Abstract
Laminopathies are a group of rare disorders due to mutation in LMNA gene. Depending on the mutation, they may affect striated muscles, adipose tissues, nerves or are multisystemic with various accelerated ageing syndromes. Although the diverse pathomechanisms responsible for laminopathies are not fully understood, several therapeutic approaches have been evaluated in patient cells or animal models, ranging from gene therapies to cell and drug therapies. This review is focused on these therapies with a strong focus on striated muscle laminopathies and premature ageing syndromes.
1. Introduction
Type V intermediate filaments, also known as lamins, are the main constituents of the nuclear lamina, a protein meshwork underlining the inner face of the nuclear envelope (NE) and facing chromatin and nucleoplasm. Lamins are divided into two categories: A-type lamins, encoded by the LMNA gene, and B-type lamins, encoded by the LMNB1 and LMNB2 genes. They display an N-terminal unstructured head domain, a central helical rod domain involved in their assembly into filaments and a globular C-terminal tail that contains a nuclear localization signal and an immunoglobulin-like (IgG-like) fold involved in protein-protein interactions [1].
The LMNA gene has 12 exons, among which exon 10 contains an alternative splice site giving rise to two major isoforms: lamin A and C. These two isoforms are identical in their first 566 amino-acids and vary in their C-terminal tail, with six unique carboxyl-terminal amino-acids for lamin C and 98 for lamin A [2]. Unlike lamin C, lamin A is synthesized as a precursor named prelamin A, that contains a C-terminus “CaaX” motif (“C” for cysteine; “a” for aliphatic amino acid; “X” for any amino acid). The C-terminal tail of prelamin A undergoes several post-translational modifications to become mature: (a) farnesylation of the cysteine from the CaaX motif responsible for the anchorage of prelamin A to the NE, (b) the cleavage of the “aaX” motif by zinc metallopeptidase STE24 (ZMPSTE24) and Ras-converting CAAX endopeptidase 1 (RCE1), (c) methylation of the farnesylated cysteine by isoprenylcysteine carboxyl methyltransferase (ICMT), and (d) the cleavage of the last 15 amino-acids including the farnesylated cysteine by ZMPSTE24, releasing mature lamin A from the NE. Like prelamin A, B-type lamins are synthesized as precursors, and while the three first steps of their maturation are similar to lamin A, the last step of maturation, corresponding to the second cleavage by ZMPSTE24, does not occur. Consequently, B-type lamins are suggested to be more closely associated to the NE than lamin A/C [3].
The lamins form parallel dimers that assemble longitudinally in a head-to-tail manner to form a long polar polymer that further associate laterally forming ~3.5 nm thick mature apolar filaments within the ~14 nm thick nuclear lamina under the NE [4,5]. A-type lamins are also found in the nucleoplasm, in a less structured and less complex organization. It has been proposed that nucleoplasmic lamins form dimers or short polymers interacting with intranuclear binding partners [6].
Over 500 mutations, mainly dominant, have been identified throughout the LMNA gene and linked to a broad spectrum of diseases called laminopathies. Different groups of diseases have been described based on the main affected tissue: striated (skeletal and cardiac) muscle laminopathies (SML), peripheral neuropathies, familial partial lipodystrophy and multisystemic disorders including premature aging syndromes [6]. In this review, we describe the pathophysiological mechanisms implicated in laminopathies, i.e., the diseases due to LMNA gene mutations, with a focus on SML and premature ageing syndromes, and associated preclinical therapies that have been developed over the years.
2. Laminopathies’ Clinical Spectrum
2.1. The Striated Muscle Laminopathies
SML are defined as a group of diseases generally characterized by dilated cardiomyopathy with conduction and/or rhythm defects (DCM-CD) associated or not with muscular dystrophy of variable type and age of onset. In fact, since the identification of the first laminopathy, the autosomal dominant Emery-Dreifuss muscular dystrophy (EDMD) [7], a range of muscular dystrophies of various clinical severity related to LMNA mutations has been described, from the most severe and early onset congenital form, the LMNA-related Congenital Muscular Dystrophy (L-CMD [8]) to less severe and almost adult onset form, the autosomal dominant Limb-Girdle Muscular Dystrophy type 1B (LGMD1B [9]). Patients with SML display varying severity of four limbs atrophy and weakness with or without joint contractures and neck/spine rigidity [10]. SML share a common life-threatening cardiac disease characterized by conduction and/or rhythm defects associated with dilated cardiomyopathy resulting in a high frequency of cardiac sudden death and end-stage hearth failure (corresponding to a clinical stage of advanced heart failure with pronounced symptoms at rest and refractory to maximal medical treatment). This cardiac disease can be the only clinical presentation of the disease without any skeletal muscle involvement [11,12].
Both dominant negative effect and haploinsufficiency have been suggested as disease mechanisms [12,13]. A large proportion of LMNA mutations causing SML are point mutations and it has been suggested that such mutations have a dominant-negative effect causing disruption of the lamina and compromising nuclear integrity. Indeed, lamin A/C mutants induce mislocalization of lamin A/C interacting proteins, such as lamin B1, lamin-associated protein 2 (LAP2), emerin or nucleoporin 153 (NUP153) [14,15]. In addition, A-type lamin mutants may form altered filaments [16] that aggregate in the nucleoplasm with wild-type (WT) lamin A/C, prelamin A and nuclear factors (such as pRb or SREBP1), which contribute to the pathogenesis of laminopathies [17,18,19,20]. More recently, a study showed that prelamin A was upregulated in hearts of DCM-CD patients and significantly associated with left ventricular (LV) remodeling, suggesting its potential involvement in the progression of cardiac disease [21].
Lamin A/C haploinsufficiency can also cause the nuclear defect underlying the pathogenesis of the disease. Nonsense mutations, out-of-frame insertions/deletions and/or splice site LMNA mutations generate truncated proteins that are not detected in patient fibroblasts or in mouse models probably because truncated mRNAs are degraded through nonsense-mediated RNA decay and truncated proteins through proteasome degradation or autophagy [12,13]. The reduced level of lamin A/C has been associated with misshapen nuclei, nuclear envelope disruption, chromatin rearrangement and DNA damage [22,23,24]. Moreover, lamin A/C haploinsufficiency led to early-onset programmed cell death of cardiomyocytes, causing DCM in mice [22,25,26,27]. Interestingly, a genotype-phenotype correlation study performed in 27 patients carrying LMNA mutations suggested that late-onset phenotypes were associated preferably with truncating mutations whereas more severe and early-onset phenotypes were associated with dominant-negative non truncating mutations [28]. These findings may help in patient management.
2.2. Progeria and Other Premature Aging Syndromes
Another group of laminopathies corresponds to premature ageing syndromes, including those involving children, i.e., restrictive dermopathy (RD) [29] and Hutchinson-Gilford Progeria syndrome (HGPS), reported by Hutchinson and Gilford in the late 1880s [30,31], and those involving adults such as mandibuloacral dysplasia type A (MAD-A) and atypical Werner’s syndrome [32,33]. HGPS, an extremely rare disorder (with a prevalence of approximately 1 in 20 million children), is the far most studied premature ageing syndrome. It is characterized by severe growth retardation, failure to thrive, alopecia, osteoporosis, severe atherosclerosis with cardiovascular decline, abnormal skin pigmentation, lipodystrophy, and joint contractures [34,35]. It is mainly caused by aberrant splicing of the LMNA gene resulting from a de novo synonymous LMNA variation in exon 11. This aberrant splicing induces the deletion of 50 amino acids in prelamin A, including the second cleavage site for ZMPSTE24, hence leading to a truncated lamin A that remains farnesylated and named progerin [36,37]. Accumulation of progerin is toxic for the cell and responsible for structural changes in the nucleus [38].
2.3. Lipodystrophies of Dunnigan Type
LMNA mutations are the most common gene defect responsible for lipodystrophy syndrome of Dunnigan type (type 2 Familial Partial Lipodystrophy or FPLD2), characterized by lack of adipose tissue in the four limbs and its accumulation within the neck and the face, accompanied by metabolic abnormalities. Symptoms of lipodystrophy may partially overlap with adult progeroid syndromes, underlying the important role of LMNA in the development and function of fat-storing adipocytes. Seventy-five percent of LMNA mutations that causes FPLD2 are missense mutations encompassing the IgG-like domain [39,40,41]. These mutations have been shown to perturb the interaction of lamin A/C with several partners, including SREBP1, a transcription factor involved in adipocyte differentiation [42].
2.4. Neuropathies
Only one homozygous LMNA mutation (p.Arg298Cys) has been reported in an axonal form of autosomal recessive Charcot-Marie-Tooth type 2 (CMT2B) peripheral neuropathy [43,44] in families originating from North Africa. Patients had distal axonal sensorimotor neuropathy with a proximal involvement of the lower limb muscles in some cases. A wide range of age of onset, course and severities have been reported suggesting that modifier genes may be involved [45].
3. Therapies for Striated Muscle Laminopathies
The use of patient materials and animal models (mouse knock-out (KO) and knock-in (KI) models reproducing human mutations, C. elegans and drosophila) have greatly helped understanding the pathophysiological mechanisms of laminopathies (Figure 1) [46]. Different strategies have been developed over the years that act either on the primary cause of the diseases or on their consequences (i.e., altered pathways), using gene, cells or drug therapies (Table 1). However, for now, apart from one molecule that is currently under clinical trial (see Section 3.3.4), these therapies are only at the preclinical stage for SML.
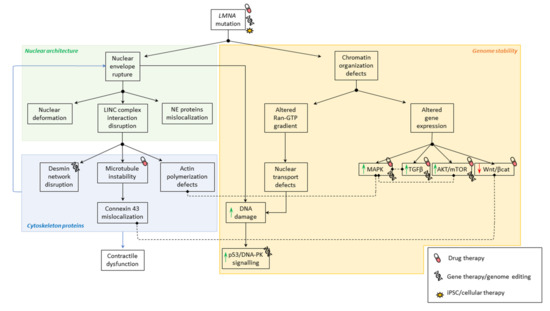
Figure 1.
Pathophysiological mechanisms involved in SML. Summary of physiological mechanisms affected in striated muscle laminopathies due to LMNA mutations. Black solid arrows indicate the consequence of altered mechanisms. Doted lines indicate correlation between mechanisms.

Table 1.
Literature review of preclinical therapeutic strategies in striated muscle laminopathies in vivo and in vitro.
3.1. Gene and RNA-Based Therapies
3.1.1. Targeting Lamin A/C
Homozygous LmnaΔ8–11/Δ8–11 mice, first thought to be a knock-out (KO) model for Lmna, display growth retardation, skeletal dystrophy and DCM-CD characterized by left ventricular (LV) dilatation and reduced systolic contraction, and die around 8 weeks of age due to the expression of a truncated lamin A mutant at low level [25,47]. Work performed on genetically modified mice has shown that expression of one isoform only (either lamin A or lamin C) can prevent the onset of deleterious phenotypes of Lmna Δ8–11/Δ8–11 mice [48,49]. In line with this, cardiomyocyte-specific expression of WT-lamin A transgene partially restored cardiac function of these mice. It significantly increased contractility and myocardial performance but had no effect on cardiac dilatation. Improvements of cardiac function have a beneficial effect on lifespan (12% median extension) but are limited by the heterogenic expression of Lmna transgene in cardiomyocytes (30 to 40% positive cells) [50].
As overexpression of mutant lamin A/C is often associated with toxicity [51], alternative gene therapy approaches for laminopathies tested the possibility to use exon-skipping strategy to remove the exon bearing the mutation. Scharner et al. evaluated the potential of LMNA exon 5 skipping by antisense oligonucleotide (AON) in HeLa cells and WT human dermal fibroblasts but trials in affected cells or animal models are still missing [52].
