; Gaarenstroom, K.N.; Peters, A.A.; Verbeek, F.P.; de Kroon, C.D.; Trimbos, J.B.M.; van Poelgeest, M.I.; Frangioni, J.V.; van de Velde, C.J.; et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol. Oncol. 2012, 127, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Vorst, J.R.; Hutteman, M.; Gaarenstroom, K.N.; Peters, A.A.W.; Mieog, J.S.D.; Schaafsma, B.E.; Kuppen, P.; Frangioni, J.V.; Van De Velde, C.J.H.; Vahrmeijer, A.L. Optimization of near-infrared fluorescent sentinel lymph node mapping in cervical cancer patients. Int. J. Gynecol. Cancer 2011, 21, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querleu, D.; Cibula, D.; Concin, N.; Fagotti, A.; Ferrero, A.; Fotopoulou, C.; Knapp, P.; Kurdiani, D.; Ledermann, J.A.; Mirza, M.R.; et al. Laparoscopic radical hysterectomy: A European Society of Gynaecological Oncology (ESGO) statement. Int. J. Gynecol. Cancer 2020, 30, 15. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Silva-Filho, A.L.; Traiman, P.; Triginelli, S.A.; de Lima, C.F.; Siqueira, C.F.; Barroso, A.; Rossi, T.M.F.; Pedrosa, M.S.; Miranda, D.; et al. Sentinel node detection in cervical cancer with (99m)Tc-phytate. Gynecol. Oncol. 2005, 97, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Wydra, D.; Sawicki, S.; Wojtylak, S.; Bandurski, T.; Emerich, J. Sentinel node identification in cervical cancer patients undergoing transperitoneal radical hysterectomy: A study of 100 cases. Int. J. Gynecol. Cancer 2006, 16, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Altgassen, C.; Paseka, A.; Urbanczyk, H.; Dimpfl, T.; Diedrich, K.; Dahmen, G.; Hertel, H. Dilution of dye improves parametrial SLN detection in patients with cervical cancer. Gynecol. Oncol. 2007, 105, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kara, P.P.; Ayhan, A.; Caner, B.; Gültekin, M.; Ugur, O.; Bozkurt, M.F.; Usubutun, A. Sentinel lymph node detection in early stage cervical cancer: A prospective study comparing preoperative lymphoscintigraphy, intraoperative gamma probe, and blue dye. Ann. Nucl. Med. 2008, 22, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, L.; Zikan, M.; Fischerova, D.; Kocian, R.; Germanova, A.; Frühauf, F.; Dušek, L.; Slama, J.; Dundr, P.; Němejcová, K.; et al. SLN biopsy in cervical cancer patients with tumors larger than 2cm and 4cm. Gynecol. Oncol. 2018, 148, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z.; Armbrust, R.; Neymeyer, J.; Miranda, A.; Muallem, J. Nerve Sparing Radical Hysterectomy: Short-Term Oncologic, Surgical, and Functional Outcomes. Cancers 2020, 12, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom, Langversion, 2.1, 2021, AWMF-Registernummer: 032/033OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom/ (accessed on 7 June 2021).