More recently, our group demonstrated the promising role of spliceosome-mediated RNA trans-splicing (SMaRT) as a therapeutic strategy to replace the mutated pre-mRNA by the corresponding WT transcript using an exogenous RNA called pre-trans-spliced molecules (PTM) [53]. PTM molecules designed to replace exons 1 to 5 of the mutated Lmna pre-mRNA, allowing for the targeting of 51% of the described LMNA mutations, were tested in vitro and in vivo in the LmnaΔK32/ΔK32 mouse model, a KI mouse reproducing a LMNA mutation found in severe EDMD and L-CMD [54]. This strategy rescued part of the nuclear phenotype of LmnaΔK32/ΔK32 mouse myotubes in vitro, however the efficiency of PTM’s adeno-associated virus (AAV) delivery was particularly low, leading to an extremely modest increase in lamin A/C mRNA expression preventing any conclusion regarding the survival analysis in vivo [53]. Despite mixed results, this strategy is a promising tool that could be a potential replacement to classical gene therapy [51]. Finally, Salvarani et al. used CRISPR-Cas9 editing tool to decipher the conduction abnormalities associated with LMNA-cardiomyopathies of iPSC-derived cardiomyocytes harboring LMNA p.Lys219Thr (LMNA-K219T) mutation. They showed that LMNA-K219T mutation affects excitability and cardiac impulse propagation by repressing SCN5A expression, encoding the sodium channel gene, NAV1.5, hallmarks that are restored after CRISPR/Cas9 correction [55].
3.1.2. Targeting Lamin-Associated Proteins
In the nucleus, lamins interact with numerous proteins thus involving them in a wide range of nuclear functions such as cell proliferation, genome organization and DNA repair. Lamina-associated polypeptide 2α (LAP2α) is a nucleoplasmic protein that interacts with A-type lamin in the nucleoplasm. Interestingly, mutation in LAP2α gene causes autosomal-dominant cardiomyopathy and altered its interaction with A-type lamin [56]. In 2013, Cohen et al. showed that Lap2α and retinoblastoma protein (pRB) signaling were up-regulated in LmnaΔ8–11/Δ8–11 mice which could contribute to the phenotype of these mice. Therefore, they generated LmnaΔ8–11/Δ8–11/Lap2α−/− mice in which the depletion of Lap2α increased lifespan and bodyweight but was not sufficient to completely rescue LmnaΔ8–11/Δ8–11 mouse phenotype since cardiac defect remained the cause of death of these mice. These results highlight the role of Lap2α/pRB pathway in the deleterious phenotype of these mice [57]. However, Pilat et al. performed a similar study in LmnaΔK32/ΔK32 but did not show any beneficial effect of Lap2α depletion on the phenotype of these mice [58].
3.2. Cell Therapies
Cellular therapies are also a promising tool in treatment of cardiovascular disease, and notably in heart failure. The functional benefits of these therapies are mainly based on the propriety of implanted cells to release paracrine factors that would activate myocardial repair pathways [59]. In 2013, Catelain et al. compared transplantation efficiency of murine embryonic stem cells (ESC) induced into cardiac lineage, and a murine myoblast cell line (D7LNB1) considered at that time as “gold-standard” for cell-based therapy, into the LV wall of LmnaH222P/H222P mouse, a KI mouse model reproducing a mutation found in EDMD patients and mainly responsible for DCM in homozygous mice [60]. Myoblast engraft had a greater transplantation efficacy and improved cardiac functions (stabilization of LV fractional shortening), whereas ESCs failed to integrate in the myocardium of LmnaH222P/H222P mice [61]. Clearly, more research is needed in order to find the best cell type to use for cell therapy in the heart. Many groups are actively working on multipotent and pluripotent stem cells with promising results [62].
3.3. Drug Therapies
Drug therapies are the most advanced therapies for SML. The different molecules tested aimed either at reading through a premature STOP codon or at slowing down the progression of the diseases via modulating altered signaling pathways identified mainly by transcriptomic analyses and RNA sequencing of patient material, KO or KI mouse models.
3.3.1. Molecule Targeting LMNA mRNA
Lee et al. generated human iPSC-derived cardiomyocytes from patients carrying different premature termination codon (PTC) mutations in LMNA gene that reproduced the pathological hallmarks of LMNA-associated cardiomyopathy. In these models, they tested PTC124, a molecule that induces translational read-through over the PTC to restore the production of the full-length protein and evaluated its potential therapeutic effect. PTC124 treatment showed beneficial effect in only one of the two mutants tested by reducing nuclear blebbing, excitation-contraction coupling and apoptosis [63].
3.3.2. Modulation of Chromatin-Associated Protein Activity
Lamin A/C interacts with chromatin and organizes the genome into large territories called lamin-associated domains (LADs) that influence gene expression in a cell type-specific manner [64]. Therefore, it is not surprising that LMNA mutations affect LAD organization and modify gene expression [65,66,67]. Numerous transcriptomic analyses performed on the heart of various animal models have revealed a wide variety of altered signaling pathways, even before the appearance of any pathological features [68,69].
Auguste et al. performed RNA sequencing in a mouse model with a cardiac specific depletion of Lmna gene (Myh6-Cre:LmnaF/F mice), before the onset of cardiac dysfunction, identifying over 2300 differentially expressed genes. Among them, BRD4 (Bromodomain-containing protein 4) gene, a regulator of chromatin-associated protein, was upregulated. Daily treatment of Myh6-Cre:LmnaF/F mice with JQ1, a specific BET bromodomain inhibitor, improved cardiac function, fibrosis, apoptosis and prolonged lifespan. These findings highlight BET bromodomain inhibition as a potential new therapeutic strategy for LMNA-associated cardiomyopathy [70].
Similarly, cardiac differentiation defects of ESCs from heterozygous LmnaH222P/+ mouse have been correlated to altered expression of genes involved in the epithelial to mesenchymal transition. Analysis of the regulatory regions of genes revealed a decreased H3K4me1 deposit on Twist and Mesp1 that was reversed by inhibiting LSD1, the enzyme responsible for H3K4 demethylation. Treatment restored cardiac differentiation of LmnaH222P/+ ESC, and ameliorated heart formation and function in embryos and post-natal LmnaH222P/H222P mice [71].
3.3.3. DNA Repair and Oxidative Stress
DNA damage in laminopathies have been associated with increased nuclear envelope rupture, altered Ran-GTP gradient or oxidative stress [72,73,74]. Cells respond to stress by activating redox-sensitive transcription factors (TF) such as pRb, p53 (tumor suppressor) and forkhead box O (FOXO) TF [75]. Transcriptomic and RNA-sequencing analyses performed on mouse embryonic fibroblasts (MEF) or heart tissue from various mouse models (LmnaΔ8–11/Δ8–11, LmnaH222P/H222P, Tg-LMNAD300N) all led to the identification of a major up-regulation of p53 [33,68,76,77]. Up-regulation of FOXO, NF-κB or TGF-β were also reported in some models [68,78]. These results were corroborated by a transcriptional analysis of cells from patient with LMNA-cardiomyopathy, EDMD and FPLD2 [67,79,80,81]. Modulation of FOXO by shRNA or supplementation with NAD+, with its precursor Nicotinamide Riboside, or with AP endonuclease 1 (APE1] required for base excision repair led to increased DNA repair, and ameliorated altered pathways and mouse survival [68,76,82].
We examined the involvement of oxidative stress in the progression of cardiac disease in LmnaH222P/H222P mice and showed that LMNA cardiomyopathy is associated with increased oxidative stress and depletion of glutathione (GSH). Treatment of LmnaH222P/H222P mice with N-acetyl cysteine (NAC), a precursor of GSH, restored the redox homeostasis and delayed the onset of LV dilatation and cardiac dysfunction [77].
3.3.4. Inhibition of MAPK Pathways
Lamin A/C has been shown to play a dynamic role in regulating signal transduction by tethering proteins at the NE. Lamin A/C interacts directly with ERK1/2 (Extracellular signal-regulated kinase 1/2), which highlights a potential role of lamin A/C on the regulation of ERK1/2 signaling pathway [83]. Transcriptomic analyses performed on the hearts of pre-symptomatic LmnaH222P/H222P mice and on explanted hearts of patients with LMNA-associated dilated cardiomyopathy showed an increased expression of genes implicated in 3 of the 4 MAPK signaling pathways: ERK1/2, JNK and p38α [69,84]. Inhibition of ERK1/2 was achieved using several MEK1/2 inhibitors (PD098059, Selumetinib, compound 8 allosteric macrocyclic MEK1/2 inhibitor). They all target MEK1/2 kinases responsible for ERK1/2 phosphorylation. Treated LmnaH222P/H222P mice showed a significant slow-down of LV dilatation progression, improved cardiac contractility and functions and increased survival [84,85,86,87,88]. Selumetinib was also shown to have a synergic effect when combined with benazepril, an angiotensin II converting enzyme (ACE) inhibitor, a standard medical therapy in heart failure. Of note, ACE inhibition alone delayed the onset of cardiac disease [89]. Treatment with JNK inhibitor SP600125, or p38α inhibitor ARRY-371797, also slowed down the development of cardiac contractile dysfunction [84,90,91]. The beneficial effects of ARRY-371797 in mice led to the first clinical trial, still on going, on the p38α inhibitor in patients with LMNA-associated dilated cardiomyopathy (clinicaltrials.gov #NCT02057341). The results of these studies demonstrate that MAPK activation contributes to the pathogenesis of dilated cardiomyopathy caused by LMNA mutation but the mechanism leading to MAPK activation remains unknown.
3.3.5. Inhibition of TGF-β Signaling Pathway
Transcriptome and secretome analyses revealed the hyperactivation of TGF-β signaling in hearts of LmnaH222P/H222P mice, prior to the onset of the cardiac disease and leading to elevated TGF-β2 levels in the majority of the patients (EDMD and LGMD1B and other neuromuscular diseases) and in LmnaH222P/H222P mouse sera [80,92]. TGF-β2 neutralizing antibody avoided activation of fibrogenic markers and myogenesis impairment in vitro [80], while the TGF-β receptor (ALK5] inhibitor SB-431542 reduces fibrosis and improves LV functions in LmnaH222P/H222P mouse hearts, in part via lowering the level of active ERK1/2. These findings highlighted TGF-β as a mediator in the pathogenesis of Lmna-associated cardiomyopathy [92].
Inhibition of TGF-β signaling was also tested in another mouse model: the Lmna-DCM mice, an inducible and cardiomyocyte-specific model of lamin A/C depletion created by Tan et al. by AAV delivery of shRNA targeting Lmna mRNA under cardiac specific promoter in 1.5-week-old mice. These mice exhibit marked fibrosis, cardiac dilation and dysfunction, rescued upon treatment with Yy1 (Ying Yang 1], a transcription factor associated with cell cycle progression. Upregulation of Yy1 led to the upregulation of Bmp7 expression and the downregulation of Ctgf expression, inhibiting TGF-β signaling pathway [93]. These studies provide several lines of evidence supporting TGF-β signaling as potential targets for DCM-CD and cardiac fibrosis.
3.3.6. Targeting Cytoskeleton Proteins
Recently, it has been reported that ERK1/2 interacts directly with cofilin-1, an actin-depolymerizing factor, that lead to the alteration of the sarcomeric actin polymerization, participating in the development of LV dysfunction in LMNA-associated cardiomyopathy and muscle weakness. Inhibition of ERK1/2 using selumetinib or other MEK1/2 inhibitors suppressed cofilin-1 phosphorylation and restored LV functions [78,94].
Microtubule, another cytoskeleton constituent, polymer of tubulin proteins, was shown to be impaired in SML. Impairment of the microtubule network triggered abnormal electrical communication between cardiomyocytes and induced cardiac conduction defects in LmnaH222P/H222P, LmnaN195K/N195K and LmnaΔ8–11/Δ8–11 mice [95,96,97]. Increased phosphorylation and aberrant localization of Cx43 have been reported, in vivo and in vitro, due to microtubule instability [96,97,98]. Stabilization of microtubules using paclitaxel, a microtubule-stabilization agent commonly used in chemotherapy, improved intraventricular conduction defects in LmnaH222P/H222P mice, demonstrating a novel pathophysiological mechanism based on microtubule network and Cx43 displacement [97].
Disorganized desmin network is also observed in SML, triggering nuclear deformation and contractile dysfunction [25,99]. In LmnaH222P/H222P mice, cardiac-specific expression of αB-crystallin (αBCry), a chaperone protein interacting with desmin to maintain cytoskeletal integrity, has cardioprotective effects by improving desmin network, mitochondrial and nuclear defects and ERK1/2 abnormal activation. Overall, LmnaH222P/H222P/αBCry+/− mice displayed significantly improved cardiac functions. Interestingly, similar results were observed in desmin-depleted LmnaH222P/H222P mice [100]. Increased of desmin protein levels and disorganization of the desmin network were also rescued in LmnaΔ8–11/Δ8–11 mice expressing the cardiomyocyte-specific expression of WT-lamin A transgene [50].
3.3.7. Inhibition of WNT/β-Catenin Signaling
Wnt proteins are secreted cysteine-rich glycoproteins involved in several cellular processes such as proliferation, differentiation, apoptosis and senescence. In the absence of Wnt ligand, β-catenin is phosphorylated by glycogen synthase kinase 3-β (GSK3-β) and degraded by the proteasome. When Wnt binds to its receptors, β-catenin accumulates in the cytosol and translocates to the nucleus where it activates gene expression such as connexin 43 (CX43) [101]. In LmnaH222P/H222P mouse hearts, Wnt, β-catenin and Cx43 expressions are decreased [69,84,102]. The pharmacological activation of WNT/β-catenin signaling using 6-bromoindirubin-3′-oxime (BIO), a GSK3-β inhibitor, restored connexin 43, Wnt-1 and β-catenin expressions and improved cardiac functions of LmnaH222P/H222P mice [102]. Similar results were observed in HL-1 cardiomyocytes transfected with LMNA p.Asp243Glyfs*4 mutant, where decreased connexin 43 level was restored by lithium treatment, another well-known GSK3 inhibitor [103].
3.3.8. Activation of Autophagy
The mammalian target of rapamycin (mTOR) pathway plays a key regulatory function in cardiovascular physiology (embryonic development, maintenance of cardiac structure and function) and pathology (cardiac hypertrophy, ischemia). mTOR is an atypical serine/threonine kinase that forms two distinct multiprotein complexes, mTORC1 and mTORC2, to exert specific functions in response to environmental stimuli. mTORC1 plays a central role in protein synthesis, cell growth/proliferation and autophagy while mTORC2 regulates cell survival and polarity [104]. Hyperactivation of mTOR signaling has been reported in mouse models of LMNA-associated cardiomyopathy [105,106]. Treatment of 4-week old LmnaΔ8–11/Δ8–11 mice with rapamycin, a specific inhibitor of mTORC1, or treatment of 14-week-old LmnaH222P/H222P mice with temsirolimus, a rapamycin analog, showed improvement of cardiac functions [105,106]. Similarly, everolimus treatment, another Rapamycin analog, improves fibroblasts phenotypes of patients carrying various LMNA mutations associated with EDMD, HGPS and atypical Werner syndrome [107].
4. Therapies for Premature Aging Syndromes
Similarly to SML, different strategies have been developed over the years to understand the pathophysiological mechanisms underlying premature aging syndrome (Figure 2) as well as developing strategies to prevent the progression of disease (Table 2).
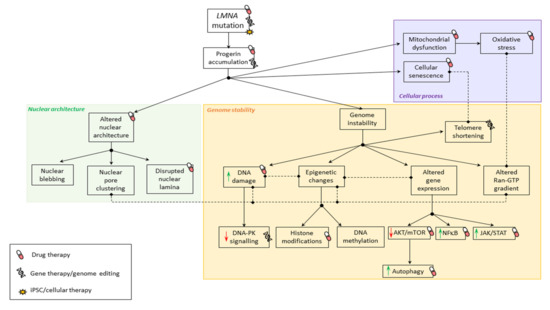
Figure 2.
Pathophysiological mechanisms involved in premature aging syndromes. Summary of physiological mechanisms affected in premature aging syndromes due to LMNA mutations. Black solid arrows indicate the consequence of altered mechanisms. Doted lines indicate correlation between mechanisms.

Table 2.
Literature review of preclinical therapeutic strategies in premature aging syndromes and lipodystrophies.
4.1. Gene and RNA Based Therapies
Gene therapy strategy started in 2005, with the use of morpholino antisense oligonucleotides (MAOs) targeting lamin A cryptic splice site, thus restoring normal nuclear morphology in HGPS fibroblasts [112]. Efficacy of this approach was proven in vivo, and splicing modulation even demonstrated a beneficial upregulation of lamin C transcripts compensating for the absence of lamin A [113,114,115]. Additionally, MAOs’ success extends to other progeroid syndromes, including HGPS-like and MAD-B syndromes [116]. Another strategy relies on the suppression of the specific disease-causing LMNA transcript using shRNA, which also showed reduction of abnormal nuclear morphology and cell senescence, and improvement of proliferative potential [117]. RNA interference using microRNAs such as miR-9, specifically targeting lamin A for degradation, exerts a protective effect in HGPS neurons [118,119] reviewed in [120]. CRISPR/Cas for direct genome editing [121,122] could also, after evaluation and minimization of their off-target effects, represent a new potential therapeutic strategy for the clinics [123]. Currently, several groups are working towards the development of efficient tools to deliver such gene therapy, with promising results using AAV or lentiviral vectors [121,124,125]. Lentiviral delivery to induce base editing with adenine base editors in cultured HGPS fibroblasts and mouse models resulted in improved cellular phenotype and rescued vascular pathology in vivo [126,127]. More recently, Mojiri et al. tested the impact of lentiviral delivery of telomerase mRNA (TERT) on senescence in human HGPS iPSC-derived endothelial cells and HGPS mouse model. Both models showed improved phenotypes placing hTERT therapy as a viable option for treating vascular disease in HGPS patients [128].
4.2. Drug Therapies
4.2.1. Targeting Post-Translational Processing
Because progerin lacks the target site for ZMPSTE24 endoprotease encoded by the missing exon 11, it remains permanently farnesylated and hence anchored to the inner nuclear membrane. The first drugs tested for treatment aimed at inhibiting protein farnesylation. Farnesyltransferase inhibitors (FTIs), the most commonly used therapeutic agent in the field of HGPS treatment, have shown efficacy against disease phenotype in both HGPS cells and mouse models [129,130,131,132,133,134,135,136,137,138], though mitigated by an alternative post-translational modification of the precursor protein in place of farnesylation: the geranylgeranylation [139]. To overcome this, Varela et al. used a combination of statins and aminobisphosphonates that improved nuclear morphology, lifespan, skeletal properties, and reduced growth retardation and weight loss in the previously mentioned models. Based on a similar approach, Blondel et al., identified mono-aminopyrimidines (mono-APs) as inhibitors of farnesyl pyrophosphate synthase and farnesyltransferase to prevent farnesylation, and rescue progeria cell phenotype [140]. Finally, another way progerin may bind to the lamina is through its carboxy methylation by ICMT. Thus, targeting ICMT represents another option to address progerin post-translational processing, and already showed to be promising in the context of HGPS [141]. Preclinical studies and clinical trials using FTIs (lonafarnib) alone or in combination with statins (Pravastatin) and bisphosphonates (zoledronate) highlighted that triple-combination therapies did not add beneficial effect compared to the single-drug treatment [142,143,144,145,146]. Nevertheless, the combination and cocktail therapies of FTIs with mono-APs and/or ICMT inhibitors may potentially be the right strategy for care improvement. In a continuing effort to find the right treatment for each patient and to make the above mentioned literature data readily available to clinicians, databases such as the treatabolome are being developed, compiling an exhaustive list of existing treatments for laminopathies and other rare disorders [147].
4.2.2. Targeting the Protein
Many of the drugs used in therapies for SML were also tested for HGPS cellular and mice models. It is the case for the mTORC1 inhibitor rapamycin, used to induce autophagy and resulting in restored nuclear morphology, delayed onset of cellular senescence and progerin clearance in HGPS cells [148,149,150]. Rapamycin was also shown to restore peripheral heterochromatin and cell cycle dynamics in cells from MAD patients [151]. Other autophagy inducers such as sulforaphane, a vegetable-derived antioxidant, and temsirolimus both showed comparable beneficial results [152,153].
The combination of FTIs with rapamycin induced correction of aberrant genome organization and reduction of DNA damage [154] while its combination with sulforaphane induced progerin clearance, rescued cellular phenotype, increased ATP level, decreased DNA damage and lowered the number of dysmorphic nuclei, despite an enhanced cytotoxicity [155]. The last FTI-based therapy that has been evaluated clinically in combination with rapamycin (everolimus) is still under clinical trial (clinicaltrials.gov #NCT02579044).
Additionally, all-trans retinoic acid (ATRA), for which the LMNA promoter contains response elements, has been shown to induce progerin autophagy in combination with rapamycin in HGPS fibroblasts [156]. Injections of the proteasome inhibitor MG132 also resulted in an autophagy-mediated enhanced progerin turnover in HGPS patient fibroblasts, HGPS patient iPSC-derived mesenchymal stem cells, vascular smooth muscle cells, and LmnaG609G/G609G progeric mouse model [157]. MG132 also downregulates serine and arginine rich splicing factor 1 (SRSF-1) and SRSF-5, two RNA binding proteins favouring LMNA aberrant splicing, which could also explain decreased progerin expression and ameliorated nuclear defects [157]. This phenomenon is also observed in presence of Metformin, an antidiabetic drug known to downregulate mTOR signalling and SRSF-1 [158,159]. Lastly, it has been shown that progerin disrupts nuclear lamina in interaction with lamin A/C, and that this specific binding was inhibited by JH1, JH4 and JH13, compounds identified through a chemical library screening. JH4 in particular, alleviates nucleus distortion, senescence-associated β-gal activity, increases H3K9me3 level and proliferation in HGPS patient cells, LmnaG609G/G609G and LmnaG609G/+ mice [113]. These effects are even more pronounced in the optimized version of JH4 called progerinin [160].
4.2.3. Targeting Downstream Toxic Effects of Progerin Accumulation
Oxidative Stress
Antioxidants such as NAC and methylene blue, Rho-associated protein kinase (ROCK) inhibitors (Y-27632, fasudil) or ataxia-telangiectasia-mutated (ATM) inhibitors (KU-60019), all alleviated mitochondrial dysfunction and improved HGPS phenotype in vitro [161,162,163,164]. Impaired mitochondrial function results in vascular calcification, which was improved in vivo with pyrophosphate treatment [165]. Similarly, olipraz, CPDT, compound AI-1 and TAT-14, small molecules that either activate or stabilize the redox sensor NRF2, significantly reduced oxidative stress, ROS levels and HGPS-associated nuclear defects [166]. MG132, previously shown to reduce progerin expression, also activates NRF2 signalling pathway [167]. Of note, reduction of ROS level was also observed using previously mentioned drugs, namely pravastatin/zolenodrate and metformin [133,159]. An in-human clinical trial (Clinical Trials.gov, NCT00879034) involving 37 HGPS patients followed a previous lonafarnib-only trial [145] and employed a combination of lonafarnib, pravastatin and zoledronic acid. This trial showed only additional bone mineral density benefit beyond the previously demonstrated survival improvement already seen with lonafarnib monotherapy [146].
NF-κB Pathway
Based on its link with aging, hyperactivation of NF-κB through the JAK/STAT inflammatory signalling pathway was also explored in search of therapeutic molecules. Hence, sodium salicylate, an inhibitor of ATM, NEMO (NF-κB essential modulator) and baricitinib, an inhibitor of JAK1/2, successfully prevented progeroid features [168,169]. Inflammation could also be alleviated via NF-κB activation using an inhibitor of the reprogramming repressor DOT1L (epz-4777) and MG132, which inhibits the secretion of proinflammatory cytokines [170,171].
Other Molecules
Protection and restoration of the nuclear lamina was also achieved in HGPS cells and mouse models after administration of remodelin, a chemical inhibitor of the N-acetyltransferase NAT10 [172,173]. In the same way, cellular senescence was addressed by senolytic drugs like ABT-737 [174] or quercetin and vitamin C [175]. Enhanced cellular proliferation was obtained using S-adenosyl-methionine and spermidine [176,177], and nuclear export balance was restored with leptomycin B, a pharmacological inhibitor of exportin-1, which is overexpressed in HGPS [178]. Improvements in DNA damage repair machinery and epigenetic modifications associated with HGPS was achieved after restoration of vitamin D receptors signalling, using 1α,25-dihydroxy vitamin D3 in HGPS fibroblasts [179]. Other promising treatments such as resveratrol and chloroquine, improved DNA damage response in cellular and mouse models by activation and stabilization of SIRT1 and SIRT6 respectively [180,181,182]. Another study demonstrated that in vivo induction of Oct4, Sox2, Klf4 and c-Myc in LmnaG609G/G609G mice ameliorated age-associated hallmarks [183]. Alternatively, growth hormone treatment (GH and IGF-1), given their impact on aging, also provides beneficial results in progeric conditions [34,184,185].
Dietary supplementation and even fecal transplantation were proposed and already show promising results against HGPS [186,187,188,189,190].
Although the therapeutic effects of numerous compounds have been demonstrated in vitro, some of them still need to be validated in vivo. Yet, altogether, these studies demonstrate that therapeutic benefits can be achieved without targeting progerin itself. Addressing therapies in HGPS associated to progerin accumulation may thus rely on multi-approaches combination.