- Muallem, M.Z. A New Anatomic and Staging-Oriented Classification of Radical Hysterectomy. Cancers 2021, 13, 3326. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Altgassen, C.; Hertel, H.; Brandstädt, A.; Köhler, C.; Dürst, M.; Schneider, A.; AGO Study Group. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J. Clin. Oncol. 2008, 26, 2943–5291. [Google Scholar] [CrossRef] [PubMed]
- Lécuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, J.; Jung, L.; Juhasz-Böss, I. Status of Sentinel Lymph Node Biopsy in Vulvar and Cervical Cancer. Geburtshilfe Frauenheilkd. 2020, 80, 1212–1220. [Google Scholar] [PubMed]
- Buda, A.; Papadia, A.; Zapardiel, I. From Conventional Radiotracer Tc-99(m) with Blue Dye to Indocyanine Green Fluorescence: A Comparison of Methods Towards Optimization of Sentinel Lymph Node Mapping in Early Stage Cervical Cancer for a Laparoscopic Approach. Ann. Surg. Oncol. 2016, 23, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Wuntakal, R.; Papadopoulos, A.J.; Montalto, S.A.; Perovic, M.; Coutts, M.; Devaja, O. Location of Sentinel Lymph Node in Cervical Carcinoma and Factors Associated with Unilateral Detection. Int. J. Gynecol. Cancer 2015, 25, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. The efficacy of sentinel lymph node mapping with indocyanine green in cervical cancer. World J. Surg. Oncol. 2018, 16, 52. [Google Scholar] [CrossRef] [PubMed]
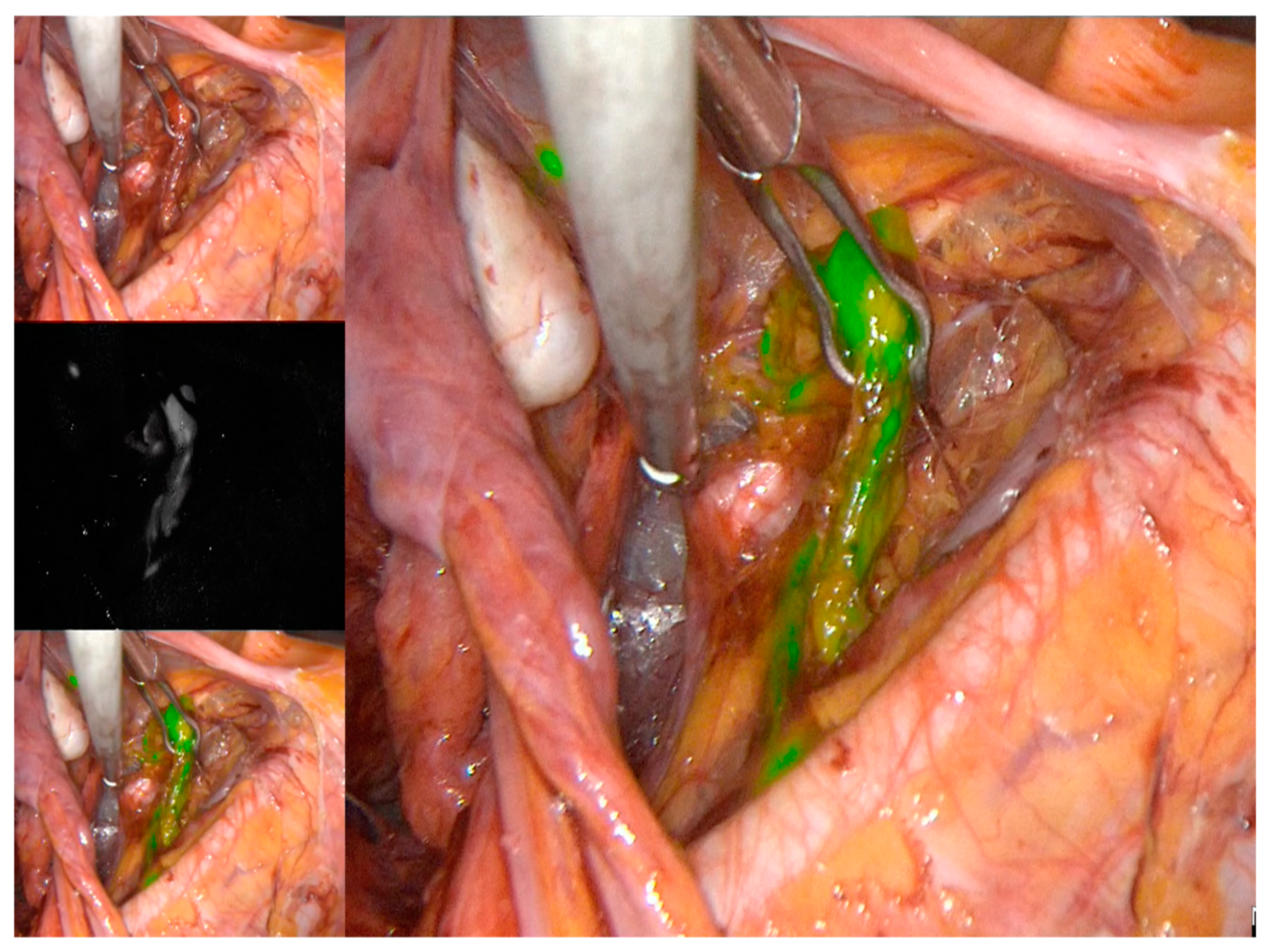
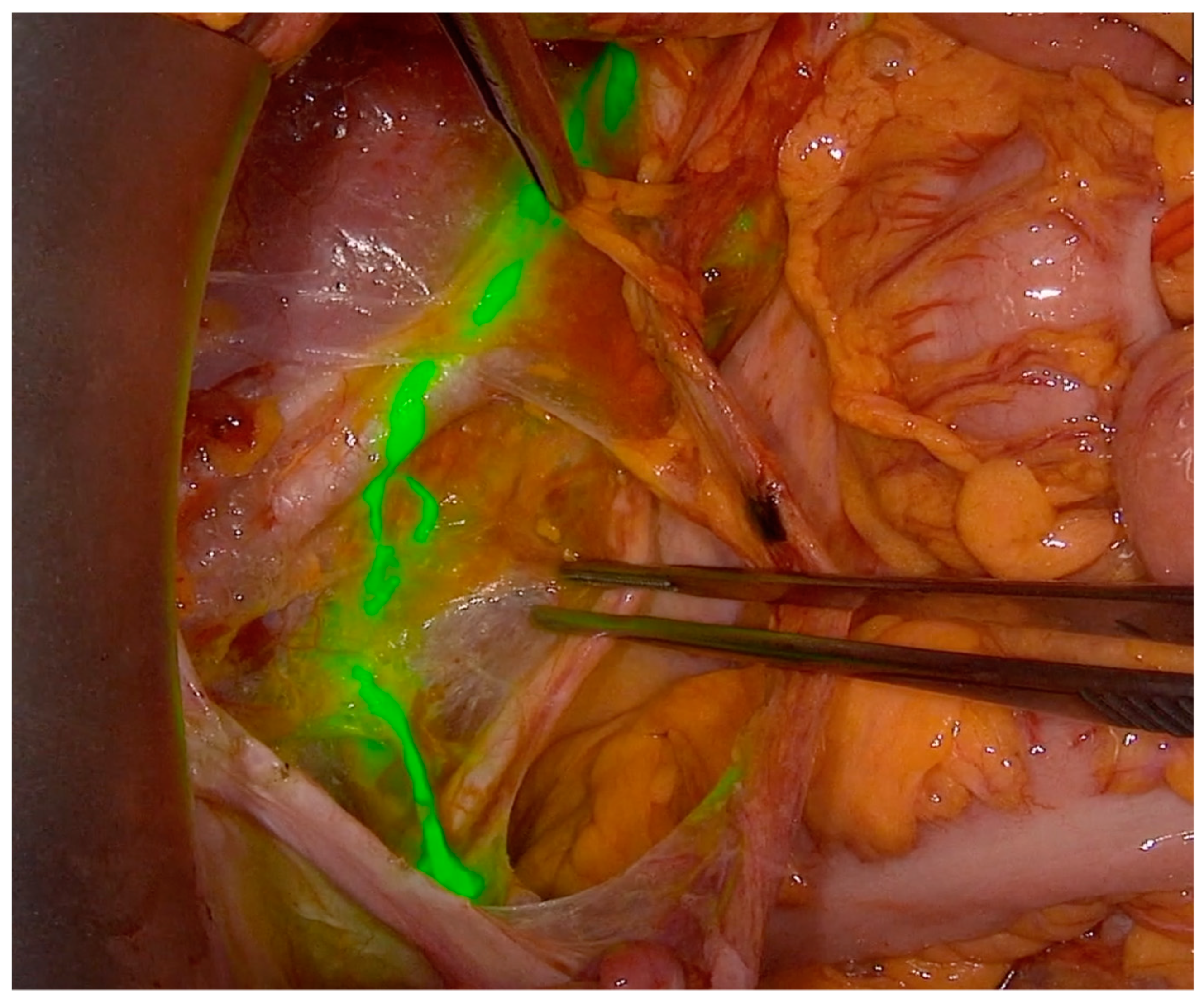
| Characteristic | N = 76, Median (Range, %) | Laparotomy = 38 (Range, %) | Laparoscopy = 38 (Range, %) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 45.6 (25.5–72.9) | 46.1 (25.5–70.7) | 45.6 (28.8–72.9) | 0.192 | |