5. Therapies for Other Laminopathies
5.1. Lipodystrophies
Dietary modifications and daily physical activity can help improving the metabolic complications of lipodystrophy, as well as insulin sensitizers (such as metformin) and lipid-lowering drugs (statins, fibrates). However, this risk associated with atherosclerotic vascular disease in patients with lipodystrophies promotes the need for novel therapy development and better patient care management. Currently, the most promising treatment for this disease is metreleptin, a recombinant leptin, however it is not widely approved at the present time, and newer leptin analogues are still being developed [197] reviewed in [147].
To allow potential drug screening, Wojtanik et al. [198] developed a mouse model, which highlighted an inability of the adipose tissue to self-renew, unlike the loss of fat suggested in the literature. Preclinical studies suggest the use of PPARγ agonists Thiazolidinediones and adiponectin upregulators as potential therapies, with only modest improvements observed in patients [194,199]. As described in HGPS, autophagy induction, statin and antioxidant treatments represent potential therapies also for FPLD2. Indeed, autophagy modulation mediated by Itm2a silencing rescued differentiation of 3T3-L1 mouse preadipocytes through the stabilization of PPARγ proteins [195]; and both Pravastatin and NAC reversed ROS production, inflammatory secretions and DNA damages in vitro [196].
5.2. Neuropathies
Similarly to what was done for SML and HGPS, a mouse model homozygous for the LMNA-related CMT2 mutation (p.Arg298Cys) has been developed. However, despite abnormalities in peripheral nerves, it did not show any disease phenotype [200], therefore slowing the search for therapeutically active molecules. Advances in treatments for lamin-associated neuropathies will have to rely on other CMT models and therapeutic approaches discussed in [201,202].
6. Concluding Remarks
After the first identification of a LMNA mutation in 1999, quickly followed by the implication of LMNA mutations in other disorders, research has rapidly focused on therapeutic approaches. The identification of numerous altered pathways opened pharmacological possibilities and led to the first clinical trial on HGPS patients in 2007 (#NCT00425607) with mitigated results. Despite huge phenotypical variabilities in laminopathies, similar preclinical approaches have been performed in SML, progeria or lipodystrophies. Hence, drugs used with success in a specific laminopathy might be of interest as well in other laminopathies.
Pharmacological approaches may lead quickly to clinical trials as some drugs are already approved by medical authorities in other diseases. However, considering the numerous altered pathways identified, these approaches are only able to slow down the progression of the diseases as they only tackle one or few of the altered pathways.
As for other genetic disorders, great hope is arising from gene therapy. As researchers continue developing in vivo gene editing and reducing the benefit risk ratio of such strategies, future investigations will determine if genetic correction can supplement drug therapies to tackle both LMNA mutations and downstream consequences.
Author Contributions
Writing—Original Draft Preparation: L.B., E.C.; Production of the review literature of preclinical works include in the manuscript: A.A.; Review & Editing: A.A., R.B.Y., G.B.; Writing—Review & Editing; supervision A.T.B.; Funding Acquisition A.T.B., G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the AFM-Telethon, the Institut National de la Santé et de la Recherche Médicale (INSERM), Sorbonne Université, Cure-CMD and Muscular Dystrophy-UK. L.B. received fellowship from MD-UK (#18GROI-PG24-0140) and Fondation Lefoulon-Delalande-Institut de France (2021 Awards). A.A., E.C. and G.B. are supported via the Solve-RD project funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 779257. R.B.Y. and A.T.B. are supported by AFM-Telethon. The APC was funded by MD-UK (#18GROI-PG24-0140).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- McKeon, F.D.; Kirschner, M.W.; Caput, D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 1986, 319, 463–468. [Google Scholar] [CrossRef]
- Hutchison, C.J. B-type lamins in health and disease. Semin. Cell Dev. Biol. 2014, 29, 158–163. [Google Scholar] [CrossRef]
- Tenga, R.; Medalia, O. Structure and unique mechanical aspects of nuclear lamin filaments. Curr. Opin. Struct. Biol. 2020, 64, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Turgay, Y.; Eibauer, M.; Goldman, A.E.; Shimi, T.; Khayat, M.; Ben-Harush, K.; Dubrovsky-Gaupp, A.; Sapra, K.T.; Goldman, R.D.; Medalia, O. The molecular architecture of lamins in somatic cells. Nature 2017, 543, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Naetar, N.; Ferraioli, S.; Foisner, R. Lamins in the nuclear interior–Life outside the lamina. J. Cell Sci. 2017, 130, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Bonne, G.; Barletta, M.R.D.; Varnous, S.; Bécane, H.-M.; Hammouda, E.-H.; Merlini, L.; Muntoni, F.; Greenberg, C.R.; Gary, F.; Urtizberea, J.-A.; et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999, 21, 285–288. [Google Scholar] [CrossRef]
- Quijano-Roy, S.; Mbieleu, B.; Bönnemann, C.G.; Jeannet, P.-Y.; Colomer, J.; Clarke, N.F.; Cuisset, J.-M.; Roper, H.; De Meirleir, L.; D’Amico, A.; et al. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann. Neurol. 2008, 64, 177–186. [Google Scholar] [CrossRef]
- Muchir, A. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum. Mol. Genet. 2000, 9, 1453–1459. [Google Scholar] [CrossRef]
- Madej-Pilarczyk, A. Clinical aspects of Emery-Dreifuss muscular dystrophy. Nucleus 2018, 9, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; MacRae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J.; Spudich, S.; De Girolami, U.; et al. Missense Mutations in the Rod Domain of the Lamin A/C Gene as Causes of Dilated Cardiomyopathy and Conduction-System Disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef]
- Becane, H.-M.; Bonne, G.; Varnous, S.; Muchir, A.; Ortega, V.; Hammouda, E.H.; Urtizberea, J.-A.; Lavergne, T.; Fardeau, M.; Eymard, B.; et al. High Incidence of Sudden Death with Conduction System and Myocardial Disease Due to Lamins A and C Gene Mutation. Pacing Clin. Electrophysiol. 2000, 23, 1661–1666. [Google Scholar] [CrossRef]
- Geiger, S.K.; Bär, H.; Ehlermann, P.; Wälde, S.; Rutschow, D.; Zeller, R.; Ivandic, B.T.; Zentgraf, H.; Katus, H.A.; Herrmann, H.; et al. Incomplete nonsense-mediated decay of mutant lamin A/C mRNA provokes dilated cardiomyopathy and ventricular tachycardia. J. Mol. Med. 2008, 86, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; Medioni, J.; Laluc, M.; Massart, C.; Arimura, T.; Kooi, A.J.V.D.; Desguerre, I.; Mayer, M.; Ferrer, X.; Briault, S.; et al. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve 2004, 30, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Favreau, C. Expression of Lamin A Mutated in the Carboxyl-Terminal Tail Generates an Aberrant Nuclear Phenotype Similar to That Observed in Cells from Patients with Dunnigan-Type Partial Lipodystrophy and Emery-Dreifuss Muscular Dystrophy. Exp. Cell Res. 2003, 282, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zwerger, M.; Jaalouk, D.E.; Lombardi, M.L.; Isermann, P.; Mauermann, M.; Dialynas, G.; Herrmann, H.; Wallrath, L.L.; Lammerding, J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 2013, 22, 2335–2349. [Google Scholar] [CrossRef]
- Raharjo, W.H.; Enarson, P.; Sullivan, T.; Stewart, C.L.; Burke, B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 2001, 114, 4447–4457. [Google Scholar] [CrossRef] [PubMed]
- Hubner, S.; Eam, J.; Hubner, A.; Jans, D. Laminopathy-inducing lamin A mutants can induce redistribution of lamin binding proteins into nuclear aggregates. Exp. Cell Res. 2006, 312, 171–183. [Google Scholar] [CrossRef]
- Sylvius, N.; Hathaway, A.; Boudreau, E.; Gupta, P.; Labib, S.; Bolongo, P.; Rippstein, P.; Mcbride, H.; Bilinska, Z.; Tesson, F. Specific contribution of lamin A and lamin C in the development of laminopathies. Exp. Cell Res. 2008, 314, 2362–2375. [Google Scholar] [CrossRef]
- Östlund, C.; Bonne, G.; Schwartz, K.; Worman, H.J. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J. Cell Sci. 2001, 114, 4435–4445. [Google Scholar] [CrossRef]
- Messner, M.; Ghadge, S.K.; Goetsch, V.; Wimmer, A.; Dörler, J.; Pölzl, G.; Zaruba, M.-M. Upregulation of the aging related LMNA splice variant progerin in dilated cardiomyopathy. PLoS ONE 2018, 13, e0196739. [Google Scholar] [CrossRef]
- Gupta, P.; Bilinska, Z.T.; Sylvius, N.; Boudreau, E.; Veinot, J.P.; Labib, S.; Bolongo, P.M.; Hamza, A.; Jackson, T.; Ploski, R.; et al. Genetic and ultrastructural studies in dilated cardiomyopathy patients: A large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res. Cardiol. 2010, 105, 365–377. [Google Scholar] [CrossRef]
- Bertero, A.; Fields, P.A.; Smith, A.S.T.; Leonard, A.; Beussman, K.; Sniadecki, N.J.; Kim, D.-H.; Tse, H.-F.; Pabon, L.; Shendure, J.; et al. Chromatin compartment dynamics in a haploinsufficient model of cardiac laminopathy. J. Cell Biol. 2019, 218, 2919–2944. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hunt, C.R.; Pandita, R.K.; Kumar, R.; Yang, C.-R.; Horikoshi, N.; Bachoo, R.; Serag, S.; Story, M.D.; Shay, J.W.; et al. Lamin A/C Depletion Enhances DNA Damage-Induced Stalled Replication Fork Arrest. Mol. Cell. Biol. 2013, 33, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of a-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.M.; Wang, L.; Alcalai, R.; Pizard, A.; Burgon, P.G.; Ahmad, F.; Sherwood, M.; Branco, D.M.; Wakimoto, H.; Fishman, G.I.; et al. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell. Cardiol. 2008, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Cattin, M.-E.; Bertrand, A.T.; Schlossarek, S.; Le Bihan, M.-C.; Skov Jensen, S.; Neuber, C.; Crocini, C.; Maron, S.; Lainé, J.; Mougenot, N.; et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum. Mol. Genet. 2013, 22, 3152–3164. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Menditto, I.; Degano, M.; Rodolico, C.; Merlini, L.; D’Amico, A.; Palmucci, L.; Berardinelli, A.; Pegoraro, E.; Trevisan, C.P.; et al. Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology 2007, 69, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.L.; De Sandre-Giovannoli, A.; Bernard, R.; Boccaccio, I.; Boyer, A.; Geneviève, D.; Hadj-Rabia, S.; Gaudy-Marqueste, C.; Smitt, H.S.; Vabres, P.; et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum. Mol. Genet. 2004, 13, 2493–2503. [Google Scholar] [CrossRef]
- Hutchinson, J. Congenital Absence of Hair and Mammary Glands with Atrophic Condition of the Skin and its Appendages in a Boy Whose Mother Had Been Almost Wholly Bald from Alopecia Areata from the Age of Six. J. R. Soc. Med. 1886, MCT-69, 473–477. [Google Scholar] [CrossRef]
- Gilford, H.; Hutchinson, J. On a Condition of Mixed Premature and Immature Development. J. R. Soc. Med. 1897, MCT-80, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Muchir, A.; Sangiuolo, F.; Helbling-Leclerc, A.; D’Apice, M.R.; Massart, C.; Capon, F.; Sbraccia, P.; Federici, M.; Lauro, R.; et al. Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C. Am. J. Hum. Genet. 2002, 71, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, L.; Kudlow, B.A.; Dos Santos, H.G.; Sletvold, O.; Shafeghati, Y.; Botha, E.G.; Garg, A.; Hanson, N.B.; Martin, G.M.; et al. LMNA mutations in atypical Werner’s syndrome. The Lancet 2003, 362, 440–445. [Google Scholar] [CrossRef]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.M.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B.; et al. Phenotype and Course of Hutchinson–Gilford Progeria Syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Hennekam, R.C.M. Hutchinson–Gilford progeria syndrome: Review of the phenotype. Am. J. Med. Genet. A 2006, 140A, 2603–2624. [Google Scholar] [CrossRef] [PubMed]
- De Sandre-Giovannoli, A. Lamin A Truncation in Hutchinson-Gilford Progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef]
- Goldman, R.D.; Shumaker, D.K.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Gordon, L.B.; Gruenbaum, Y.; Khuon, S.; Mendez, M.; Varga, R.; et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8963–8968. [Google Scholar] [CrossRef]
- Cao, H. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 2000, 9, 109–112. [Google Scholar] [CrossRef]
- Shackleton, S.; Lloyd, D.J.; Jackson, S.N.J.; Evans, R.; Niermeijer, M.F.; Singh, B.M.; Schmidt, H.; Brabant, G.; Kumar, S.; Durrington, P.N.; et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat. Genet. 2000, 24, 153–156. [Google Scholar] [CrossRef]
- Bonne, G.; Quijano-Roy, S. Emery–Dreifuss muscular dystrophy, laminopathies, and other nuclear envelopathies. In Handbook of Clinical Neurology; Elsevier B. V.: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1367–1376. ISBN 978-0-444-59565-2. [Google Scholar]
- Vadrot, N.; Duband-Goulet, I.; Cabet, E.; Attanda, W.; Barateau, A.; Vicart, P.; Gerbal, F.; Briand, N.; Vigouroux, C.; Oldenburg, A.R.; et al. The p.R482W substitution in A-type lamins deregulates SREBP1 activity in Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 2015, 24, 2096–2109. [Google Scholar] [CrossRef]
- De Sandre-Giovannoli, A.; Chaouch, M.; Kozlov, S.; Vallat, J.-M.; Tazir, M.; Kassouri, N.; Szepetowski, P.; Hammadouche, T.; Vandenberghe, A.; Stewart, C.L.; et al. Homozygous Defects in LMNA, Encoding Lamin A/C Nuclear-Envelope Proteins, Cause Autosomal Recessive Axonal Neuropathy in Human (Charcot-Marie-Tooth Disorder Type 2) and Mouse. Am. J. Hum. Genet. 2002, 70, 726–736. [Google Scholar] [CrossRef]
- Chaouch, M.; Allal, Y.; De Sandre-Giovannoli, A.; Vallat, J.M.; Amer-el-Khedoud, A.; Kassouri, N.; Chaouch, A.; Sindou, P.; Hammadouche, T.; Tazir, M.; et al. The phenotypic manifestations of autosomal recessive axonalCharcot–Marie–Tooth due to a mutation in Lamin A/C gene. Neuromuscul. Disord. 2003, 13, 60–67. [Google Scholar] [CrossRef]
- Tazir, M. Phenotypic variability in autosomal recessive axonal Charcot-Marie-Tooth disease due to the R298C mutation in lamin A/C. Brain 2004, 127, 154–163. [Google Scholar] [CrossRef]
- Azibani, F.; Muchir, A.; Vignier, N.; Bonne, G.; Bertrand, A.T. Striated muscle laminopathies. Semin. Cell Dev. Biol. 2014, 29, 107–115. [Google Scholar] [CrossRef]
- Jahn, D.; Schramm, S.; Schnölzer, M.; Heilmann, C.J.; De Koster, C.G.; Schütz, W.; Benavente, R.; Alsheimer, M. A truncated lamin A in the Lmna −/− mouse line: Implications for the understanding of laminopathies. Nucleus 2012, 3, 463–474. [Google Scholar] [CrossRef]
- Fong, L.G. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Investig. 2006, 116, 743–752. [Google Scholar] [CrossRef]
- Davies, B.S.J.; Barnes, R.H.; Tu, Y.; Ren, S.; Andres, D.A.; Spielmann, H.P.; Lammerding, J.; Wang, Y.; Young, S.G.; Fong, L.G. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum. Mol. Genet. 2010, 19, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Frock, R.L.; Chen, S.C.; Da, D.-F.; Frett, E.; Lau, C.; Brown, C.; Pak, D.N.; Wang, Y.; Muchir, A.; Worman, H.J.; et al. Cardiomyocyte-Specific Expression of Lamin A Improves Cardiac Function in Lmna−/− Mice. PLoS ONE 2012, 7, e42918. [Google Scholar] [CrossRef]
- Wang, Y. Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 2006, 15, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Scharner, J.; Figeac, N.; Ellis, J.A.; Zammit, P.S. Ameliorating pathogenesis by removing an exon containing a missense mutation: A potential exon-skipping therapy for laminopathies. Gene Ther. 2015, 22, 503–515. [Google Scholar] [CrossRef]
- Azibani, F.; Brull, A.; Arandel, L.; Beuvin, M.; Nelson, I.; Jollet, A.; Ziat, E.; Prudhon, B.; Benkhelifa-Ziyyat, S.; Bitoun, M.; et al. Gene Therapy via Trans-Splicing for LMNA-Related Congenital Muscular Dystrophy. Mol. Ther.—Nucleic Acids 2018, 10, 376–386. [Google Scholar] [CrossRef]
- Bertrand, A.T.; Renou, L.; Papadopoulos, A.; Beuvin, M.; Lacène, E.; Massart, C.; Ottolenghi, C.; Decostre, V.; Maron, S.; Schlossarek, S.; et al. DelK32-lamin A/C has abnormal location and induces incomplete tissue maturation and severe metabolic defects leading to premature death. Hum. Mol. Genet. 2012, 21, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, N.; Crasto, S.; Miragoli, M.; Bertero, A.; Paulis, M.; Kunderfranco, P.; Serio, S.; Forni, A.; Lucarelli, C.; Dal Ferro, M.; et al. The K219T-Lamin mutation induces conduction defects through epigenetic inhibition of SCN5A in human cardiac laminopathy. Nat. Commun. 2019, 10, 2267. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.G.; Slavov, D.; Gajewski, A.; Vlcek, S.; Ku, L.; Fain, P.R.; Carniel, E.; Di Lenarda, A.; Sinagra, G.; Boucek, M.M.; et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum. Mutat. 2005, 26, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.V.; Gnocchi, V.F.; Cohen, J.E.; Phadke, A.; Liu, H.; Ellis, J.A.; Foisner, R.; Stewart, C.L.; Zammit, P.S.; Partridge, T.A. Defective skeletal muscle growth in lamin A/C-deficient mice is rescued by loss of Lap2α. Hum. Mol. Genet. 2013, 22, 2852–2869. [Google Scholar] [CrossRef]
- Pilat, U.; Dechat, T.; Bertrand, A.T.; Woisetschläger, N.; Gotic, I.; Spilka, R.; Biadasiewicz, K.; Bonne, G.; Foisner, R. Muscle dystrophy-causing ΔK32 lamin A/C mutant does not impair functions of nucleoplasmic LAP2α—lamin A/C complexes in mice. J. Cell Sci. 2013, jcs.115246. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Rhee, J.-W.; Wu, J.C. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. 2016, 1, 831. [Google Scholar] [CrossRef]
- Arimura, T.; Helbling-Leclerc, A.; Massart, C.; Varnous, S.; Niel, F.; Lacène, E.; Fromes, Y.; Toussaint, M.; Mura, A.-M.; Keller, D.I.; et al. Mouse model carrying H222P- Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005, 14, 155–169. [Google Scholar] [CrossRef]
- Catelain, C.; Riveron, S.; Papadopoulos, A.; Mougenot, N.; Jacquet, A.; Vauchez, K.; Yada, E.; Pucéat, M.; Fiszman, M.; Butler-Browne, G.; et al. Myoblasts and Embryonic Stem Cells Differentially Engraft in a Mouse Model of Genetic Dilated Cardiomyopathy. Mol. Ther. 2013, 21, 1064–1075. [Google Scholar] [CrossRef]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Lee, Y.; Lau, Y.; Cai, Z.; Lai, W.; Wong, L.; Tse, H.; Ng, K.; Siu, C. Modeling Treatment Response for Lamin A/C Related Dilated Cardiomyopathy in Human Induced Pluripotent Stem Cells. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Briand, N.; Collas, P. Lamina-associated domains: Peripheral matters and internal affairs. Genome Biol. 2020, 21, 85. [Google Scholar] [CrossRef]
- Paulsen, J.; Sekelja, M.; Oldenburg, A.R.; Barateau, A.; Briand, N.; Delbarre, E.; Shah, A.; Sørensen, A.L.; Vigouroux, C.; Buendia, B.; et al. Chrom3D: Three-dimensional genome modeling from Hi-C and nuclear lamin-genome contacts. Genome Biol. 2017, 18, 21. [Google Scholar] [CrossRef]
- Perovanovic, J.; Dell’Orso, S.; Gnochi, V.F.; Jaiswal, J.K.; Sartorelli, V.; Vigouroux, C.; Mamchaoui, K.; Mouly, V.; Bonne, G.; Hoffman, E.P. Laminopathies disrupt epigenomic developmental programs and cell fate. Sci. Transl. Med. 2016, 8, 335ra58. [Google Scholar] [CrossRef]
- Cheedipudi, S.M.; Matkovich, S.J.; Coarfa, C.; Hu, X.; Robertson, M.J.; Sweet, M.; Taylor, M.; Mestroni, L.; Cleveland, J.; Willerson, J.T.; et al. Genomic Reorganization of Lamin-Associated Domains in Cardiac Myocytes Is Associated With Differential Gene Expression and DNA Methylation in Human Dilated Cardiomyopathy. Circ. Res. 2019, 124, 1198–1213. [Google Scholar] [CrossRef] [PubMed]
- Auguste, G.; Gurha, P.; Lombardi, R.; Coarfa, C.; Willerson, J.T.; Marian, A.J. Suppression of Activated FOXO Transcription Factors in the Heart Prolongs Survival in a Mouse Model of Laminopathies. Circ. Res. 2018, 122, 678–692. [Google Scholar] [CrossRef]
- Muchir, A.; Pavlidis, P.; Decostre, V.; Herron, A.J.; Arimura, T.; Bonne, G.; Worman, H.J. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J. Clin. Investig. 2007, 117, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Auguste, G.; Rouhi, L.; Matkovich, S.J.; Coarfa, C.; Robertson, M.J.; Czernuszewicz, G.; Gurha, P.; Marian, A.J. BET bromodomain inhibition attenuates cardiac phenotype in myocyte-specific lamin A/C–deficient mice. J. Clin. Investig. 2020, 130, 4740–4758. [Google Scholar] [CrossRef] [PubMed]
- Guénantin, A.-C.; Jebeniani, I.; Leschik, J.; Watrin, E.; Bonne, G.; Vignier, N.; Pucéat, M. Targeting the histone demethylase LSD1 prevents cardiomyopathy in a mouse model of laminopathy. J. Clin. Investig. 2021, 131, e136488. [Google Scholar] [CrossRef] [PubMed]
- Earle, A.J.; Kirby, T.J.; Fedorchak, G.R.; Isermann, P.; Patel, J.; Iruvanti, S.; Moore, S.A.; Bonne, G.; Wallrath, L.L.; Lammerding, J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2020, 19, 464–473. [Google Scholar] [CrossRef]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920–935. [Google Scholar] [CrossRef]
- Dworak, N.; Makosa, D.; Chatterjee, M.; Jividen, K.; Yang, C.-S.; Snow, C.; Simke, W.C.; Johnson, I.G.; Kelley, J.B.; Paschal, B.M. A nuclear lamina-chromatin-Ran GTPase axis modulates nuclear import and DNA damage signaling. Aging Cell 2019, 18, e12851. [Google Scholar] [CrossRef]