| BMI (kg/m2) | 25.8 (17.6–43) | 25.7 (18.2–43) | 25.8 (17.6–34) | 0.921 | |
| Preoperative conization | 13 (17.1) | 6 (16) | 7 (18) | 1.000 | |
| Type of surgery | Radical hysterectomy | 70 (92) | 38 (100) | 32 (84.2) | 0.025 |
| Radical trachelectomy | 6 (8) | 0 | 6 (15.8) | 0.025 | |
| Sentinel lymph node | No | 3 (4) | 1 (2.6) | 2 (5.2) | 1.000 |
| Unilateral | 2 (2.6) | 1 (2.6) | 1 (2.6) | ||
| Bilateral | 71 (93.4) | 36 (94.7) | 35 (92.1) | ||
| Number of SLN | 11 (1–33) | 11 (2–29) | 11 (1–33) | 0.926 | |
| Number of removed lymph nodes | 52 (8–124) | 56 (12–124) | 47 (8–100) | 0.766 | |
| Histology | Squamous cell cancer | 63 (82.9) | 31 (82) | 33 (87) | 0.754 |
| Adenocarcinoma | 12 (15.8) | 7 (18) | 5 (13) | ||
| Adenosquamous | 1 (1.3) | 0 | 0 | ||
| Grading | 1 | 2 (2.6) | 0 | 2 (5.3) | 0.277 |
| 2 | 44 (58) | 21 (55.3) | 23 (60.5) | ||
| 3 | 29 (38) | 17 (44.7) | 12 (31.6) | ||
| Unknown | 1 (1.3) | 0 | 1 (2.6) | ||
| LVSI | Negative | 38 (50) | 16 (42.1) | 22 (57.9) | 0.073 |
| Positive | 36 (47.4) | 22 (57.9) | 14 (36.8) | ||
| Unknown | 2 (2.6) | 0 | 2 (5.3) | ||
| Tumor volume | ≤20 mm | 15 (19.7) | 3 (8) | 12 (32) | 0.012 |
| >20–≤40 mm | 25 (32.9) | 17 (45) | 8 (21) | ||
| >40 mm | 36 (47.4) | 18 (47) | 18 (47) | ||
| Mapping results | No SLN-involvement | 54 (71) | 27 (71) | 27 (71) | 1.000 |
| Positive in frozen section | 17 (22.4) | 9 (23.7) | 8 | ||
| Positive in ultra-staging | 2 (2.6) | 1 (2.6) | 1 (2.6) | ||
| Non-sentinel * | 1 (1.3) | 1 (2.6) | 0 | ||
| Mapping without SLN | 3 (3.9) | 1 (2.6) | 2 (5.2) | ||
| Pathologic parametrial infiltration | Bilateral | 18 (23.7) | 12 (31.6) | 6 (15.8) | <0.001 |