- Shimi, T.; Goldman, R.D. Nuclear Lamins and Oxidative Stress in Cell Proliferation and Longevity. In Cancer Biology and the Nuclear Envelope; Schirmer, E.C., De las Heras, J.I., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 773, pp. 415–430. ISBN 978-1-4899-8031-1. [Google Scholar]
- Maynard, S.; Keijzers, G.; Akbari, M.; Ezra, M.B.; Hall, A.; Morevati, M.; Scheibye-Knudsen, M.; Gonzalo, S.; Bartek, J.; Bohr, V.A. Lamin A/C promotes DNA base excision repair. Nucleic Acids Res. 2019, 4747, 11709–11728. [Google Scholar] [CrossRef]
- Rodriguez, B.M.; Khouzami, L.; Decostre, V.; Varnous, S.; Pekovic-Vaughan, V.; Hutchison, C.J.; Pecker, F.; Bonne, G.; Muchir, A. N-acetyl cysteine alleviates oxidative stress and protects mice from dilated cardiomyopathy caused by mutations in nuclear A-type lamins gene. Hum. Mol. Genet. 2018, 27, 3353–3360. [Google Scholar] [CrossRef]
- Chatzifrangkeskou, M.; Yadin, D.; Marais, T.; Chardonnet, S.; Cohen-Tannoudji, M.; Mougenot, N.; Schmitt, A.; Crasto, S.; Di Pasquale, E.; Macquart, C.; et al. Cofilin-1 phosphorylation catalyzed by ERK1/2 alters cardiac actin dynamics in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018, 27, 3060–3078. [Google Scholar] [CrossRef]
- Le Dour, C.; Wu, W.; Béréziat, V.; Capeau, J.; Vigouroux, C.; Worman, H.J. Extracellular matrix remodeling and transforming growth factor-β signaling abnormalities induced by lamin A/C variants that cause lipodystrophy. J. Lipid Res. 2017, 58, 151–163. [Google Scholar] [CrossRef]
- Bernasconi, P.; Carboni, N.; Ricci, G.; Siciliano, G.; Politano, L.; Maggi, L.; Mongini, T.; Vercelli, L.; Rodolico, C.; Biagini, E.; et al. Elevated TGF β2 serum levels in Emery-Dreifuss Muscular Dystrophy: Implications for myocyte and tenocyte differentiation and fibrogenic processes. Nucleus 2018, 9, 337–349. [Google Scholar] [CrossRef]
- Chen, S.N.; Sbaizero, O.; Taylor, M.R.G.; Mestroni, L. Lamin A/C Cardiomyopathy: Implications for Treatment. Curr. Cardiol. Rep. 2019, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Vignier, N.; Chatzifrangkeskou, M.; Morales Rodriguez, B.; Mericskay, M.; Mougenot, N.; Wahbi, K.; Bonne, G.; Muchir, A. Rescue of biosynthesis of nicotinamide adenine dinucleotide protects the heart in cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018, 27, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Navarro-Puche, A.; Casar, B.; Crespo, P.; Andrés, V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J. Cell Biol. 2008, 183, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; Wu, W.; Choi, J.C.; Iwata, S.; Morrow, J.; Homma, S.; Worman, H.J. Abnormal p38 mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2012, 21, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; Reilly, S.A.; Wu, W.; Iwata, S.; Homma, S.; Bonne, G.; Worman, H.J. Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc. Res. 2012, 93, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; Kim, Y.; Reilly, S.A.; Wu, W.; Choi, J.C.; Worman, H.J. Inhibition of extracellular signal-regulated kinase 1/2 signaling has beneficial effects on skeletal muscle in a mouse model of Emery-Dreifuss muscular dystrophy caused by lamin A/C gene mutation. Skelet. Muscle 2013, 3, 17. [Google Scholar] [CrossRef]
- Wu, W.; Chordia, M.D.; Hart, B.P.; Kumarasinghe, E.S.; Ji, M.K.; Bhargava, A.; Lawlor, M.W.; Shin, J.-Y.; Sera, F.; Homma, S.; et al. Macrocyclic MEK1/2 inhibitor with efficacy in a mouse model of cardiomyopathy caused by lamin A/C gene mutation. Bioorg. Med. Chem. 2017, 25, 1004–1013. [Google Scholar] [CrossRef][Green Version]
- Muchir, A.; Shan, J.; Bonne, G.; Lehnart, S.E.; Worman, H.J. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet. 2008, 18, 241–247. [Google Scholar] [CrossRef]
- Muchir, A.; Wu, W.; Sera, F.; Homma, S.; Worman, H.J. Mitogen-activated protein kinase kinase 1/2 inhibition and angiotensin II converting inhibition in mice with cardiomyopathy caused by lamin A/C gene mutation. Biochem. Biophys. Res. Commun. 2014, 452, 958–961. [Google Scholar] [CrossRef]
- Wu, W.; Muchir, A.; Shan, J.; Bonne, G.; Worman, H.J. Mitogen-Activated Protein Kinase Inhibitors Improve Heart Function and Prevent Fibrosis in Cardiomyopathy Caused by Mutation in Lamin A/C Gene. Circulation 2011, 123, 53–61. [Google Scholar] [CrossRef]
- Wu, W.; Shan, J.; Bonne, G.; Worman, H.J.; Muchir, A. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2010, 1802, 632–638. [Google Scholar] [CrossRef]
- Chatzifrangkeskou, M.; Le Dour, C.; Wu, W.; Morrow, J.P.; Joseph, L.C.; Beuvin, M.; Sera, F.; Homma, S.; Vignier, N.; Mougenot, N.; et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet. 2016, 25, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Wong, J.X.; Chan, P.S.; Tan, H.; Liao, D.; Chen, W.; Tan, L.W.; Ackers-Johnson, M.; Wakimoto, H.; Seidman, J.G.; et al. Yin Yang 1 Suppresses Dilated Cardiomyopathy and Cardiac Fibrosis Through Regulation of Bmp7 and Ctgf. Circ. Res. 2019, 125, 834–846. [Google Scholar] [CrossRef]
- Vignier, N.; Chatzifrangkeskou, M.; Pinton, L.; Wioland, H.; Marais, T.; Lemaitre, M.; Le Dour, C.; Peccate, C.; Cardoso, D.; Schmitt, A.; et al. The non-muscle ADF/cofilin-1 controls sarcomeric actin filament integrity and force production in striated muscle laminopathies. Cell Rep. 2021, 36, 109601. [Google Scholar] [CrossRef]
- Mounkes, L.C.; Kozlov, S.V.; Rottman, J.N.; Stewart, C.L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 2005, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kennedy, B.K.; Lampe, P.D. Phosphorylation of connexin43 on S279/282 may contribute to laminopathy-associated conduction defects. Exp. Cell Res. 2013, 319, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Macquart, C.; Jüttner, R.; Morales Rodriguez, B.; Le Dour, C.; Lefebvre, F.; Chatzifrangkeskou, M.; Schmitt, A.; Gotthardt, M.; Bonne, G.; Muchir, A. Microtubule cytoskeleton regulates Connexin 43 localization and cardiac conduction in cardiomyopathy caused by mutation in A-type lamins gene. Hum. Mol. Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Borin, D.; Peña, B.; Chen, S.N.; Long, C.S.; Taylor, M.R.G.; Mestroni, L.; Sbaizero, O. Altered microtubule structure, hemichannel localization and beating activity in cardiomyocytes expressing pathologic nuclear lamin A/C. Heliyon 2020, 6, e03175. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.; Leimena, C.; McMahon, A.C.; Tan, J.C.; Chandar, S.; Jogia, D.; Kesteven, S.H.; Michalicek, J.; Otway, R.; Verheyen, F.; et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice. J. Clin. Investig. 2004, 113, 357–369. [Google Scholar] [CrossRef]
- Galata, Z.; Kloukina, I.; Kostavasili, I.; Varela, A.; Davos, C.H.; Makridakis, M.; Bonne, G.; Capetanaki, Y. Amelioration of desmin network defects by αB-crystallin overexpression confers cardioprotection in a mouse model of dilated cardiomyopathy caused by LMNA gene mutation. J. Mol. Cell. Cardiol. 2018, 125, 73–86. [Google Scholar] [CrossRef]
- Moon, R.T.; Kohn, A.D.; Ferrari, G.V.D.; Kaykas, A. WNT and β-catenin signalling: Diseases and therapies. Nat. Rev. Genet. 2004, 5, 691–701. [Google Scholar] [CrossRef]
- Le Dour, C.; Macquart, C.; Sera, F.; Homma, S.; Bonne, G.; Morrow, J.P.; Worman, H.J.; Muchir, A. Decreased WNT/β-catenin signalling contributes to the pathogenesis of dilated cardiomyopathy caused by mutations in the lamin a/C gene. Hum. Mol. Genet. 2017, 26, 333–343. [Google Scholar] [CrossRef]
- Gerbino, A.; Bottillo, I.; Milano, S.; Lipari, M.; Zio, R.D.; Morlino, S.; Mola, M.G.; Procino, G.; Re, F.; Zachara, E.; et al. Functional Characterization of a Novel Truncating Mutation in Lamin A/C Gene in a Family with a Severe Cardiomyopathy with Conduction Defects. Cell. Physiol. Biochem. 2017, 44, 1559–1577. [Google Scholar] [CrossRef]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian Target of Rapamycin Signaling in Cardiac Physiology and Disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; Chen, S.C.; Garelick, M.G.; Dai, D.-F.; Liao, C.-Y.; Schreiber, K.H.; MacKay, V.L.; An, E.H.; Strong, R.; Ladiges, W.C.; et al. Rapamycin Reverses Elevated mTORC1 Signaling in Lamin A/C-Deficient Mice, Rescues Cardiac and Skeletal Muscle Function, and Extends Survival. Sci. Transl. Med. 2012, 4, 144ra103. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.C.; Muchir, A.; Wu, W.; Iwata, S.; Homma, S.; Morrow, J.P.; Worman, H.J. Temsirolimus Activates Autophagy and Ameliorates Cardiomyopathy Caused by Lamin A/C Gene Mutation. Sci. Transl. Med. 2012, 4, 144ra102. [Google Scholar] [CrossRef] [PubMed]
- DuBose, A.J.; Lichtenstein, S.T.; Petrash, N.M.; Erdos, M.R.; Gordon, L.B.; Collins, F.S. Everolimus rescues multiple cellular defects in laminopathy-patient fibroblasts. Proc. Natl. Acad. Sci. USA 2018, 115, 4206–4211. [Google Scholar] [CrossRef]
- Siu, C.-W.; Lee, Y.-K.; Ho, J.C.-Y.; Lai, W.-H.; Chan, Y.-C.; Ng, K.-M.; Wong, L.-Y.; Au, K.-W.; Lau, Y.-M.; Zhang, J.; et al. Modeling of lamin A/C mutation premature cardiac aging using patient-specific induced pluripotent stem cells. Aging 2012, 4, 803–822. [Google Scholar] [CrossRef]
- Wu, W.; Iwata, S.; Homma, S.; Worman, H.J.; Muchir, A. Depletion of extracellular signal-regulated kinase 1 in mice with cardiomyopathy caused by lamin A/C gene mutation partially prevents pathology before isoenzyme activation. Hum. Mol. Genet. 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Anderson, S.S.; Chicoine, N.H.; Mayfield, J.R.; Garrett, B.J.; Kwok, C.S.; Academia, E.C.; Hsu, Y.-M.; Miller, D.M.; Bair, A.M.; et al. Evidence that S6K1, but not 4E-BP1, mediates skeletal muscle pathology associated with loss of A-type lamins. Cell Discov. 2017, 3, 17039. [Google Scholar] [CrossRef]
- Choi, J.C.; Wu, W.; Phillips, E.; Plevin, R.; Sera, F.; Homma, S.; Worman, H.J. Elevated dual specificity protein phosphatase 4 in cardiomyopathy caused by lamin A/C gene mutation is primarily ERK1/2-dependent and its depletion improves cardiac function and survival. Hum. Mol. Genet. 2018, 27, 2290–2305. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat. Med. 2005, 11, 440–445. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jung, Y.-S.; Yoon, M.-H.; Kang, S.; Oh, A.-Y.; Lee, J.-H.; Jun, S.-Y.; Woo, T.-G.; Chun, H.-Y.; Kim, S.K.; et al. Interruption of progerin–lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J. Clin. Investig. 2016, 126, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.G.; Vickers, T.A.; Farber, E.A.; Choi, C.; Yun, U.J.; Hu, Y.; Yang, S.H.; Coffinier, C.; Lee, R.; Yin, L.; et al. Activating the synthesis of progerin, the mutant prelamin A in Hutchinson–Gilford progeria syndrome, with antisense oligonucleotides. Hum. Mol. Genet. 2009, 18, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.G.; Navarro, C.L.; Cadinanos, J.; Lopez-Mejia, I.C.; Quiros, P.M.; Bartoli, C.; Rivera, J.; Tazi, J.; Guzman, G.; Varela, I.; et al. Splicing-Directed Therapy in a New Mouse Model of Human Accelerated Aging. Sci. Transl. Med. 2011, 3, 106ra107. [Google Scholar] [CrossRef] [PubMed]
- Harhouri, K.; Navarro, C.; Baquerre, C.; Da Silva, N.; Bartoli, C.; Casey, F.; Mawuse, G.; Doubaj, Y.; Lévy, N.; De Sandre-Giovannoli, A. Antisense-Based Progerin Downregulation in HGPS-Like Patients’ Cells. Cells 2016, 5, 31. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L.; Libina, N.; Janes, J.; Martin, G.M.; Campisi, J.; Oshima, J. Correction of cellular phenotypes of Hutchinson-Gilford Progeria cells by RNA interference. Hum. Genet. 2005, 118, 444–450. [Google Scholar] [CrossRef]
- Jung, H.-J.; Tu, Y.; Yang, S.H.; Tatar, A.; Nobumori, C.; Wu, D.; Young, S.G.; Fong, L.G. New Lmna knock-in mice provide a molecular mechanism for the ‘segmental aging’ in Hutchinson–Gilford progeria syndrome. Hum. Mol. Genet. 2014, 23, 1506–1515. [Google Scholar] [CrossRef]
- Jung, H.-J.; Coffinier, C.; Choe, Y.; Beigneux, A.P.; Davies, B.S.J.; Yang, S.H.; Barnes, R.H.; Hong, J.; Sun, T.; Pleasure, S.J.; et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. USA 2012, 109, E423–E431. [Google Scholar] [CrossRef]
- Frankel, D.; Delecourt, V.; Harhouri, K.; De Sandre-Giovannoli, A.; Lévy, N.; Kaspi, E.; Roll, P. MicroRNAs in hereditary and sporadic premature aging syndromes and other laminopathies. Aging Cell 2018, 17, e12766. [Google Scholar] [CrossRef]
- Santiago-Fernández, O.; Osorio, F.G.; Quesada, V.; Rodríguez, F.; Basso, S.; Maeso, D.; Rolas, L.; Barkaway, A.; Nourshargh, S.; Folgueras, A.R.; et al. Development of a CRISPR/Cas9-based therapy for Hutchinson–Gilford progeria syndrome. Nat. Med. 2019, 25, 423–426. [Google Scholar] [CrossRef]
- Beyret, E.; Liao, H.-K.; Yamamoto, M.; Hernandez-Benitez, R.; Fu, Y.; Erikson, G.; Reddy, P.; Izpisua Belmonte, J.C. Single-dose CRISPR–Cas9 therapy extends lifespan of mice with Hutchinson–Gilford progeria syndrome. Nat. Med. 2019, 25, 419–422. [Google Scholar] [CrossRef]
- Harhouri, K.; Frankel, D.; Bartoli, C.; Roll, P.; De Sandre-Giovannoli, A.; Lévy, N. An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus 2018, 9, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-H.; Suzuki, K.; Qu, J.; Sancho-Martinez, I.; Yi, F.; Li, M.; Kumar, S.; Nivet, E.; Kim, J.; Soligalla, R.D.; et al. Targeted Gene Correction of Laminopathy-Associated LMNA Mutations in Patient-Specific iPSCs. Cell Stem Cell 2011, 8, 688–694. [Google Scholar] [CrossRef]
- Endisha, H.; Merrill-Schools, J.; Zhao, M.; Bristol, M.; Wang, X.; Kubben, N.; Elmore, L.W. Restoring SIRT6 Expression in Hutchinson-Gilford Progeria Syndrome Cells Impedes Premature Senescence and Formation of Dysmorphic Nuclei. Pathobiology 2015, 82, 9–20. [Google Scholar] [CrossRef]
- Koblan, L.W.; Erdos, M.R.; Wilson, C.; Cabral, W.A.; Levy, J.M.; Xiong, Z.-M.; Tavarez, U.L.; Davison, L.M.; Gete, Y.G.; Mao, X.; et al. In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature 2021, 589, 608–614. [Google Scholar] [CrossRef]
- Gete, Y.G.; Koblan, L.W.; Mao, X.; Trappio, M.; Mahadik, B.; Fisher, J.P.; Liu, D.R.; Cao, K. Mechanisms of angiogenic incompetence in Hutchinson–Gilford progeria via downregulation of endothelial NOS. Aging Cell 2021, 20. [Google Scholar] [CrossRef]
- Mojiri, A.; Walther, B.K.; Jiang, C.; Matrone, G.; Holgate, R.; Xu, Q.; Morales, E.; Wang, G.; Gu, J.; Wang, R.; et al. Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. Eur. Heart J. 2021, ehab547. [Google Scholar] [CrossRef] [PubMed]
- Capell, B.C.; Erdos, M.R.; Madigan, J.P.; Fiordalisi, J.J.; Varga, R.; Conneely, K.N.; Gordon, L.B.; Der, C.J.; Cox, A.D.; Collins, F.S. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 12879–12884. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.G. A Protein Farnesyltransferase Inhibitor Ameliorates Disease in a Mouse Model of Progeria. Science 2006, 311, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.W.; Glover, T.W. Incomplete processing of mutant lamin A in Hutchinson–Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 2005, 14, 2959–2969. [Google Scholar] [CrossRef]
- Pacheco, L.M.; Gomez, L.A.; Dias, J.; Ziebarth, N.M.; Howard, G.A.; Schiller, P.C. Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging 2014, 6, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Torres, J.; Acín-Perez, R.; Cabezas-Sánchez, P.; Osorio, F.G.; Gonzalez-Gómez, C.; Megias, D.; Cámara, C.; López-Otín, C.; Enríquez, J.A.; Luque-García, J.L.; et al. Identification of mitochondrial dysfunction in Hutchinson–Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture. J. Proteom. 2013, 91, 466–477. [Google Scholar] [CrossRef]
- Toth, J.I.; Yang, S.H.; Qiao, X.; Beigneux, A.P.; Gelb, M.H.; Moulson, C.L.; Miner, J.H.; Young, S.G.; Fong, L.G. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA 2005, 102, 12873–12878. [Google Scholar] [CrossRef]
- Yang, S.H.; Bergo, M.O.; Toth, J.I.; Qiao, X.; Hu, Y.; Sandoval, S.; Meta, M.; Bendale, P.; Gelb, M.H.; Young, S.G.; et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. USA 2005, 102, 10291–10296. [Google Scholar] [CrossRef]
- Capell, B.C.; Olive, M.; Erdos, M.R.; Cao, K.; Faddah, D.A.; Tavarez, U.L.; Conneely, K.N.; Qu, X.; San, H.; Ganesh, S.K.; et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 15902–15907. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Investig. 2006, 116, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Treatment with a farnesyltransferase inhibitor improves survival in mice with a Hutchinson–Gilford progeria syndrome mutation. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2008, 1781, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Varela, I.; Pereira, S.; Ugalde, A.P.; Navarro, C.L.; Suárez, M.F.; Cau, P.; Cadiñanos, J.; Osorio, F.G.; Foray, N.; Cobo, J.; et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat. Med. 2008, 14, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Blondel, S.; Egesipe, A.-L.; Picardi, P.; Jaskowiak, A.-L.; Notarnicola, M.; Ragot, J.; Tournois, J.; Le Corf, A.; Brinon, B.; Poydenot, P.; et al. Drug screening on Hutchinson Gilford progeria pluripotent stem cells reveals aminopyrimidines as new modulators of farnesylation. Cell Death Dis. 2016, 7, e2105. [Google Scholar] [CrossRef]
- Ibrahim, M.X.; Sayin, V.I.; Akula, M.K.; Liu, M.; Fong, L.G.; Young, S.G.; Bergo, M.O. Targeting Isoprenylcysteine Methylation Ameliorates Disease in a Mouse Model of Progeria. Science 2013, 340, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Blondel, S.; Jaskowiak, A.-L.; Egesipe, A.-L.; Le Corf, A.; Navarro, C.; Cordette, V.; Martinat, C.; Laabi, Y.; Djabali, K.; De Sandre-Giovannoli, A.; et al. Induced Pluripotent Stem Cells Reveal Functional Differences Between Drugs Currently Investigated in Patients With Hutchinson-Gilford Progeria Syndrome. STEM CELLS Transl. Med. 2014, 3, 510–519. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Miller, D.T.; Neuberg, D.S.; Giobbie-Hurder, A.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.; Snyder, B.D.; et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16666–16671. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, N.J.; Kieran, M.W.; Miller, D.T.; Gordon, L.B.; Cho, Y.-J.; Silvera, V.M.; Giobbie-Hurder, A.; Neuberg, D.; Kleinman, M.E. Neurologic features of Hutchinson-Gilford progeria syndrome after lonafarnib treatment. Neurology 2013, 81, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Shappell, H.; Massaro, J.; D’Agostino, R.B.; Brazier, J.; Campbell, S.E.; Kleinman, M.E.; Kieran, M.W. Association of Lonafarnib Treatment vs No Treatment with Mortality Rate in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA 2018, 319, 1687. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B.; Shappell, H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Atalaia, A.; Ben Yaou, R.; Wahbi, K.; De Sandre-Giovannoli, A.; Vigouroux, C.; Bonne, G. Laminopathies’ Treatments Systematic Review: A Contribution Towards a ‘Treatabolome’. J. Neuromuscul. Dis. 2021, 8, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Graziotto, J.J.; Blair, C.D.; Mazzulli, J.R.; Erdos, M.R.; Krainc, D.; Collins, F.S. Rapamycin Reverses Cellular Phenotypes and Enhances Mutant Protein Clearance in Hutchinson-Gilford Progeria Syndrome Cells. Sci. Transl. Med. 2011, 3, 89ra58. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Columbaro, M.; Ortolani, M.; D’Apice, M.R.; Novelli, G.; Fini, M.; Marmiroli, S.; Scarano, E.; Maraldi, N.M.; et al. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur. J. Histochem. 2011, 55, 36. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Hambright, W.S.; Takayama, K.; Mu, X.; Lu, A.; Cummins, J.H.; Matsumoto, T.; Yurube, T.; Kuroda, R.; Kurosaka, M.; et al. Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice. Mol. Ther.—Methods Clin. Dev. 2019, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Mattioli, E.; Columbaro, M.; Wehnert, M.; Ortolani, M.; Fini, M.; Novelli, G.; Bertacchini, J.; Maraldi, N.M.; et al. Rapamycin treatment of Mandibuloacral Dysplasia cells rescues localization of chromatin-associated proteins and cell cycle dynamics. Aging 2014, 6, 755–769. [Google Scholar] [CrossRef]
- Gabriel, D.; Roedl, D.; Gordon, L.B.; Djabali, K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell 2015, 14, 78–91. [Google Scholar] [CrossRef]
- Gabriel, D.; Gordon, L.B.; Djabali, K. Temsirolimus Partially Rescues the Hutchinson-Gilford Progeria Cellular Phenotype. PLoS ONE 2016, 11, e0168988. [Google Scholar] [CrossRef]
- Bikkul, M.U.; Clements, C.S.; Godwin, L.S.; Goldberg, M.W.; Kill, I.R.; Bridger, J.M. Farnesyltransferase inhibitor and rapamycin correct aberrant genome organisation and decrease DNA damage respectively, in Hutchinson–Gilford progeria syndrome fibroblasts. Biogerontology 2018, 19, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Shafry, D.D.; Gordon, L.B.; Djabali, K. Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson-Gilford progeria fibroblasts. Oncotarget 2017, 8, 64809–64826. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Columbaro, M.; Capanni, C.; D’Apice, M.R.; Cavallo, C.; Murdocca, M.; Lattanzi, G.; Squarzoni, S. All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype. Oncotarget 2015, 6, 29914–29928. [Google Scholar] [CrossRef] [PubMed]
- Harhouri, K.; Navarro, C.; Depetris, D.; Mattei, M.; Nissan, X.; Cau, P.; De Sandre-Giovannoli, A.; Lévy, N. MG 132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol. Med. 2017, 9, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Egesipe, A.-L.; Blondel, S.; Lo Cicero, A.; Jaskowiak, A.-L.; Navarro, C.; Sandre-Giovannoli, A.D.; Levy, N.; Peschanski, M.; Nissan, X. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson–Gilford progeria syndrome cells. NPJ Aging Mech. Dis. 2016, 2, 16026. [Google Scholar] [CrossRef]