| Only right | 7 (9.2) | 7 (18.4) | 0 | ||
| Only left | 7 (9.2) | 5 (13.2) | 2 (5.3) | ||
| Total | 32 (42.1) | 24 (63.2) | 8 (21) | ||
| FIGO | IA1 | 0 | - | - | n.a. |
| IA2 | 2 (2.6) | 1 (2.6) | 1 (2.6) | ||
| IB1 | 14 (18.4) | 4 (10.5) | 10 (26.3) | ||
| IB2 | 24 (31.6) | 12 (31.6) | 12 (31.6) | ||
| IB3 | 14 (18.4) | 6 (15.8) | 8 (21) | ||
| IIA1 | 3 (3.9) | 1 (2.6) | 2 (5.3) | ||
| IIA2 | 1 (1.3) | 1 (2.6) | 0 | ||
| IIB | 9 (11.8) | 5 (13.2) | 4 (10.5) | ||
| IIIA | 1 (1.3) | 1 (2.6) | 0 | ||
| IIIB | 0 | - | - | ||
| IIIC1 | 5 (6.6) | 4 (10.5) | 1 (2.6) | ||
| IIIC2 | 0 | - | - | ||
| IVA | 3 (3.9) | 3 (7.9) | 0 | ||
| IVB | 0 | - | - | ||
| TNM | pT1b1 pN0 | 13 (17.1) | 4 (10.5) | 9 (23.7) | n.a. |
| pT1b1 pN1 | 6 (7.9) | 3 (7.9) | 3 (7.9) | ||
| pT1b2 pN0 | 6 (7.9) | 3 (7.9) | 3 (7.9) | ||
| pT1b2 pN1 | 2 (2.6) | 0 | 2 (5.3) | ||
| pT2a1 pN0 | 5 (6.6) | 2 (5.3) | 3 (7.9) | ||
| pT2a1 pN1 | 0 | - | - | ||
| pT2a2 pN0 | 1 (1.3) | 0 | 1 (2.6) | ||
| pT2a2 pN1 | 6 (7.9) | 4 (10.5) | 2 (5.3) | ||
| pT2b pN0 | 18 (23.7) | 12 (31.6) | 6 (15.8) | ||
| pT2b pN1 | 14 (18.4) | 10 (26.3) | 4 (10.5) | ||
| No residual tumour after conization | 5 (6.6) | 0 | 5 (13.2) | 0.054 | |
| N = 71 | Laparotomy = 36 (%) | Laparoscopy = 35 (%) | p-Value | ||
|---|---|---|---|---|---|
| Location of SLN in the right pelvic sidewall † | Parametrial | 4 (5.6) | 4 (11) | 0 | 0.115 |
| Obturator | 15 (21) | 9 (25) | 6 (17.1) | 0.565 | |
| Internal iliac | 5 (7) | 2 (5.6) | 3 (8.6) | 1.000 | |
| External iliac | 21 (29.6) | 10 (27.8) | 11 (31.4) | 1.000 | |
| Common iliac | 55 (77.5) | 27 (75) | 28 (80) | 1.000 | |
| Location of SLN in the left pelvic sidewall † | Parametrial | 3 (4.2) | 2 (5.6) | 1 (2.9) | 1.000 |
| Obturator | 22 (31) | 8 (22.2) | 14 (40) | 0.206 | |
| Internal iliac | 8 (11.3) | 4 (11.1) | 4 (11.4) | 1.000 | |
| External iliac | 23 (32.4) | 12 (33.3) | 11 (31.4) | 1.000 | |
| Common iliac | 48 (67.6) | 28 (77.8) | 20 (57.1) | 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muallem, M.Z.; Sayasneh, A.; Armbrust, R.; Sehouli, J.; Miranda, A. Sentinel Lymph Node Staging with Indocyanine Green for Patients with Cervical Cancer: The Safety and Feasibility of Open Approach Using SPY-PHI Technique. J. Clin. Med. 2021, 10, 4849. https://doi.org/10.3390/jcm10214849
Muallem MZ, Sayasneh A, Armbrust R, Sehouli J, Miranda A. Sentinel Lymph Node Staging with Indocyanine Green for Patients with Cervical Cancer: The Safety and Feasibility of Open Approach Using SPY-PHI Technique. Journal of Clinical Medicine. 2021; 10(21):4849. https://doi.org/10.3390/jcm10214849
Chicago/Turabian StyleMuallem, Mustafa Zelal, Ahmad Sayasneh, Robert Armbrust, Jalid Sehouli, and Andrea Miranda. 2021. "Sentinel Lymph Node Staging with Indocyanine Green for Patients with Cervical Cancer: The Safety and Feasibility of Open Approach Using SPY-PHI Technique" Journal of Clinical Medicine 10, no. 21: 4849. https://doi.org/10.3390/jcm10214849
APA StyleMuallem, M. Z., Sayasneh, A., Armbrust, R., Sehouli, J., & Miranda, A. (2021). Sentinel Lymph Node Staging with Indocyanine Green for Patients with Cervical Cancer: The Safety and Feasibility of Open Approach Using SPY-PHI Technique. Journal of Clinical Medicine, 10(21), 4849. https://doi.org/10.3390/jcm10214849