- Park, S.-K.; Shin, O.S. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp. Dermatol. 2017, 26, 889–895. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, M.-H.; Ahn, J.; Kim, J.-E.; Kim, S.Y.; Kang, S.Y.; Joo, J.; Park, S.; Cho, J.-H.; Woo, T.-G.; et al. Progerinin, an optimized progerin-lamin A binding inhibitor, ameliorates premature senescence phenotypes of Hutchinson-Gilford progeria syndrome. Commun. Biol. 2021, 4, 5. [Google Scholar] [CrossRef]
- Richards, S.A.; Muter, J.; Ritchie, P.; Lattanzi, G.; Hutchison, C.J. The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum. Mol. Genet. 2011, 20, 3997–4004. [Google Scholar] [CrossRef]
- Xiong, Z.; Choi, J.Y.; Wang, K.; Zhang, H.; Tariq, Z.; Wu, D.; Ko, E.; LaDana, C.; Sesaki, H.; Cao, K. Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell 2016, 15, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Park, J.T.; Choi, K.; Choi, H.J.C.; Jung, C.W.; Kim, G.R.; Lee, Y.-S.; Park, S.C. Chemical screening identifies ROCK as a target for recovering mitochondrial function in Hutchinson-Gilford progeria syndrome. Aging Cell 2017, 16, 541–550. [Google Scholar] [CrossRef]
- Kuk, M.U.; Kim, J.W.; Lee, Y.-S.; Cho, K.A.; Park, J.T.; Park, S.C. Alleviation of Senescence via ATM Inhibition in Accelerated Aging Models. Mol. Cells 2019, 42, 210–217. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Rivera-Torres, J.; Osorio, F.G.; Acín-Pérez, R.; Enriquez, J.A.; López-Otín, C.; Andrés, V. Defective Extracellular Pyrophosphate Metabolism Promotes Vascular Calcification in a Mouse Model of Hutchinson-Gilford Progeria Syndrome That Is Ameliorated on Pyrophosphate Treatment. Circulation 2013, 127, 2442–2451. [Google Scholar] [CrossRef]
- Kubben, N.; Zhang, W.; Wang, L.; Voss, T.C.; Yang, J.; Qu, J.; Liu, G.-H.; Misteli, T. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell 2016, 165, 1361–1374. [Google Scholar] [CrossRef]
- Cui, W.; Bai, Y.; Luo, P.; Miao, L.; Cai, L. Preventive and Therapeutic Effects of MG132 by Activating Nrf2-ARE Signaling Pathway on Oxidative Stress-Induced Cardiovascular and Renal Injury. Oxid. Med. Cell. Longev. 2013, 2013, 306073. [Google Scholar] [CrossRef]
- Osorio, F.G.; López-Otín, C.; Freije, J.M.P. NF-κB in premature aging. Aging 2012, 4, 726–727. [Google Scholar] [CrossRef]
- Liu, C.; Arnold, R.; Henriques, G.; Djabali, K. Inhibition of JAK-STAT Signaling with Baricitinib Reduces Inflammation and Improves Cellular Homeostasis in Progeria Cells. Cells 2019, 8, 1276. [Google Scholar] [CrossRef]
- Ortiz-Lazareno, P.C.; Hernandez-Flores, G.; Dominguez-Rodriguez, J.R.; Lerma-Diaz, J.M.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A.; Gomez-Contreras, P.C.; Scott-Algara, D.; Bravo-Cuellar, A. MG132 proteasome inhibitor modulates proinflammatory cytokines production and expression of their receptors in U937 cells: Involvement of nuclear factor-κB and activator protein-1. Immunology 2008, 124, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Ahmed, M.; Li, J.; Gu, H.F.; Bakalkin, G.; Stark, A.; Harris, H.E. Proteasome inhibitor MG132 modulates inflammatory pain by central mechanisms in adjuvant arthritis. Int. J. Rheum. Dis. 2017, 20, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, D.; Britton, S.; Demir, M.; Rodriguez, R.; Jackson, S.P. Chemical Inhibition of NAT10 Corrects Defects of Laminopathic Cells. Science 2014, 344, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Balmus, G.; Larrieu, D.; Barros, A.C.; Collins, C.; Abrudan, M.; Demir, M.; Geisler, N.J.; Lelliott, C.J.; White, J.K.; Karp, N.A.; et al. Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat. Commun. 2018, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, Z.; Zhang, W.; Li, W.; Wu, Z.; Wang, W.; Ren, R.; Su, Y.; Wang, P.; Sun, L.; et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.; Fafián-Labora, J.; Morente-López, M.; Lesende-Rodriguez, I.; Monserrat, L.; Ódena, M.A.; De Oliveira, E.; De Toro, J.; Arufe, M.C. Next-Generation Sequencing and Quantitative Proteomics of Hutchinson-Gilford progeria syndrome-derived cells point to a role of nucleotide metabolism in premature aging. PLoS ONE 2018, 13, e0205878. [Google Scholar] [CrossRef]
- Ao, Y.; Zhang, J.; Liu, Z.; Qian, M.; Li, Y.; Wu, Z.; Sun, P.; Wu, J.; Bei, W.; Wen, J.; et al. Lamin A buffers CK2 kinase activity to modulate aging in a progeria mouse model. Sci. Adv. 2019, 5, eaav5078. [Google Scholar] [CrossRef]
- García-Aguirre, I.; Alamillo-Iniesta, A.; Rodríguez-Pérez, R.; Vélez-Aguilera, G.; Amaro-Encarnación, E.; Jiménez-Gutiérrez, E.; Vásquez-Limeta, A.; Samuel Laredo-Cisneros, M.; Morales-Lázaro, S.L.; Tiburcio-Félix, R.; et al. Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity. Aging Cell 2019, 18, e13002. [Google Scholar] [CrossRef]
- Kreienkamp, R.; Croke, M.; Neumann, M.A.; Bedia-Diaz, G.; Graziano, S.; Dusso, A.; Dorsett, D.; Carlberg, C.; Gonzalo, S. Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes. Oncotarget 2016, 7, 30018–30031. [Google Scholar] [CrossRef]
- Liu, B.; Ghosh, S.; Yang, X.; Zheng, H.; Liu, X.; Wang, Z.; Jin, G.; Zheng, B.; Kennedy, B.K.; Suh, Y.; et al. Resveratrol Rescues SIRT1-Dependent Adult Stem Cell Decline and Alleviates Progeroid Features in Laminopathy-Based Progeria. Cell Metab. 2012, 16, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Liu, Z.; Peng, L.; Tang, X.; Meng, F.; Ao, Y.; Zhou, M.; Wang, M.; Cao, X.; Qin, B.; et al. Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria. eLife 2018, 7, e34836. [Google Scholar] [CrossRef]
- Mattioli, E.; Andrenacci, D.; Mai, A.; Valente, S.; Robijns, J.; De Vos, W.H.; Capanni, C.; Lattanzi, G. Statins and Histone Deacetylase Inhibitors Affect Lamin A/C—Histone Deacetylase 2 Interaction in Human Cells. Front. Cell Dev. Biol. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Ugalde, A.P.; Fernandez, A.F.; Osorio, F.G.; Fueyo, A.; Freije, J.M.P.; Lopez-Otin, C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. USA 2010, 107, 16268–16273. [Google Scholar] [CrossRef] [PubMed]
- Abdenur, J.E.; Brown, W.T.; Friedman, S.; Smith, M.; Lifshitz, F. Response to nutritional and growth hormone treatment in progeria. Metabolism 1997, 46, 851–856. [Google Scholar] [CrossRef]
- Bárcena, C.; Quirós, P.M.; Durand, S.; Mayoral, P.; Rodríguez, F.; Caravia, X.M.; Mariño, G.; Garabaya, C.; Fernández-García, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; López-Otín, C.; Kroemer, G. Methionine restriction for improving progeria: Another autophagy-inducing anti-aging strategy? Autophagy 2019, 15, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef]
- Del Campo, L.; Sánchez-López, A.; Salaices, M.; Von Kleeck, R.A.; Expósito, E.; González-Gómez, C.; Cussó, L.; Guzmán-Martínez, G.; Ruiz-Cabello, J.; Desco, M.; et al. Vascular smooth muscle cell-specific progerin expression in a mouse model of Hutchinson–Gilford progeria syndrome promotes arterial stiffness: Therapeutic effect of dietary nitrite. Aging Cell 2019, 18, e12936. [Google Scholar] [CrossRef]
- Kreienkamp, R.; Billon, C.; Bedia-Diaz, G.; Albert, C.J.; Toth, Z.; Butler, A.A.; McBride-Gagyi, S.; Ford, D.A.; Baldan, A.; Burris, T.P.; et al. Doubled lifespan and patient-like pathologies in progeria mice fed high-fat diet. Aging Cell 2019, 18. [Google Scholar] [CrossRef]
- Yang, S.H.; Qiao, X.; Farber, E.; Chang, S.Y.; Fong, L.G.; Young, S.G. Eliminating the Synthesis of Mature Lamin A Reduces Disease Phenotypes in Mice Carrying a Hutchinson-Gilford Progeria Syndrome Allele. J. Biol. Chem. 2008, 283, 7094–7099. [Google Scholar] [CrossRef]
- Lee, J.M.; Nobumori, C.; Tu, Y.; Choi, C.; Yang, S.H.; Jung, H.-J.; Vickers, T.A.; Rigo, F.; Bennett, C.F.; Young, S.G.; et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J. Clin. Investig. 2016, 126, 1592–1602. [Google Scholar] [CrossRef]
- Park, J.T.; Kang, H.T.; Park, C.H.; Lee, Y.-S.; Cho, K.A.; Park, S.C. A crucial role of ROCK for alleviation of senescence-associated phenotype. Exp. Gerontol. 2018, 106, 8–15. [Google Scholar] [CrossRef]
- Subauste, A.R.; Das, A.K.; Li, X.; Elliot, B.; Evans, C.; El Azzouny, M.; Treutelaar, M.; Oral, E.; Leff, T.; Burant, C.F. Alterations in Lipid Signaling Underlie Lipodystrophy Secondary to AGPAT2 Mutations. Diabetes 2012, 61, 2922–2931. [Google Scholar] [CrossRef]
- Davies, S.J.; Ryan, J.; O’Connor, P.B.F.; Kenny, E.; Morris, D.; Baranov, P.V.; O’Connor, R.; McCarthy, T.V. Itm2a silencing rescues lamin A mediated inhibition of 3T3-L1 adipocyte differentiation. Adipocyte 2017, 6, 259–276. [Google Scholar] [CrossRef]
- Bidault, G.; Garcia, M.; Vantyghem, M.-C.; Ducluzeau, P.-H.; Morichon, R.; Thiyagarajah, K.; Moritz, S.; Capeau, J.; Vigouroux, C.; Béréziat, V. Lipodystrophy-Linked LMNA p.R482W Mutation Induces Clinical Early Atherosclerosis and In Vitro Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2162–2171. [Google Scholar] [CrossRef]
- Vatier, C.; Kalbasi, D.; Vantyghem, M.-C.; Lascols, O.; Jéru, I.; Daguenel, A.; Gautier, J.-F.; Buyse, M.; Vigouroux, C. Adherence with metreleptin therapy and health self-perception in patients with lipodystrophic syndromes. Orphanet J. Rare Dis. 2019, 14, 177. [Google Scholar] [CrossRef]
- Wojtanik, K.M.; Edgemon, K.; Viswanadha, S.; Lindsey, B.; Haluzik, M.; Chen, W.; Poy, G.; Reitman, M.; Londos, C. The role of LMNA in adipose: A novel mouse model of lipodystrophy based on the Dunnigan-type familial partial lipodystrophy mutation. J. Lipid Res. 2009, 50, 1068–1079. [Google Scholar] [CrossRef]
- Luedtke, A.; Boschmann, M.; Colpe, C.; Engeli, S.; Adams, F.; Birkenfeld, A.; Haufe, S.; Rahn, G.; Luft, F.; Schmidt, H.; et al. Thiazolidinedione Response in Familial Lipodystrophy Patients with LMNA Mutations: A Case Series. Horm. Metab. Res. 2012, 44, 306–311. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kozlov, S.; Devaux, J.; Vallat, J.-M.; Jamon, M.; Roubertoux, P.; Rabarimeriarijaona, S.; Baudot, C.; Hamadouche, T.; Stewart, C.L.; et al. Behavioral and Molecular Exploration of the AR-CMT2A Mouse Model Lmna R298C/R298C. NeuroMol. Med. 2012, 14, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Morelli, K.H.; Hatton, C.L.; Harper, S.Q.; Burgess, R.W. Gene therapies for axonal neuropathies: Available strategies, successes to date, and what to target next. Brain Res. 2020, 1732, 146683. [Google Scholar] [CrossRef] [PubMed]
- Rossor, A.M.; Tomaselli, P.J.; Reilly, M.M. Recent advances in the genetic neuropathies. Curr. Opin. Neurol. 2016, 29, 537–548. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).